Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
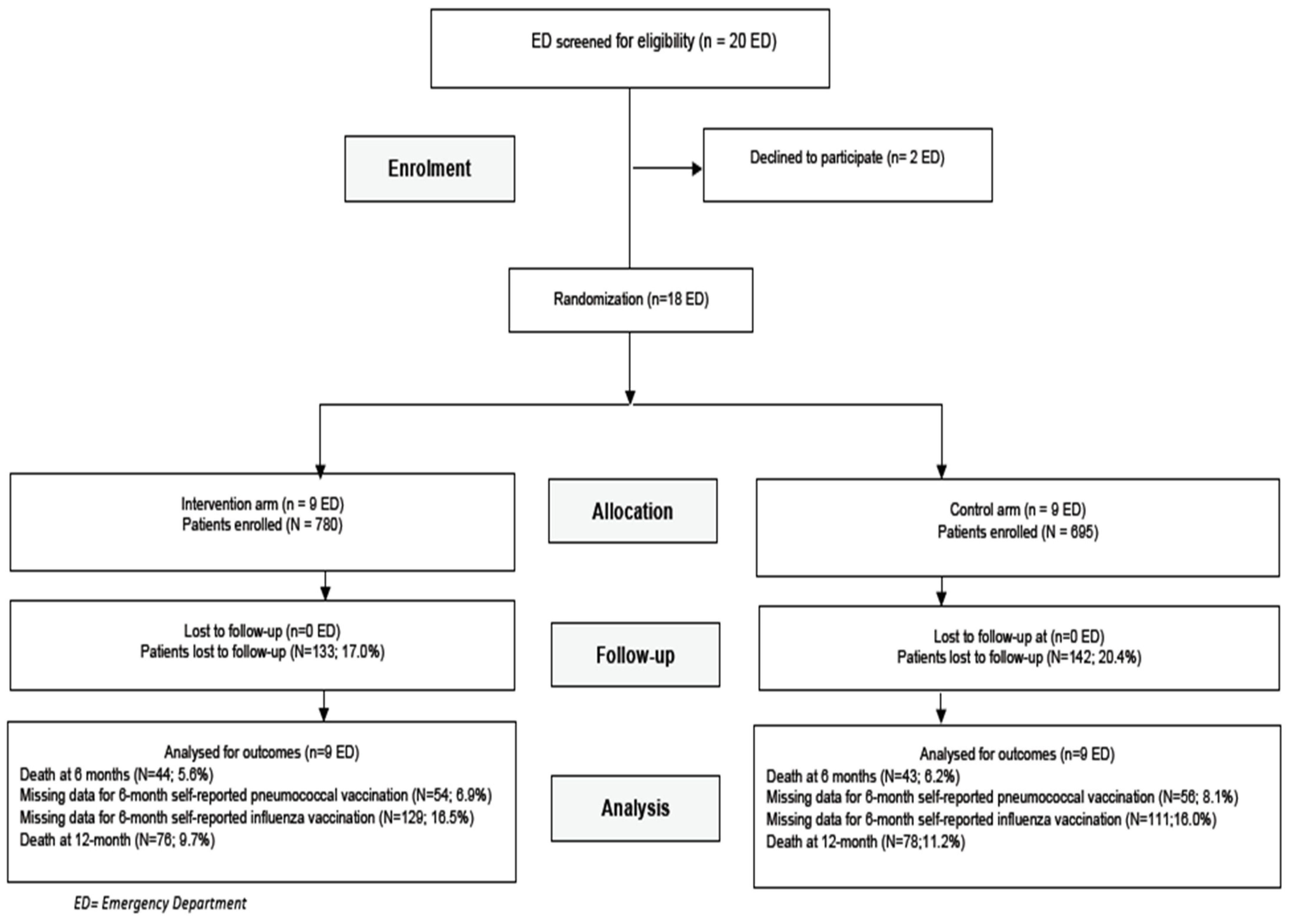
2.2. Participants
2.3. Randomization
2.4. Study Intervention
2.5. Blinding
2.6. Data Collection
2.7. Outcomes
2.8. Sample Size
2.9. Statistical Analysis
2.10. Ethics and Regulatory Issues
3. Results
3.1. Study Population
3.2. Self-Reported Pneumococcal Vaccination
3.3. Self-Reported Influenza Vaccination
3.4. 6-Month and 12-Month All-Cause Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Falkenhorst, G.; Remschmidt, C.; Harder, T.; Hummers-Pradier, E.; Wichmann, O.; Bogdan, C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169368. [Google Scholar] [CrossRef]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; Van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018, 2, CD004876. [Google Scholar] [CrossRef] [Green Version]
- Vaccines against influenza WHO position paper—November 2012. Relev. Epidemiol. Hebd. 2012, 87, 461–476.
- Tomczyk, S.; Bennett, N.M.; Stoecker, C.; Gierke, R.; Moore, M.R.; Whitney, C.G.; Hadler, S.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 822–825. [Google Scholar] [PubMed]
- Vietri, J.; Harnett, J.; Emir, B.; Chilson, E. Uptake of 13-Valent Pneumococcal Conjugate Vaccine among US Adults Aged 19 to 64 Years with Immunocompromising Conditions. Hum. Vaccines Immunother. 2020, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Mangin, O.; Gantzer, L.; Lekens, B.; Simoneau, G.; Ravelomanantsoa, M.; Evans, J.; Bergmann, J.-F.; Sellier, P. Pneumococcal vaccination coverage in France by general practitioners in adults with a high risk of pneumococcal disease. Hum. Vaccines Immunother. 2021, 17, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, B.; Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015, 33, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef] [Green Version]
- Habersaat, K.B.; Jackson, C. Understanding vaccine acceptance and demand—and ways to increase them. Bundesgesundheitsblatt Gesundh. Gesundh. 2019, 63, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.E.; Lorenzetti, D.L. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst. Rev. 2018, 5, CD005188. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Hall, M.E.; Malo, T.; Gilkey, M.B.; Quinn, B.; Lathren, C. Announcements Versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics 2016, 139, e20161764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, E.O.; Stockwell, M.S.; Martinez, R.A.; Vargas, C.Y.; Vawdrey, D.K.; Camargo, S. Effect of a Text Messaging Intervention on Influenza Vaccination in an Urban, Low-Income Pediatric and Adolescent Population: A randomized controlled trial. JAMA 2012, 307, 1702–1708. [Google Scholar] [CrossRef] [Green Version]
- Rimple, D.; Weiss, S.J.; Brett, M.; Ernst, A.A. An Emergency Department-based Vaccination Program: Overcoming the Barriers for Adults at High Risk for Vaccine-preventable Diseases. Acad. Emerg. Med. 2006, 13, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K.; Piaggio, G.; Elbourne, D.R.; Altman, D.G. For the CONSORT Group Consort 2010 statement: Extension to cluster randomised trials. BMJ 2012, 345, e5661. [Google Scholar] [CrossRef] [Green Version]
- Les Personnes âgées aux Urgences: Une patientèle au Profil Particulier—Ministère des Solidarités et de la Santé. Available online: https://drees.solidarites-sante.gouv.fr/etudes-et-statistiques/publications/etudes-et-resultats/article/les-personnes-agees-aux-urgences-une-patientele-au-profil-particulier (accessed on 10 December 2020).
- Données de Couverture Vaccinale Grippe par Groupe d’âge. Available online: /determinants-de-sante/vaccination/donnees-de-couverture-vaccinale-grippe-par-groupe-d-age (accessed on 10 December 2020).
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Naouri, D.; El Khoury, C.; Vincent-Cassy, C.; Vuagnat, A.; Schmidt, J.; Yordanov, Y. For the French Society of Emergency Medicine Evaluation and Quality Committee the French Emergency National Survey: A description of emergency departments and patients in France. PLoS ONE 2018, 13, e0198474. [Google Scholar] [CrossRef]
- Pallin, D.J.; Muennig, P.A.; Emond, J.A.; Kim, S.; Camargo, C.A. Vaccination practices in U.S. emergency departments, 1992–2000. Vaccine 2005, 23, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.J.; Tan, Y.-R.; Cook, A.; Koh, G.; Tham, T.Y.; Anwar, E.; Chiang, G.S.H.; Lwin, M.; Chen, M.I.; McFps, T.Y.T.; et al. Increasing Influenza and Pneumococcal Vaccination Uptake in Seniors Using Point-of-Care Informational Interventions in Primary Care in Singapore: A Pragmatic, Cluster-Randomized Crossover Trial. Am. J. Public Health 2019, 109, 1776–1783. [Google Scholar] [CrossRef]
- Vann, J.C.J.; Jacobson, R.M.; Coyne-Beasley, T.; Asafu-Adjei, J.K.; Szilagyi, P.G. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst. Rev. 2018, 2018, CD003941. [Google Scholar] [CrossRef]
- Herrett, E.; Williamson, E.; Van Staa, T.; Ranopa, M.; Free, C.; Chadborn, T.; Goldacre, B.; Smeeth, L. Text messaging reminders for influenza vaccine in primary care: A cluster randomised controlled trial (TXT4FLUJAB). BMJ Open 2016, 6, e010069. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.K.; Bloomfield, L.; Peters, I.; Effler, P.V. Randomized Controlled Trial of Text Message Reminders for Increasing Influenza Vaccination. Ann. Fam. Med. 2017, 15, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubiana, S.; Launay, O.; Galtier, F.; Tattevin, P.; Postil, D.; Vanhems, P.; Lenzi, N.; Verger, P.; Duval, X. Attitudes, knowledge, and willingness to be vaccinated against seasonal influenza among patients hospitalized with influenza-like-illness: Impact of diagnostic testing. Hum. Vaccines Immunother. 2020, 16, 851–857. [Google Scholar] [CrossRef]
- Loubet, P.; Kernéis, S.; Groh, M.; Loulergue, P.; Blanche, P.; Verger, P.; Launay, O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine 2015, 33, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.; Fronek, P.; Quinn, V.; Wilde, T. Perceptions of influenza and pneumococcal vaccine uptake by older persons in Australia. Vaccine 2019, 37, 4454–4459. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | All (n = 1475) n or med (% or IQR) | Intervention (n = 780) n or med (% or IQR) | Control (n = 695) n or med (% or IQR) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Age (year) | 74 | (69–82) | 74 | (68–80) | 76 | (69–83) | <0.001 |
| Male gender | 739 | (50.1) | 389 | (49.9) | 350 | (50.4) | 0.85 |
| Study site | |||||||
| Location in Paris and suburbs | 517 | (35.1) | 300 | (38.5) | 217 | (31.2) | 0.004 |
| University hospital | 874 | (59.3) | 499 | (64.0) | 375 | (54.0) | <0.001 |
| ED annual volume ≥ 40,000 | 916 | (62.1) | 499 | (64.0) | 417 | (60.0) | 0.12 |
| Enrolled between Nov. and Apr. | 865 | (58.6) | 371 | (47.6) | 494 | (71.1) | <0.001 |
| Reason for ED visit | 0.002 | ||||||
| Respiratory tract infection | 93 | (6.3) | 41 | (5.3) | 52 | (7.5) | |
| Nonrespiratory tract infection | 42 | (2.8) | 20 | (2.6) | 22 | (3.2) | |
| Medical reason | 934 | (63.3) | 525 | (67.3) | 409 | (58.8) | |
| Traumatic reason | 291 | (19.7) | 151 | (19.4) | 140 | (20.1) | |
| Nontraumatic surgery | 23 | (1.6) | 9 | (1.1) | 14 | (2.0) | |
| Psychiatry | 7 | (0.5) | 4 | (0.5) | 3 | (0.4) | |
| Other | 85 | (5.8) | 30 | (3.8) | 55 | (7.9) | |
| Medical history | |||||||
| At least one underlying condition | 515 | (34.9) | 271 | (34.7) | 244 | (35.1) | 0.88 |
| Chronic respiratory disease | 96 | (6.5) | 37 | (4.7) | 59 | (8.5) | 0.004 |
| Heart failure | 209 | (14.2) | 119 | (15.3) | 90 | (12.9) | 0.20 |
| Immunosuppression | 17 | (1.1) | 7 | (0.9) | 10 | (1.4) | 0.33 |
| History of stroke | 97 | (6.6) | 48 | (6.1) | 49 | (7.0) | 0.49 |
| Chronic renal failure | 70 | (4.7) | 30 | (3.8) | 40 | (5.8) | 0.08 |
| Cirrhosis | 17 | (1.1) | 10 | (1.3) | 7 | (1.0) | 0.62 |
| Cancer | 137 | (9.3) | 83 | (10.6) | 54 | (7.8) | 0.06 |
| History of pneumonia | 87 | (5.9) | 47 | (6.0) | 40 | (5.8) | 0.83 |
| History of meningitis | 9 | (0.6) | 5 | (0.6) | 4 | (0.6) | >0.999 |
| History of chronic sinusitis | 41 | (2.8) | 31 | (4.0) | 10 | (1.4) | 0.003 |
| Institution dwelling | 19 | (1.3) | 10 | (1.3) | 9 | (1.3) | >0.999 |
| History of influenza vaccination | 0.26 | ||||||
| Current season influenza vaccination | 739 | (50.1) | 387 | (49.6) | 352 | (50.6) | |
| influenza vaccination > 1 year | 240 | (16.3) | 134 | (17.2) | 106 | (15.2) | |
| No history of influenza vaccination | 493 | (33.4) | 259 | (33.2) | 234 | (33.7) | |
| Missing data | 3 | (0.2) | 0 | (0.0) | 3 | (0.4) | |
| At risk of invasive pneumococcal infection * | 453 | (30.7) | 241 | (30.9) | 212 | (30.5) | 0.87 |
| Hospitalization | 0.91 | ||||||
| Yes | 766 | (51.9) | 409 | (52.4) | 357 | (51.4) | |
| No | 702 | (47.6) | 367 | (47.0) | 335 | (48.2) | |
| Missing data | 7 | (0.5) | 4 | (0.5) | 3 | (0.4) | |
| Outcomes | All (n = 1475) n (%) | Intervention (n = 780) n (%) | Control (n = 695) n (%) | p-Value | |||
|---|---|---|---|---|---|---|---|
| 6-month outcomes | |||||||
| Lost to follow-up | 275 | (18.6) | 133 | (17.0) | 142 | (20.4) | 0.10 |
| Death | 87 | (5.9) | 44 | (5.6) | 43 | (6.2) | 0.66 |
| Self-reported pneumococcal vaccination | 0.14 | ||||||
| Yes | 62 | (4.2) | 38 | (4.9) | 24 | (3.4) | |
| No | 941 | (63.8) | 511 | (65.5) | 430 | (61.9) | |
| No response | 110 | (7.5) | 54 | (6.9) | 56 | (8.1) | |
| Not specified (death or lost to follow-up) | 362 | (24.5) | 177 | (22.7) | 185 | (26.6) | |
| Self-reported influenza vaccination | <0.001 | ||||||
| Yes | 387 | (26.2) | 239 | (30.6) | 148 | (21.3) | |
| No | 486 | (32.9) | 235 | (30.1) | 251 | (36.1) | |
| No response | 240 | (16.3) | 129 | (16.5) | 111 | (16.0) | |
| Not specified (death or lost to follow-up) | 362 | (24.5) | 177 | (22.7) | 185 | (26.6) | |
| 12-month outcome | |||||||
| Death | 154 | (10.4) | 76 | (9.7) | 78 | (11.2) | 0.35 |
| Outcome | Intervention (n = 780) | Control (n = 695) | Odds Ratio * (95% CI) | Absolute Difference * (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Self-reported 6-month pneumococcal vaccination; % | 6.4 | 4.6 | 1.41 | (0.84 to 2.37) | 1.8 | (−0.9 to 4.4) | 0.19 |
| Self-reported 6-month influenza vaccination, % | 52.1 | 40.0 | 1.63 | (1.10 to 2.42) | 12.1 | (2.4 to 21.8) | 0.01 |
| 6-month all-cause mortality, % | 5.6 | 6.2 | 0.91 | (0.59 to 1.40) | −0.5 | (−3.0 to 1.9) | 0.66 |
| 12-month all-cause mortality, % | 9.0 | 10.5 | 0.85 | (0.50 to 1.43) | −1.4 | (−6.1 to 3.2) | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tubiana, S.; Labarere, J.; Levraut, J.; Michelet, P.; de Vaux, F.J.; Doumenc, B.; Hausfater, P.; Choquet, C.; Plaisance, P.; Schmidt, J.; et al. Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial. Vaccines 2021, 9, 962. https://doi.org/10.3390/vaccines9090962
Tubiana S, Labarere J, Levraut J, Michelet P, de Vaux FJ, Doumenc B, Hausfater P, Choquet C, Plaisance P, Schmidt J, et al. Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial. Vaccines. 2021; 9(9):962. https://doi.org/10.3390/vaccines9090962
Chicago/Turabian StyleTubiana, Sarah, José Labarere, Jacques Levraut, Pierre Michelet, Fleur Jourda de Vaux, Benoit Doumenc, Pierre Hausfater, Christophe Choquet, Patrick Plaisance, Jeannot Schmidt, and et al. 2021. "Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial" Vaccines 9, no. 9: 962. https://doi.org/10.3390/vaccines9090962
APA StyleTubiana, S., Labarere, J., Levraut, J., Michelet, P., de Vaux, F. J., Doumenc, B., Hausfater, P., Choquet, C., Plaisance, P., Schmidt, J., Mattei, V., Gacia, O., Storme, D., Ray, P., Der Sahakian, G., Kouka, M.-C., Jainsky, L., Raude, J., Duval, X., & Claessens, Y.-E. (2021). Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial. Vaccines, 9(9), 962. https://doi.org/10.3390/vaccines9090962






