Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Construction of Recombinant Baculovirus
2.3. Production and Purification of VLPs
2.4. Western Blotting Analyses
2.5. TEM and IEM
2.6. Immunization of Mice
2.7. ELISA
2.8. Neutralizing Antibody Assay
2.9. Flow Cytometry
2.10. MTT Assay
2.11. EliSpot
2.12. Statistical Analysis
3. Results
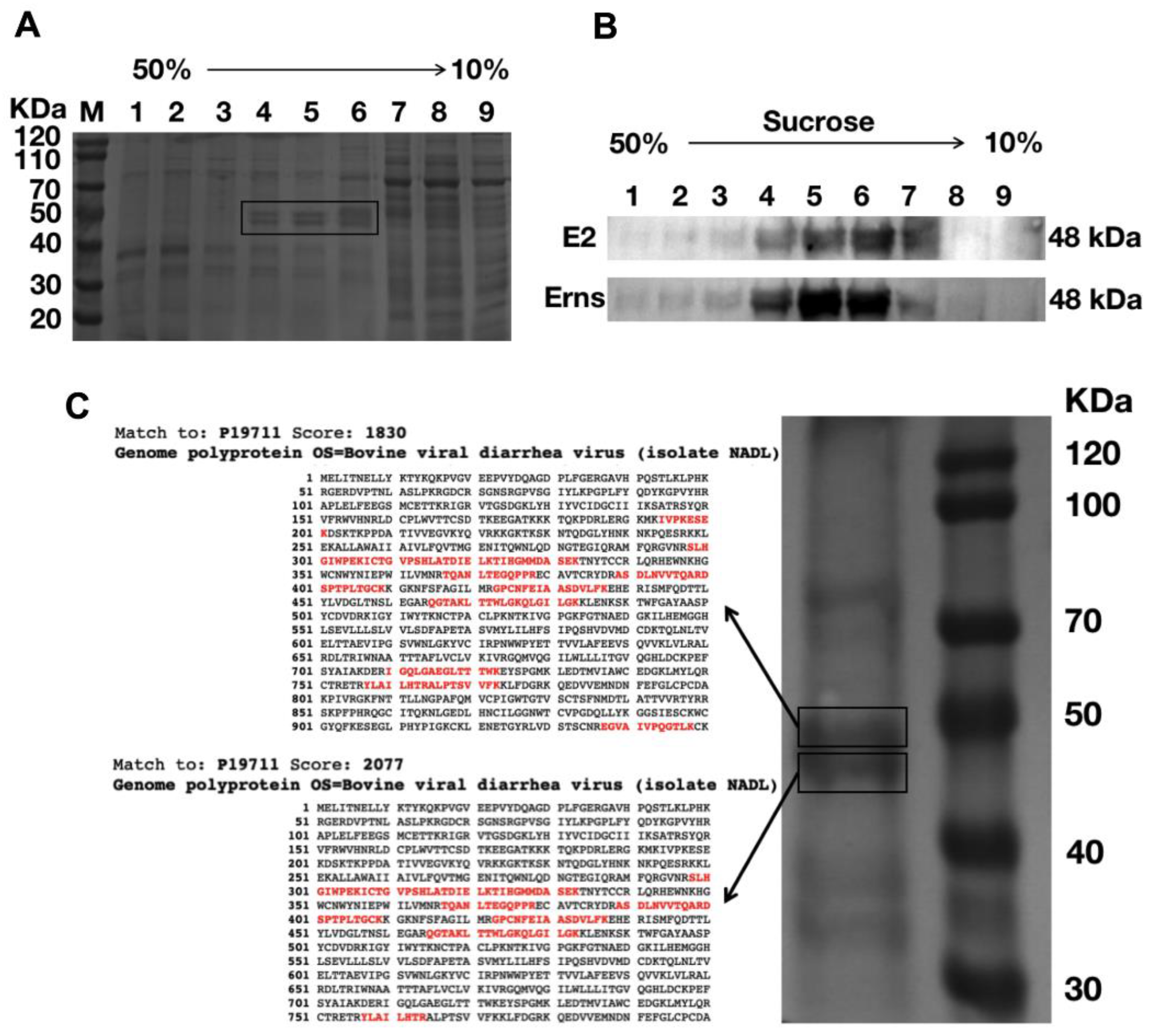
3.1. Expression of Erns and E2 Proteins in Sf9 Cells
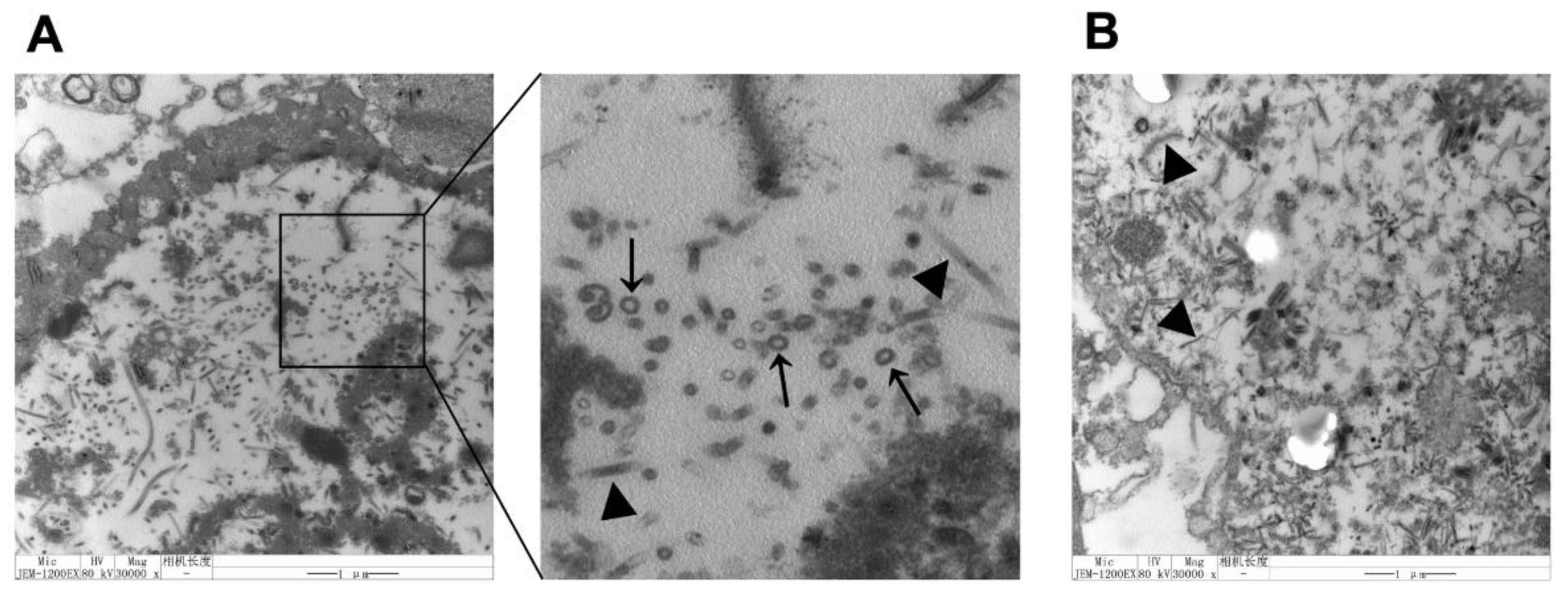
3.2. Identification of Particle Formation in Sf9 Cells
3.3. Production, Purification, and Characterization of the BVDV-VLPs
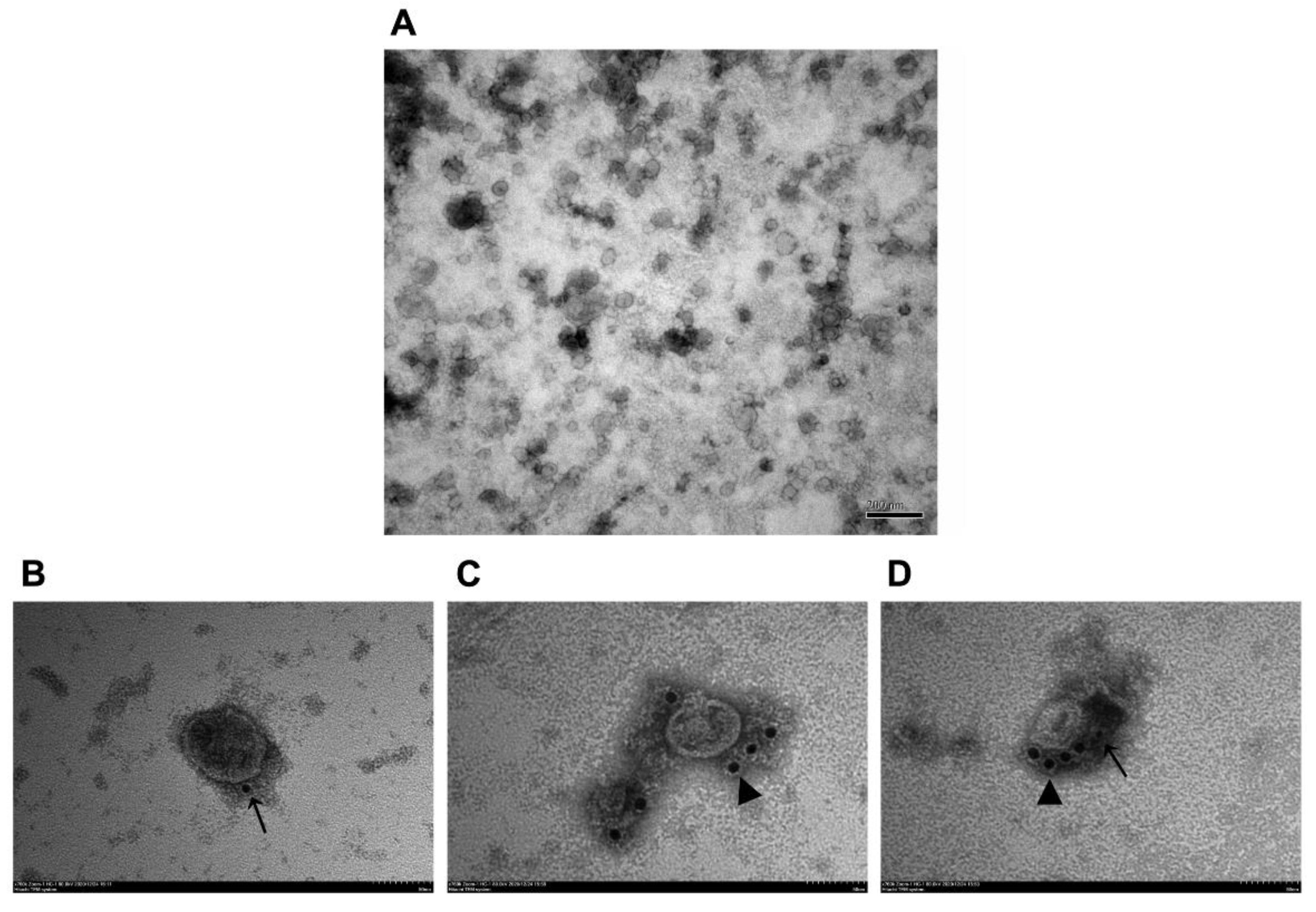
3.4. Observation of VLPs by TEM and IEM
3.5. Homodimerisation of Erns or E2 Proteins in VLPs
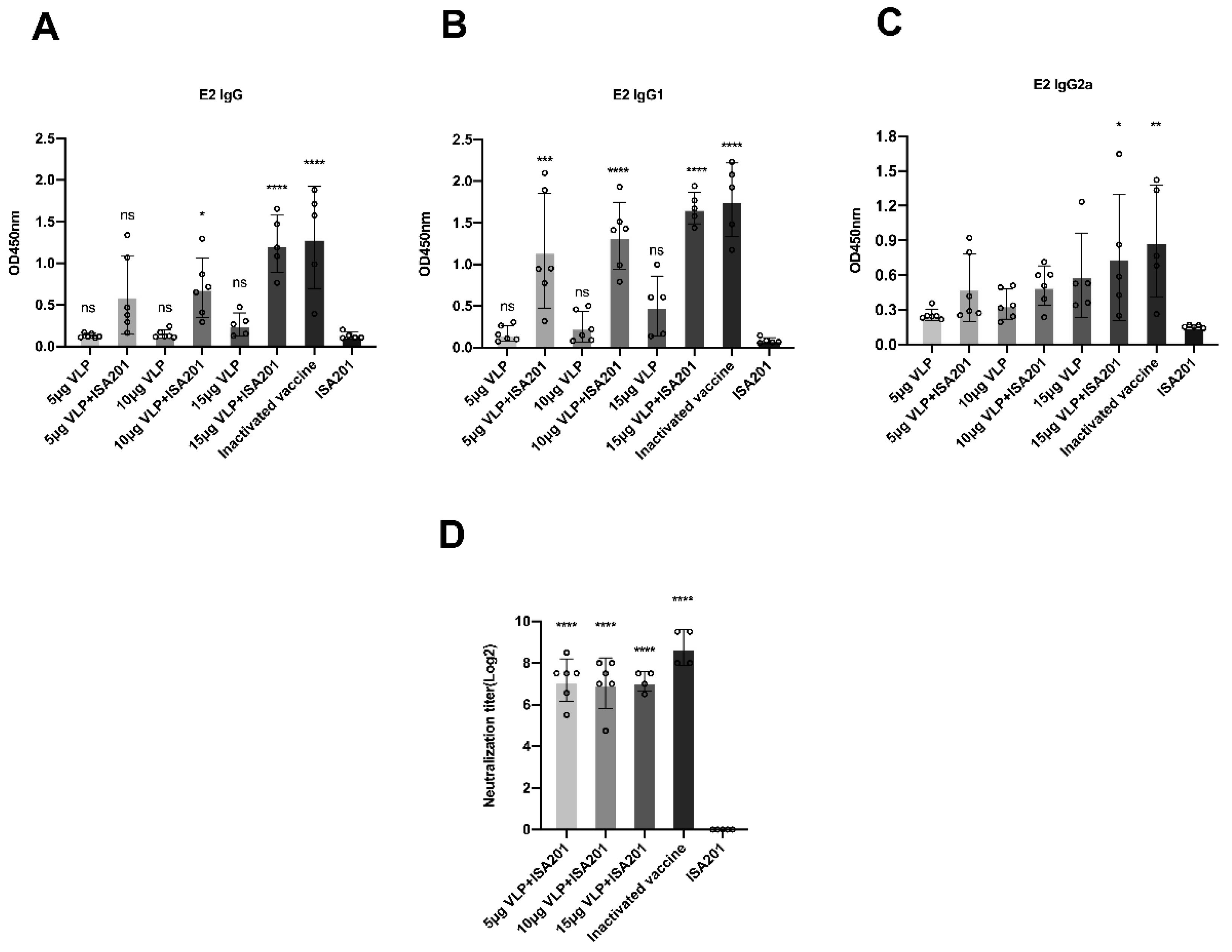
3.6. Immunization With BVDV-VLP-Induced E2-Specific IgG, IgG1, IgG2a, and BVDV-Neutralizing Antibodies
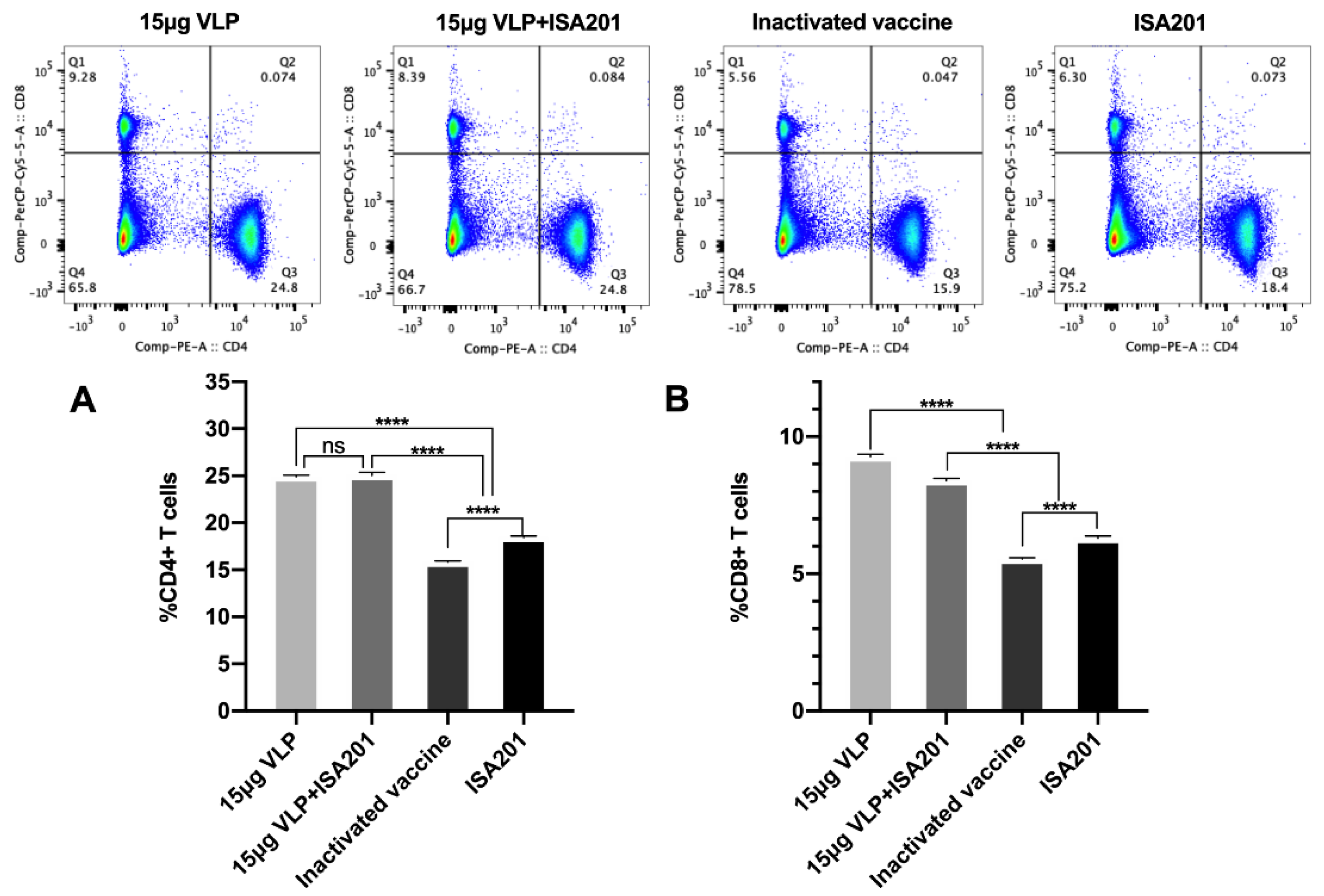
3.7. Activation of T cells by BVDV-VLPs
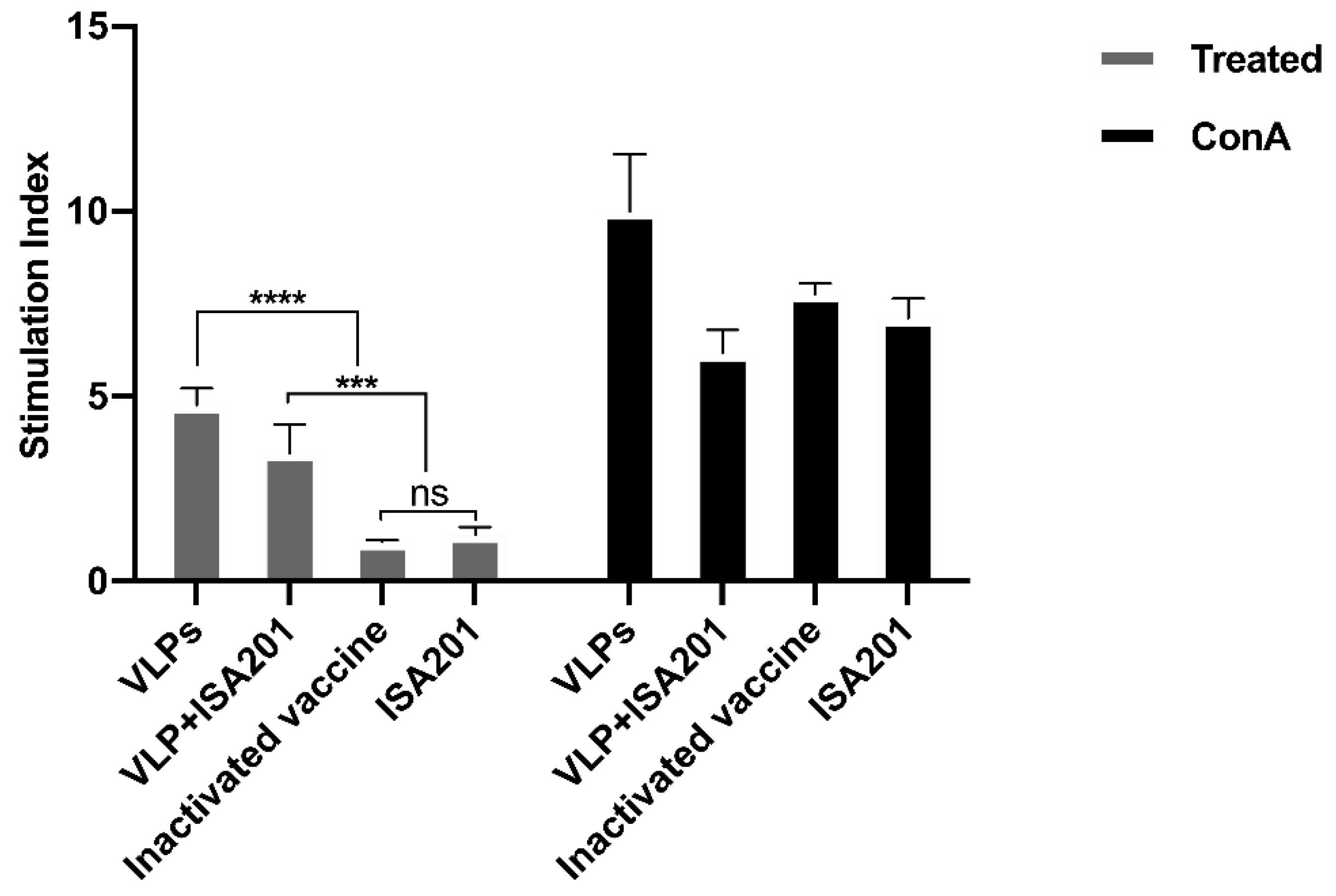
3.8. The Effect of VLPs on the Proliferation of Splenocytes
3.9. The Effect of VLPs on Cytokine Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, D.D.; Duprau, J.L.; Wolff, P.L.; Evermann, J.F. Persistent Bovine Viral Diarrhea Virus Infection in Domestic and Wild Small Ruminants and Camelids Including the Mountain Goat (Oreamnos americanus). Front. Microbiol. 2015, 6, 1415. [Google Scholar] [CrossRef] [PubMed]
- Khodakaram-Tafti, A.; Farjanikish, G.H. Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran. J. Vet. Res. 2017, 18, 154–163. [Google Scholar] [PubMed]
- Brock, K.V. The persistence of bovine viral diarrhea virus. Biologicals 2003, 31, 133–135. [Google Scholar] [CrossRef]
- Dubovi, E.J. Molecular biology of bovine virus diarrhoea virus. Rev. Sci. Tech. 1990, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yesilbag, K.; Alpay, G.; Becher, P. Variability and Global Distribution of Subgenotypes of Bovine Viral Diarrhea Virus. Viruses 2017, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Bauermann, F.V.; Ridpath, J.F.; Weiblen, R.; Flores, E.F. HoBi-like viruses: An emerging group of pestiviruses. J. Vet. Diagn. Investig. 2013, 25, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Meyers, G.; Thiel, H.J. Pathogenesis of mucosal disease, a deadly disease of cattle caused by a pestivirus. Clin. Diagn. Virol. 1998, 10, 121–127. [Google Scholar] [CrossRef]
- Colett, M.S.; Larson, R.; Gold, C.; Strick, D.; Anderson, D.K.; Purchio, A.F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 1988, 165, 191–199. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Tews, B.A.; Meyers, G. The Molecular Biology of Pestiviruses. Adv. Virus Res. 2015, 93, 47–160. [Google Scholar] [CrossRef]
- Hulst, M.M.; Moormann, R.J. Erns protein of pestiviruses. Methods Enzym. 2001, 342, 431–440. [Google Scholar] [CrossRef]
- Krey, T.; Bontems, F.; Vonrhein, C.; Vaney, M.C.; Bricogne, G.; Rumenapf, T.; Rey, F.A. Crystal structure of the pestivirus envelope glycoprotein E(rns) and mechanistic analysis of its ribonuclease activity. Structure 2012, 20, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Lazar, C.; Dwek, R.A.; Zitzmann, N. Role of N-glycan trimming in the folding and secretion of the pestivirus protein E(rns). Biochem. Biophys. Res. Commun. 2004, 319, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Branza-Nichita, N.; Lazar, C.; Durantel, D.; Dwek, R.A.; Zitzmann, N. Role of disulfide bond formation in the folding and assembly of the envelope glycoproteins of a pestivirus. Biochem. Biophys. Res. Commun. 2002, 296, 470–476. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Kanai, R.; Modis, Y. Crystal structure of glycoprotein E2 from bovine viral diarrhea virus. Proc. Natl. Acad. Sci. USA 2013, 110, 6805–6810. [Google Scholar] [CrossRef] [PubMed]
- El Omari, K.; Iourin, O.; Harlos, K.; Grimes, J.M.; Stuart, D.I. Structure of a pestivirus envelope glycoprotein E2 clarifies its role in cell entry. Cell Rep. 2013, 3, 30–35. [Google Scholar] [CrossRef]
- Donofrio, G.; Bottarelli, E.; Sandro, C.; Flammini, C.F. Expression of bovine viral diarrhea virus glycoprotein E2 as a soluble secreted form in a Mammalian cell line. Clin. Vaccine Immunol. 2006, 13, 698–701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krey, T.; Himmelreich, A.; Heimann, M.; Menge, C.; Thiel, H.J.; Maurer, K.; Rumenapf, T. Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 2006, 80, 3912–3922. [Google Scholar] [CrossRef]
- Fuenmayor, J.; Godia, F.; Cervera, L. Production of virus-like particles for vaccines. New Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Aksular, M.; Graves, L.P.; Irons, S.L.; Possee, R.D.; King, L.A. Overview of the Baculovirus Expression System. Curr. Protoc. Protein Sci. 2018, 91, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Deschuyteneer, M.; Elouahabi, A.; Plainchamp, D.; Plisnier, M.; Soete, D.; Corazza, Y.; Lockman, L.; Giannini, S.; Deschamps, M. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum. Vaccines 2010, 6, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Riedmann, E.M. Human Vaccines & Immunotherapeutics: News. Hum Vaccines Immunother. 2014, 10, 1773–1777. [Google Scholar]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hemmi, H.; Miyamoto, H.; Moriishi, K.; Tamura, S.; Takaku, H.; Akira, S.; Matsuura, Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 2005, 79, 2847–2858. [Google Scholar] [CrossRef]
- Abe, T.; Kaname, Y.; Wen, X.; Tani, H.; Moriishi, K.; Uematsu, S.; Takeuchi, O.; Ishii, K.J.; Kawai, T.; Akira, S.; et al. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J. Virol. 2009, 83, 7629–7640. [Google Scholar] [CrossRef]
- Palomares, L.A.; Joosten, C.E.; Hughes, P.R.; Granados, R.R.; Shuler, M.L. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol. Prog. 2003, 19, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Suphatrakul, A.; Yasanga, T.; Keelapang, P.; Sriburi, R.; Roytrakul, T.; Pulmanausahakul, R.; Utaipat, U.; Kawilapan, Y.; Puttikhunt, C.; Kasinrerk, W.; et al. Generation and preclinical immunogenicity study of dengue type 2 virus-like particles derived from stably transfected mosquito cells. Vaccine 2015, 33, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chiao, D.J.; Hsu, Y.L.; Lin, C.C.; Wu, H.L.; Shu, P.Y.; Chang, S.F.; Chang, J.H.; Kuo, S.C. Mosquito Cell-Derived Japanese Encephalitis Virus-Like Particles Induce Specific Humoral and Cellular Immune Responses in Mice. Viruses 2020, 12, 336. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika Virus Baculovirus-Expressed Virus-Like Particles Induce Neutralizing Antibodies in Mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef]
- Rebollo, B.; Sarraseca, J.; Rodriguez, M.J.; Sanz, A.; Jimenez-Clavero, M.A.; Venteo, A. Diagnostic aptitude of West Nile virus-like particles expressed in insect cells. Diagn. Microbiol. Infect. Dis. 2018, 91, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Elmowalid, G.A.; Qiao, M.; Jeong, S.H.; Borg, B.B.; Baumert, T.F.; Sapp, R.K.; Hu, Z.; Murthy, K.; Liang, T.J. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc. Natl. Acad. Sci. USA 2007, 104, 8427–8432. [Google Scholar] [CrossRef]
- Ran, X.; Chen, X.; Ma, L.; Wen, X.; Zhai, J.; Wang, M.; Tong, X.; Hou, G.; Ni, H. A systematic review and meta-analysis of the epidemiology of bovine viral diarrhea virus (BVDV) infection in dairy cattle in China. Acta Trop. 2019, 190, 296–303. [Google Scholar] [CrossRef]
- Deng, M.; Chen, N.; Guidarini, C.; Xu, Z.; Zhang, J.; Cai, L.; Yuan, S.; Sun, Y.; Metcalfe, L. Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet. Microbiol. 2020, 242, 108565. [Google Scholar] [CrossRef] [PubMed]
- Pinior, B.; Firth, C.L.; Richter, V.; Lebl, K.; Trauffler, M.; Dzieciol, M.; Hutter, S.E.; Burgstaller, J.; Obritzhauser, W.; Winter, P.; et al. A systematic review of financial and economic assessments of bovine viral diarrhea virus (BVDV) prevention and mitigation activities worldwide. Prev. Vet. Med. 2017, 137, 77–92. [Google Scholar] [CrossRef]
- Chimeno Zoth, S.; Leunda, M.R.; Odeon, A.; Taboga, O. Recombinant E2 glycoprotein of bovine viral diarrhea virus induces a solid humoral neutralizing immune response but fails to confer total protection in cattle. Braz. J. Med. Biol. Res. 2007, 40, 813–818. [Google Scholar] [CrossRef][Green Version]
- Thomas, C.; Young, N.J.; Heaney, J.; Collins, M.E.; Brownlie, J. Evaluation of efficacy of mammalian and baculovirus expressed E2 subunit vaccine candidates to bovine viral diarrhoea virus. Vaccine 2009, 27, 2387–2393. [Google Scholar] [CrossRef]
- Bhuyan, A.A.; Memon, A.M.; Bhuiyan, A.A.; Zhonghua, L.; Zhang, B.; Ye, S.; Mengying, L.; He, Q.G. The construction of recombinant Lactobacillus casei expressing BVDV E2 protein and its immune response in mice. J. Biotechnol. 2018, 270, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, B.; Niu, C.; Jia, S.; Sun, C.; Wang, Z.; Jiang, Y.; Cui, W.; Wang, L.; Xu, Y. Dendritic Cell Targeting of Bovine Viral Diarrhea Virus E2 Protein Expressed by Lactobacillus casei Effectively Induces Antigen-Specific Immune Responses via Oral Vaccination. Viruses 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, G.; Nie, J.; Yang, R.; Du, M.; Su, J.; Wang, J.; Wang, J.; Zhu, Y. Recombinant E(rns)-E2 protein vaccine formulated with MF59 and CPG-ODN promotes T cell immunity against bovine viral diarrhea virus infection. Vaccine 2020, 38, 3881–3891. [Google Scholar] [CrossRef]
- Pande, A.; Carr, B.V.; Wong, S.Y.; Dalton, K.; Jones, I.M.; McCauley, J.W.; Charleston, B. The glycosylation pattern of baculovirus expressed envelope protein E2 affects its ability to prevent infection with bovine viral diarrhoea virus. Virus Res. 2005, 114, 54–62. [Google Scholar] [CrossRef]
- Huang, J.; Liu, N.; Xu, F.; Ayepa, E.; Amanze, C.; Sun, L.; Shen, Y.; Yang, M.; Yang, S.; Shen, X.; et al. Efficient Expression and Processing of Ebola Virus Glycoprotein Induces Morphological Changes in BmN Cells but Cannot Rescue Deficiency of Bombyx Mori Nucleopolyhedrovirus GP64. Viruses 2019, 11, 1067. [Google Scholar] [CrossRef]
- Schmeiser, S.; Mast, J.; Thiel, H.J.; Konig, M. Morphogenesis of pestiviruses: New insights from ultrastructural studies of strain Giraffe-1. J. Virol. 2014, 88, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Callens, N.; Brugger, B.; Bonnafous, P.; Drobecq, H.; Gerl, M.J.; Krey, T.; Roman-Sosa, G.; Rumenapf, T.; Lambert, O.; Dubuisson, J.; et al. Morphology and Molecular Composition of Purified Bovine Viral Diarrhea Virus Envelope. PLoS Pathog. 2016, 12, e1005476. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Flick-Smith, H.; McCauley, J.W. Interactions of bovine viral diarrhoea virus glycoprotein E(rns) with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Asfor, A.S.; Wakeley, P.R.; Drew, T.W.; Paton, D.J. Recombinant pestivirus E2 glycoproteins prevent viral attachment to permissive and non permissive cells with different efficiency. Virus Res. 2014, 189, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tews, B.A.; Schurmann, E.M.; Meyers, G. Mutation of cysteine 171 of pestivirus E rns RNase prevents homodimer formation and leads to attenuation of classical swine fever virus. J. Virol. 2009, 83, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Sisk, W.P.; Bradley, J.D.; Leipold, R.J.; Stoltzfus, A.M.; Ponce de Leon, M.; Hilf, M.; Peng, C.; Cohen, G.H.; Eisenberg, R.J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 1994, 68, 766–775. [Google Scholar] [CrossRef]
- Tessier, D.C.; Thomas, D.Y.; Khouri, H.E.; Laliberte, F.; Vernet, T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene 1991, 98, 177–183. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Holmes, J.; Katona, I.M.; Urban, J.F., Jr.; Beckmann, M.P.; Park, L.S.; Schooley, K.A.; Coffman, R.L.; Mosmann, T.R.; Paul, W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990, 8, 303–333. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, M.; Zhao, H.; Wang, P.; Ma, W.; Zhang, Y.; Wu, W.; Peng, C. Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System. Vaccines 2021, 9, 350. https://doi.org/10.3390/vaccines9040350
Wang Z, Liu M, Zhao H, Wang P, Ma W, Zhang Y, Wu W, Peng C. Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System. Vaccines. 2021; 9(4):350. https://doi.org/10.3390/vaccines9040350
Chicago/Turabian StyleWang, Zhanhui, Mengyao Liu, Haoran Zhao, Pengpeng Wang, Wenge Ma, Yunke Zhang, Wenxue Wu, and Chen Peng. 2021. "Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System" Vaccines 9, no. 4: 350. https://doi.org/10.3390/vaccines9040350
APA StyleWang, Z., Liu, M., Zhao, H., Wang, P., Ma, W., Zhang, Y., Wu, W., & Peng, C. (2021). Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System. Vaccines, 9(4), 350. https://doi.org/10.3390/vaccines9040350




