Prophylactic and Therapeutic EBV Vaccines: Major Scientific Obstacles, Historical Progress, and Future Direction
Abstract
1. Introduction
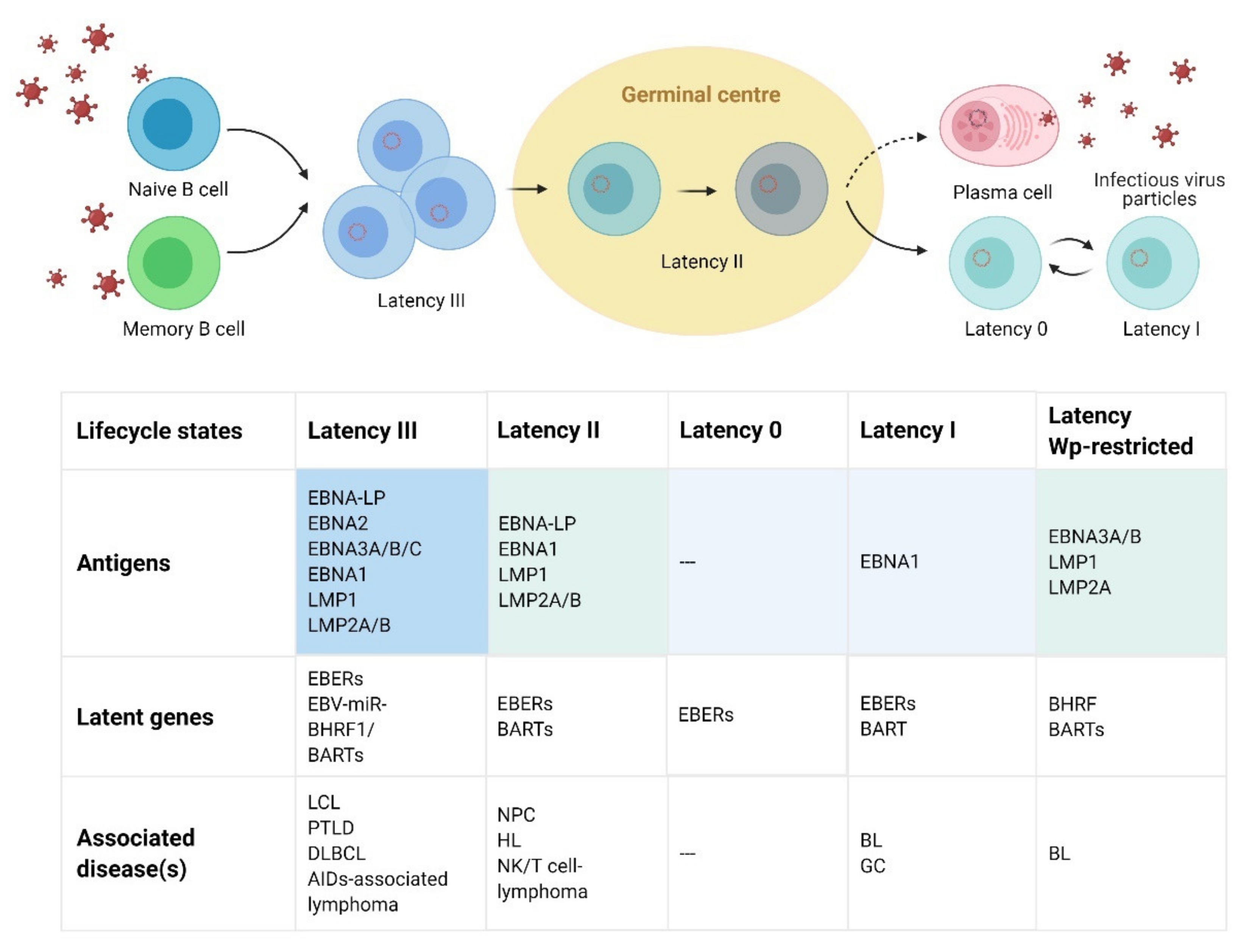
2. EBV Virologic Features and Lifecycle
3. Immune Responses to EBV Infection
3.1. Humoral Immune Responses
3.2. Cellular Immune Responses
4. EBV Transmission: Extensive and Rebellious
5. Challenges in EBV Vaccine Development
5.1. Immunization Surveillance and Efficacy Assessment
5.2. Adjuvant Selection
5.3. Appropriate Animal Models
5.4. The Complexity of EBV Infection and Vaccine Design
6. Progress: Where Are We Now?
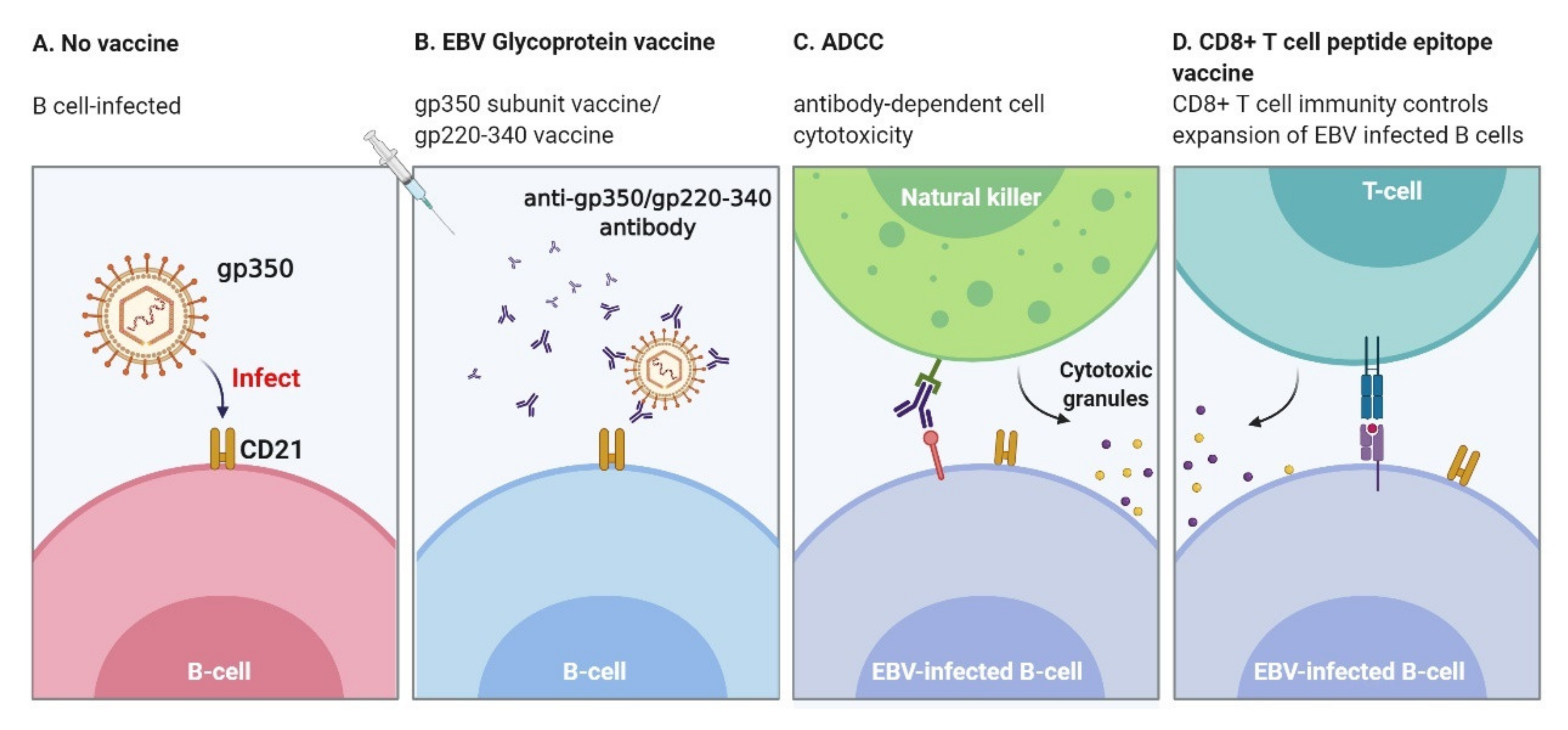
6.1. Envelope Protein Vaccines: Neutralizing Antibodies Elicited
6.2. Protein Polymers and Nano-Vaccines Enhance the Response of Antibodies
6.3. CD8+ T cell Peptide Epitope Vaccine
6.4. DC Vaccines
6.5. Viral Vector Vaccines
6.6. Virus-Like Particles (VLPs)
6.7. Nucleic Acid Vaccines
7. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Lieberman, P.M. Epstein-Barr virus turns 50. Science 2014, 343, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- De-The, G.; Geser, A.; Day, N.E.; Tukei, P.M.; Williams, E.H.; Beri, D.P.; Smith, P.G.; Dean, A.G.; Bronkamm, G.W.; Feorino, P.; et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature 1978, 274, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fachko, D.N.; Ivanov, N.S.; Skalsky, R.L. B Cell Receptor-Responsive miR-141 Enhances Epstein-Barr Virus Lytic Cycle via FOXO3 Inhibition. mSphere 2021, 6, e00093-21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fachko, D.; Ivanov, N.S.; Skinner, C.M.; Skalsky, R.L. Epstein-Barr virus microRNAs regulate B cell receptor signal transduction and lytic reactivation. PLoS Pathog. 2019, 15, e1007535. [Google Scholar] [CrossRef]
- Münz, C. Epstein-Barr Virus-Specific Immune Control by Innate Lymphocytes. Front. Immunol. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H., Jr. Primary Epstein-Barr virus infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Berliner, N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. 2018, 13, 27–49. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; Lunemann, J. The initiation and prevention of multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 602–612. [Google Scholar] [CrossRef]
- Toussirot, E.; Roudier, J. Epstein-Barr virus in autoimmune diseases. Best Practice & Research. Clin. Rheumatol. 2008, 22, 883–896. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Epstein, M.A.; Henle, G.; Achong, B.G.; Barr, Y.M. Morphological and Biological Studies on a Virus in Cultured Lymphoblasts from Burkitt’s Lymphoma. J. Exp. Med. 1965, 121, 761–770. [Google Scholar] [CrossRef]
- Lung, M.L.; So, S.; Chan, K.; Lam, W.; Ng, M. Evidence That Respiratory Tract Is Major Reservoir for Epstein-Barr Virus. Lancet 1985, 325, 889–892. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Kutok, J.; Wang, F. Spectrum of Epstein-Barr Virus–Associated Diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 375–404. [Google Scholar] [CrossRef]
- Fukayama, M. Epstein-Barr virus and gastric carcinoma. Pathol. Int. 2010, 60, 337–350. [Google Scholar] [CrossRef]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Cohen, J.I.; Fauci, A.S.; Varmus, H.; Nabel, G.J. Epstein-Barr Virus: An Important Vaccine Target for Cancer Prevention. Sci. Transl. Med. 2011, 3, 107fs7. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G. The EB virus. Annu. Rev. Microbiol. 1973, 27, 413–436. [Google Scholar] [CrossRef]
- Epstein, M.A. Epstein-Barr virus—Is it time to develop a vaccine program? J. Nat. Cancer Inst. 1976, 56, 697–700. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2020, 19, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, Z.H. Comparative virion structures of human herpesviruses. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Nii, S.; Uno, F.; Yoshida, M.; Akatsuka, K. Structure and assembly of human beta herpesviruses. Jpn. J. Clin. Med. 1998, 56, 22–28. [Google Scholar]
- Johannsen, E.; Luftig, M.; Chase, M.R.; Weicksel, S.; Cahir-McFarland, E.; Illanes, D.; Sarracino, D.; Kieff, E. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 2004, 101, 16286–16291. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, K.; Desai, P.; Winkler, D.C.; Heymann, J.B.; Belnap, D.M.; Baumeister, W.; Steven, A.C. Three-Dimensional Structure of Herpes Simplex Virus from Cryo-Electron Tomography. Science 2003, 302, 1396–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Dong, L.; Pang, J.; Xu, M.; Zhong, Q.; Zeng, M.-S.; Yu, X. CryoEM structure of the tegumented capsid of Epstein-Barr virus. Cell Res. 2020, 30, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jia, Q.; Bortz, E.; Shah, S.; Liu, J.; Atanasov, I.; Li, X.; Taylor, K.A.; Sun, R.; Zhou, Z.H. Unique structures in a tumor herpesvirus revealed by cryo-electron tomography and microscopy. J. Struct. Biol. 2008, 161, 428–438. [Google Scholar] [CrossRef]
- Halder, S.; Murakami, M.; Verma, S.C.; Kumar, P.; Yi, F.; Robertson, E.S. Early Events Associated with Infection of Epstein-Barr Virus Infection of Primary B-Cells. PLoS ONE 2009, 4, e7214. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G.; Horan, K.A.; Fitzgerald, K.A. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 2011, 11, 143–154. [Google Scholar] [CrossRef]
- Lieberman, P.M. Keeping it quiet: Chromatin control of gammaherpesvirus latency. Nat. Rev. Microbiol. 2013, 11, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. B cells under influence: Transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 2003, 3, 801–812. [Google Scholar] [CrossRef]
- McKenzie, J.; El-Guindy, A. Epstein-Barr Virus Lytic Cycle Reactivation. Curr. Top. Microbiol. Immunol. 2015, 391, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Schelcher, C.; Valencia, S.; Delecluse, H.-J.; Hicks, M.; Sinclair, A.J. Mutation of a Single Amino Acid Residue in the Basic Region of the Epstein-Barr Virus (EBV) Lytic Cycle Switch Protein Zta (BZLF1) Prevents Reactivation of EBV from Latency. J. Virol. 2005, 79, 13822–13828. [Google Scholar] [CrossRef] [PubMed]
- Murata, T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Hewetson, J.F.; Rocchi, G.; Henle, W.; Henle, G. Neutralizing Antibodies to Epstein-Barr Virus in Healthy Populations and Patients with Infectious Mononucleosis. J. Infect. Dis. 1973, 128, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, C.A.; Henle, W.; Henle, G.; Rudnick, H.; Latts, E. Long-Term Serological Follow-Up of Patients for Epstein-Barr Virus After Recovery from Infectious Mononucleosis. J. Infect. Dis. 1985, 151, 1150–1153. [Google Scholar] [CrossRef]
- Bu, W.; Hayes, G.M.; Liu, H.; Gemmell, L.; Schmeling, D.O.; Radecki, P.; Aguilar, F.; Burbelo, P.D.; Woo, J.; Balfour, H.H.; et al. Kinetics of Epstein-Barr Virus (EBV) Neutralizing and Virus-Specific Antibodies after Primary Infection with EBV. Clin. Vaccine Immunol. 2016, 23, 363–369. [Google Scholar] [CrossRef]
- Henle, W.; Henle, G.; Andersson, J.; Ernberg, I.; Klein, G.; Horwitz, C.A.; Marklund, G.; Rymo, L.; Wellinder, C.; Straus, S.E. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc. Natl. Acad. Sci. USA 1987, 84, 570–574. [Google Scholar] [CrossRef]
- Yao, Q.Y.; Rowe, M.; Morgan, A.J.; Sam, C.K.; Prasad, U.; Dang, H.; Zeng, Y.; Rickinson, A.B. Salivary and serum IgA antibodies to the epstein-barr virus glycoprotein gp340: Incidence and potential for virus neutralization. Int. J. Cancer 2007, 48, 45–50. [Google Scholar] [CrossRef]
- Yao, Q.Y.; Rowe, M.; Martin, B.; Young, L.; Rickinson, A.B. The Epstein—Barr virus carrier state: Dominance of a single growth-transforming isolate in the blood and in the oropharynx of healthy virus carriers. J. Gen. Virol. 1991, 72, 1579–1590. [Google Scholar] [CrossRef]
- Fingeroth, J.D.; Weis, J.J.; Tedder, T.F.; Strominger, J.L.; Biro, P.A.; Fearon, D.T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA 1984, 81, 4510–4514. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Bu, W.; Joyce, M.G.; Meng, G.; Whittle, J.R.; Baxa, U.; Yamamoto, T.; Narpala, S.; Todd, J.-P.; Rao, S.S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Chen, X.S.; Holers, V.M.; Hannan, J.P. Isolating the Epstein-Barr virus gp350/220 binding site on complement receptor type 2 (CR2/CD21). J. Biol. Chem. 2007, 282, 36614–36625. [Google Scholar] [CrossRef]
- Kanda, T.; Yajima, M.; Ikuta, K. Epstein-Barr virus strain variation and cancer. Cancer Sci. 2019, 110, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.A.; Dreyfus, D.H.; Jones, J.F.; Gelfand, E.W. EBV infection of T cells: Potential role in malignant transformation. Semin. Cancer Biol. 1996, 7, 197–207. [Google Scholar] [CrossRef]
- Hislop, A.D.; Taylor, G.S.; Sauce, D.; Rickinson, A.B. Cellular Responses to Viral Infection in Humans: Lessons from Epstein-Barr Virus. Annu. Rev. Immunol. 2007, 25, 587–617. [Google Scholar] [CrossRef]
- Abbott, R.J.M.; Quinn, L.L.; Leese, A.M.; Scholes, H.M.; Pachnio, A.; Rickinson, A.B. CD8+T Cell Responses to Lytic EBV Infection: Late Antigen Specificities as Subdominant Components of the Total Response. J. Immunol. 2013, 191, 5398–5409. [Google Scholar] [CrossRef]
- Benninger-Döring, G.; Pepperl, S.; Deml, L.; Modrow, S.; Wolf, H.; Jilg, W. Frequency of CD8+ T Lymphocytes Specific for Lytic and Latent Antigens of Epstein-Barr Virus in Healthy Virus Carriers. Virology 1999, 264, 289–297. [Google Scholar] [CrossRef][Green Version]
- Saulquin, X.; Ibisch, C.; Peyrat, M.-A.; Scotet, E.; Hourmant, M.; Vie, H.; Bonneville, M.; Houssaint, E. A global appraisal of immunodominant CD8 T cell responses to Epstein-Barr virus and cytomegalovirus by bulk screening. Eur. J. Immunol. 2000, 30, 2531–2539. [Google Scholar] [CrossRef]
- Bihl, F.; Frahm, N.; Di Giammarino, L.; Sidney, J.; John, M.; Yusim, K.; Woodberry, T.; Sango, K.; Hewitt, H.S.; Henry, L.; et al. Impact of HLA-B Alleles, Epitope Binding Affinity, Functional Avidity, and Viral Coinfection on the Immunodominance of Virus-Specific CTL Responses. J. Immunol. 2006, 176, 4094–4101. [Google Scholar] [CrossRef] [PubMed]
- Kalra, M.; Gerdemann, U.; Luu, J.D.; Ngo, M.C.; Leen, A.M.; Louis, C.U.; Rooney, C.M.; Gottschalk, S. Epstein-Barr Virus (EBV)-derived BARF1 encodes CD4- and CD8-restricted epitopes as targets for T-cell immunotherapy. Cytotherapy 2018, 21, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Luo, Y.; Deng, R.; Liu, X.; Wang, J.; Wang, L.; Zhang, B.; Wang, F.; Lu, J.; Li, X. EBV-EBNA1 constructs an immunosuppressive microenvironment for nasopharyngeal carcinoma by promoting the chemoattraction of Treg cells. J. Immunother. Cancer 2020, 8, e001588. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B.; Manet, E.; Gruffat, H.; Busson, P.; Blondel, M.; Fahraeus, R. EBNA1: Oncogenic Activity, Immune Evasion and Biochemical Functions Provide Targets for Novel Therapeutic Strategies against Epstein-Barr Virus- Associated Cancers. Cancers 2018, 10, 109. [Google Scholar] [CrossRef]
- Amyes, E.; Hatton, C.; Montamat-Sicotte, D.; Gudgeon, N.; Rickinson, A.B.; McMichael, A.J.; Callan, M.F. Characterization of the CD4+ T Cell Response to Epstein-Barr Virus during Primary and Persistent Infection. J. Exp. Med. 2003, 198, 903–911. [Google Scholar] [CrossRef]
- Precopio, M.L.; Sullivan, J.L.; Willard, C.; Somasundaran, M.; Luzuriaga, K. Differential Kinetics and Specificity of EBV-Specific CD4+and CD8+T Cells During Primary Infection. J. Immunol. 2003, 170, 2590–2598. [Google Scholar] [CrossRef]
- Di Trolio, R.; di Lorenzo, G.; Barbiero, E.; Iacono, A.; Franco, R.; Armiento, M.D.; Delfino, M.; Armiento, F.P.D. Expression of HECA-452 in parapsoriasis and mycosis fungoides. Int. J. Immunopathol. Pharmacol. 2006, 19, 105–110. [Google Scholar] [CrossRef]
- Adhikary, D.; Behrends, U.; Moosmann, A.; Witter, K.; Bornkamm, G.W.; Mautner, J. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J. Exp. Med. 2006, 203, 995–1006. [Google Scholar] [CrossRef]
- Wallace, L.E.; Wright, J.; Ulaeto, D.O.; Morgan, A.J.; Rickinson, A.B. Identification of two T-cell epitopes on the candidate Epstein-Barr virus vaccine glycoprotein gp340 recognized by CD4+ T-cell clones. J. Virol. 1991, 65, 3821–3828. [Google Scholar] [CrossRef]
- Klinker, M.W.; Lizzio, V.; Reed, T.J.; Fox, D.A.; Lundy, S.K. Human B Cell-Derived Lymphoblastoid Cell Lines Constitutively Produce Fas Ligand and Secrete MHCII(+)FasL(+) Killer Exosomes. Front. Immunol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Williams, H.; McAulay, K.; Macsween, K.F.; Gallacher, N.J.; Higgins, C.D.; Harrison, N.; Swerdlow, A.; Crawford, D.H. The immune response to primary EBV infection: A role for natural killer cells. Br. J. Haematol. 2005, 129, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Grimm, J.M.; Schmeling, D.O.; Balfour, H.H.; Hogquist, K.A. The Incubation Period of Primary Epstein-Barr Virus Infection: Viral Dynamics and Immunologic Events. PLoS Pathog. 2015, 11, e1005286. [Google Scholar] [CrossRef]
- Azzi, T.; Lunemann, A.; Murer, A.; Ueda, S.; Beziat, V.; Malmberg, K.J.; Staubli, G.; Gysin, C.; Berger, C.; Munz, C.; et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 2014, 124, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Pappworth, I.Y.; Wang, E.C.; Rowe, M. The Switch from Latent to Productive Infection in Epstein-Barr Virus-Infected B Cells Is Associated with Sensitization to NK Cell Killing. J. Virol. 2007, 81, 474–482. [Google Scholar] [CrossRef]
- Chijioke, O.; Muller, A.; Feederle, R.; Barros, M.H.; Krieg, C.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Landtwing, V.; Raykova, A.; Pezzino, G.; Béziat, V.; Marcenaro, E.; Graf, C.; Moretta, A.; Capaul, R.; Zbinden, A.; Ferlazzo, G.; et al. Cognate HLA absence in trans diminishes human NK cell education. J. Clin. Investig. 2016, 126, 3772–3782. [Google Scholar] [CrossRef][Green Version]
- Abolhassani, H.; Edwards, E.S.J.; Ikinciogullari, A.; Jing, H.; Borte, S.; Buggert, M.; Du, L.; Matsuda-Lennikov, M.; Romano, R.; Caridha, R.; et al. Combined immunodeficiency and Epstein-Barr virus–induced B cell malignancy in humans with inherited CD70 deficiency. J. Exp. Med. 2016, 214, 91–106. [Google Scholar] [CrossRef]
- Hoagland, R.J. The transmission of infectious mononucleosis. Am. J. Med. Sci. 1955, 229, 262–272. [Google Scholar] [CrossRef]
- Balfour, H.H., Jr.; Odumade, O.A.; Schmeling, D.O.; Mullan, B.D.; Ed, J.A.; Knight, J.A.; Vezina, H.E.; Thomas, W.; Hogquist, K.A. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J. Infect. Dis. 2013, 207, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.M.; Schmeling, D.O.; Dunmire, S.K.; Knight, J.A.; Mullan, B.D.; Ed, J.A.; Brundage, R.C.; Hogquist, K.A.; Balfour, H.H. Prospective studies of infectious mononucleosis in university students. Clin. Transl. Immunol. 2016, 5, e94. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.H.; Macsween, K.F.; Higgins, C.D.; Thomas, R.; McAulay, K.; Williams, H.; Harrison, N.; Reid, S.; Conacher, M.; Douglas, J.; et al. A Cohort Study among University Students: Identification of Risk Factors for Epstein-Barr Virus Seroconversion and Infectious Mononucleosis. Clin. Infect. Dis. 2006, 43, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; McClain, K.; Frizzera, G.; Gajl-Peczalska, K.J.; Kersey, J.H.; Blazar, B.R.; Arthur, D.C.; Patton, D.F.; Greenberg, J.S.; Burke, B. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 1988, 71, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.S.; Schmeling, D.O.; Knight, J.A.; Matas, A.J.; Balfour, H.H. Valganciclovir Administration to Kidney Donors to Reduce the Burden of Cytomegalovirus and Epstein-Barr Virus Transmission During Transplantation. Transplantation 2015, 99, 1186–1191. [Google Scholar] [CrossRef]
- Gerber, P.; Walsh, J.; Rosenblum, E.; Purcell, R. Association of Eb-Virus Infection with the Post-Perfusion Syndrome. Lancet 1969, 293, 593–596. [Google Scholar] [CrossRef]
- Alfieri, C.; Tanner, J.; Carpentier, L.; Perpete, C.; Savoie, A.; Paradis, K.; Delage, G.; Joncas, J. Epstein-Barr virus transmission from a blood donor to an organ transplant recipient with recovery of the same virus strain from the recipient’s blood and oropharynx. Blood 1996, 87, 812–887. [Google Scholar] [CrossRef]
- Trottier, H.; Buteau, C.; Robitaille, N.; Duval, M.; Tucci, M.; Lacroix, J.; Alfieri, C. Transfusion-related Epstein-Barr virus infection among stem cell transplant recipients: A retrospective cohort study in children. Transfusion 2012, 52, 2653–2663. [Google Scholar] [CrossRef]
- Sumaya, C.V.; Ench, Y. Epstein-Barr Virus Infections in Families: The Role of Children with Infectious Mononucleosis. J. Infect. Dis. 1986, 154, 842–850. [Google Scholar] [CrossRef]
- Johnson, K.H.; Webb, C.-H.; Schmeling, D.O.; Brundage, R.C.; Balfour, H.H. Epstein-Barr virus dynamics in asymptomatic immunocompetent adults: An intensive 6-month study. Clin. Transl. Immunol. 2016, 5, e81. [Google Scholar] [CrossRef]
- Butler, L.M.; Neilands, T.B.; Mosam, A.; Mzolo, S.; Martin, J.N. A population-based study of how children are exposed to saliva in KwaZulu-Natal Province, South Africa: Implications for the spread of saliva-borne pathogens to children. Trop. Med. Int. Health 2010, 15, 442–453. [Google Scholar] [CrossRef]
- Taylor, G.S.; Long, H.M.; Brooks, J.M.; Rickinson, A.B.; Hislop, A.D. The Immunology of Epstein-Barr Virus–Induced Disease. Annu. Rev. Immunol. 2015, 33, 787–821. [Google Scholar] [CrossRef]
- Sitki-Green, D.; Covington, M.; Raab-Traub, N. Compartmentalization and Transmission of Multiple Epstein-Barr Virus Strains in Asymptomatic Carriers. J. Virol. 2003, 77, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Walling, D.M.; Brown, A.L.; Etienne, W.; Keitel, W.A.; Ling, P.D. Multiple Epstein-Barr Virus Infections in Healthy Individuals. J. Virol. 2003, 77, 6546–6550. [Google Scholar] [CrossRef]
- Balfour, H.H. Progress, prospects, and problems in Epstein-Barr virus vaccine development. Curr. Opin. Virol. 2014, 6, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Zyl, D.G.; Mautner, J.; Delecluse, H.J. Progress in EBV Vaccines. Front. Oncol. 2019, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection for Inactivated Influenza Vaccines in Children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Glass, R.I.; Jiang, B.; Santosham, M.; Lopman, B.; Parashar, U. A Systematic Review of Anti-Rotavirus Serum IgA Antibody Titer as a Potential Correlate of Rotavirus Vaccine Efficacy. J. Infect. Dis. 2013, 208, 284–294. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Barouch, D.H.; Alter, G. Systems serology for evaluation of HIV vaccine trials. Immunol. Rev. 2017, 275, 262–270. [Google Scholar] [CrossRef]
- Cortese, M.; Sherman, A.C.; Rouphael, N.G.; Pulendran, B. Systems Biological Analysis of Immune Response to Influenza Vaccination. Cold Spring Harb. Perspect. Med. 2020, 11, a038596. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lo, W.-F.; Lee, T.-H.; Ren, Y.; Hwang, S.-L.; Cheng, Y.-F.; Chen, C.-L.; Chang, Y.-S.; Lee, S.P.; Rickinson, A.B.; et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002, 62, 6952–6958. [Google Scholar]
- Chia, W.K.; Wang, W.W.; Teo, M.; Tai, W.M.; Lim, W.T.; Tan, E.H.; Leong, S.S.; Sun, L.; Chen, J.J.; Gottschalk, S.; et al. A phase II study evaluating the safety and efficacy of an adenovirus-DeltaLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann. Oncol. 2012, 23, 997–1005. [Google Scholar] [CrossRef]
- Hui, E.P.; Taylor, G.S.; Jia, H.; Ma, B.; Chan, S.; Ho, R.; Wong, W.-L.; Wilson, S.; Johnson, B.; Edwards, C.; et al. Phase I Trial of Recombinant Modified Vaccinia Ankara Encoding Epstein-Barr Viral Tumor Antigens in Nasopharyngeal Carcinoma Patients. Cancer Res. 2013, 73, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein-Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients with EBV-Positive Cancer. Clin. Cancer Res. 2014, 20, 5009–5022. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Brenner, M.K. Immunotherapy against cancer-related viruses. Cell Res. 2016, 27, 59–73. [Google Scholar] [CrossRef]
- Si, Y.; Deng, Z.; Lan, G.; Du, H.; Wang, Y.; Si, J.; Wei, J.; Weng, J.; Qin, Y.; Huang, B.; et al. The Safety and Immunological Effects of rAd5-EBV-LMP2 Vaccine in Nasopharyngeal Carcinoma Patients: A Phase I Clinical Trial and Two-Year Follow-Up. Chem. Pharm. Bull. 2016, 64, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Hagn, M.; Panikkar, A.; Smith, C.; Balfour, H.H., Jr.; Khanna, R.; Voskoboinik, I.; Trapani, J.A. B cell-derived circulating granzyme B is a feature of acute infectious mononucleosis. Clin. Transl. Immunol. 2015, 4, e38. [Google Scholar] [CrossRef]
- Burrows, S.R.; Moss, D.J.; Khanna, R. Understanding human T-cell-mediated immunoregulation through herpesviruses. Immunol. Cell Biol. 2011, 89, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Panikkar, A.; Smith, C.; Hislop, A.D.; Tellam, N.; Dasari, V.; Hogquist, K.A.; Wykes, M.; Moss, D.J.; Rickinson, A.; Balfour, H.H.; et al. Impaired Epstein-Barr Virus-Specific Neutralizing Antibody Response during Acute Infectious Mononucleosis Is Coincident with Global B-Cell Dysfunction. J. Virol. 2015, 89, 9137–9141. [Google Scholar] [CrossRef]
- Panikkar, A.; Smith, C.; Hislop, A.; Tellam, N.; Dasari, V.; Hogquist, K.A.; Wykes, M.; Moss, D.J.; Rickinson, A.; Balfour, H.H., Jr.; et al. Cytokine-Mediated Loss of Blood Dendritic Cells During Epstein-Barr Virus-Associated Acute Infectious Mononucleosis: Implication for Immune Dysregulation. J. Infect. Dis. 2015, 212, 1957–1961. [Google Scholar] [CrossRef]
- Morgan, A.J.; Epstein, M.A.; North, J.R. Comparative immunogenicity studies on epstein-barr virus membrane antigen (MA) gp340 with novel adjuvants in mice, rabbits, and cotton-top tamarins. J. Med. Virol. 1984, 13, 281–292. [Google Scholar] [CrossRef]
- Morgan, A.J.; Finerty, S.; Lovgren, K.; Scullion, F.T.; Morein, B. Prevention of Epstein-Barr (EB) Virus-induced Lymphoma in Cottontop Tamarins by Vaccination with the EB Virus Envelope Glycoprotein gp340 Incorporated into Immune-stimulating Complexes. J. Gen. Virol. 1988, 69, 2093–2096. [Google Scholar] [CrossRef]
- Finerty, S.; Tarlton, J.; Mackett, M.; Conway, M.; Arrand, J.R.; Watkins, P.E.; Morgan, A.J. Protective immunization against Epstein-Barr virus-induced disease in cottontop tamarins using the virus envelope glycoprotein gp340 produced from a bovine papillomavirus expression vector. J. Gen. Virol. 1992, 73, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Finerty, S.; Mackett, M.; Arrand, J.R.; Watkins, P.E.; Tarlton, J.; Morgan, A.J. Immunization of cottontop tamarins and rabbits with a candidate vaccine against the Epstein-Barr virus based on the major viral envelope glycoprotein gp340 and alum. Vaccine 1994, 12, 1180–1184. [Google Scholar] [CrossRef]
- Klasse, P.J.; Nixon, D.F.; Moore, J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021, 7, eabe8065. [Google Scholar] [CrossRef]
- Moutschen, M.; Léonard, P.; Sokal, E.; Smets, F.; Haumont, M.; Mazzu, P.; Bollen, A.; Denamur, F.; Peeters, P.; Dubin, G.; et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 2007, 25, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.A.; Morgan, A.J.; Finerty, S.; Randle, B.J.; Kirkwood, J.K. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature 1985, 318, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Emini, E.A.; Schleif, W.A.; Silberklang, M.; Lehman, D.; Ellis, R.W. Vero cell-expressed Epstein-Barr virus (EBV) gp350/220 protects marmosets from EBV challenge. J. Med. Virol. 1989, 27, 120–123. [Google Scholar] [CrossRef]
- Leskowitz, R.; Fogg, M.H.; Zhou, X.Y.; Kaur, A.; Silveira, E.L.V.; Villinger, F.; Lieberman, P.M.; Wang, F.; Ertl, H.C. Adenovirus-Based Vaccines against Rhesus Lymphocryptovirus EBNA-1 Induce Expansion of Specific CD8+ and CD4+ T Cells in Persistently Infected Rhesus Macaques. J. Virol. 2014, 88, 4721–4735. [Google Scholar] [CrossRef]
- Leskowitz, R.M.; Zhou, X.Y.; Villinger, F.; Fogg, M.H.; Kaur, A.; Lieberman, P.M.; Wang, F.; Ertl, H.C. CD4 + and CD8 + T-Cell Responses to Latent Antigen EBNA-1 and Lytic Antigen BZLF-1 during Persistent Lymphocryptovirus Infection of Rhesus Macaques. J. Virol. 2013, 87, 8351–8362. [Google Scholar] [CrossRef]
- Rivailler, P.; Carville, A.; Kaur, A.; Rao, P.; Quink, C.; Kutok, J.L.; Westmoreland, S.; Klumpp, S.; Simon, M.; Aster, J.C.; et al. Experimental rhesus lymphocryptovirus infection in immunosuppressed macaques: An animal model for Epstein-Barr virus pathogenesis in the immunosuppressed host. Blood 2004, 104, 1482–1489. [Google Scholar] [CrossRef]
- Moghaddam, A.; Koch, J.; Annis, B.; Wang, F. Infection of human B lymphocytes with lymphocryptoviruses related to Epstein-Barr virus. J. Virol. 1998, 72, 3205–3212. [Google Scholar] [CrossRef]
- Sestak, K. Non-Human Primate Models of Enteric Viral Infections. Viruses 2018, 10, 544. [Google Scholar] [CrossRef]
- Sashihara, J.; Hoshino, Y.; Bowman, J.J.; Krogmann, T.; Burbelo, P.D.; Coffield, V.M.; Kamrud, K.; Cohen, J.I. Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge. PLoS Pathog. 2011, 7, e1002308. [Google Scholar] [CrossRef] [PubMed]
- Heeke, D.S.; Lin, R.; Rao, E.; Woo, J.C.; McCarthy, M.P.; Marshall, J.D. Identification of GLA/SE as an effective adjuvant for the induction of robust humoral and cell-mediated immune responses to EBV-gp350 in mice and rabbits. Vaccine 2016, 34, 2562–2569. [Google Scholar] [CrossRef]
- Singh, S.; Homad, L.J.; Akins, N.R.; Stoffers, C.M.; Lackhar, S.; Malhi, H.; Wan, Y.-H.; Rawlings, D.J.; McGuire, A.T. Neutralizing Antibodies Protect against Oral Transmission of Lymphocryptovirus. Cell Rep. Med. 2020, 1, 100033. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Ohashi, M.; Kitamura, Y.; Ando, K.; Nagashima, K.; Sugihara, H.; Okuno, K.; Sairenji, T.; Hayashi, K. A new animal model for primary and persistent Epstein-Barr virus infection: Human EBV-infected rabbit characteristics determined using sequential imaging and pathological analysis. J. Med. Virol. 2008, 80, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kanai, K.; Takashima, K.; Okuno, K.; Kato, K.; Sano, H.; Kuwamoto, S.; Higaki, H.; Nagata, K.; Sugihara, H.; Kato, M.; et al. Lifelong persistent EBV infection of rabbits with EBER1-positive lymphocyte infiltration and mild sublethal hemophagocytosis. Virus Res. 2010, 153, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, S.; Zhou, L.; Du, H.; Mo, W.; Zeng, Y. Specific cellular immune responses in mice immunized with DNA, adeno-associated virus and adenoviral vaccines of Epstein-Barr virus-LMP2 alone or in combination. Sci. China Life Sci. 2011, 54, 263–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, X.; Cao, Z.; Sen, G.; Chattopadhyay, G.; Fuller, D.H.; Fuller, J.T.; Snapper, D.M.; Snow, A.L.; Mond, J.J.; Snapper, C.M. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus vaccine. Vaccine 2013, 31, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Liu, X.; Wang, Y. Expression, purification, and immunogenic characterization of Epstein-Barr virus recombinant EBNA1 protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 2013, 97, 6251–6262. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Sherritt, M.; Khanna, R.; Moss, D. Contrasting Epstein-Barr virus-specific cytotoxic T cell responses to HLA A2-restricted epitopes in humans and HLA transgenic mice: Implications for vaccine design. Vaccine 2001, 19, 3769–3777. [Google Scholar] [CrossRef]
- Hartlage, A.S.; Liu, T.; Patton, J.T.; Garman, S.L.; Zhang, X.; Kurt, H.; Lozanski, G.; Lustberg, M.E.; Caligiuri, M.A.; Baiocchi, R.A. The Epstein-Barr Virus Lytic Protein BZLF1 as a Candidate Target Antigen for Vaccine Development. Cancer Immunol. Res. 2015, 3, 787–794. [Google Scholar] [CrossRef]
- Van Zyl, D.G.; Tsai, M.H.; Shumilov, A.; Schneidt, V.; Poirey, R.; Schlehe, B.; Fluhr, H.; Mautner, J.; Delecluse, H.-J. Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice. PLoS Pathog. 2018, 14, e1007464. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.-C.; Lanzavecchia, A.; Manz, M.G. Development of a Human Adaptive Immune System in Cord Blood Cell-Transplanted Mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.L.; Suhrbier, A.; Miles, J.J.; Lawrence, G.; Pye, S.J.; Le, T.T.; Rosenstengel, A.; Nguyen, T.; Allworth, A.; Burrows, S.; et al. Phase I Trial of a CD8 + T-Cell Peptide Epitope-Based Vaccine for Infectious Mononucleosis. J. Virol. 2008, 82, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, L.S.; Hutt-Fletcher, L.M. Fusion of Epstein-Barr virus with epithelial cells can be triggered by alphavbeta5 in addition to alphavbeta6 and alphavbeta8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 2011, 85, 13214–13223. [Google Scholar] [CrossRef]
- Li, Q.; Turk, S.M.; Hutt-Fletcher, L.M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 1995, 69, 3987–3994. [Google Scholar] [CrossRef]
- Turk, S.M.; Jiang, R.; Chesnokova, L.S.; Hutt-Fletcher, L.M. Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. J. Virol. 2006, 80, 9628–9633. [Google Scholar] [CrossRef]
- Snijder, J.; Ortego, M.S.; Weidle, C.; Stuart, A.B.; Gray, M.D.; McElrath, M.J.; Pancera, M.; Veesler, D.; McGuire, A.T. An Antibody Targeting the Fusion Machinery Neutralizes Dual-Tropic Infection and Defines a Site of Vulnerability on Epstein-Barr Virus. Immunity 2018, 48, 799–811. [Google Scholar] [CrossRef]
- Coleman, C.; Wohlford, E.M.; Smith, N.A.; King, C.A.; Ritchie, J.A.; Baresel, P.C.; Kimura, H.; Rochford, R. Epstein-Barr Virus Type 2 Latently Infects T Cells, Inducing an Atypical Activation Characterized by Expression of Lymphotactic Cytokines. J. Virol. 2014, 89, 2301–2312. [Google Scholar] [CrossRef]
- Coleman, C.B.; Daud, I.I.; Ogolla, S.O.; Ritchie, J.A.; Smith, N.A.; Sumba, P.O.; Dent, A.E.; Rochford, R. Epstein-Barr Virus Type 2 Infects T Cells in Healthy Kenyan Children. J. Infect. Dis. 2017, 216, 670–677. [Google Scholar] [CrossRef]
- Isobe, Y.; Sugimoto, K.; Yang, L.; Tamayose, K.; Egashira, M.; Kaneko, T.; Takada, K.; Oshimi, K. Epstein-Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res. 2004, 64, 2167–2174. [Google Scholar] [CrossRef]
- Brooks, J.M.; Long, H.M.; Tierney, R.J.; Shannon-Lowe, C.; Leese, A.M.; Fitzpatrick, M.; Taylor, G.S.; Rickinson, A.B. Early T Cell Recognition of B Cells following Epstein-Barr Virus Infection: Identifying Potential Targets for Prophylactic Vaccination. PLoS Pathog. 2016, 12, e1005549. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, S.; Feederle, R.; Gärtner, K.; Fuchs, W.; Granzow, H.; Delecluse, H.-J. An Epstein-Barr Virus Mutant Produces Immunogenic Defective Particles Devoid of Viral DNA. J. Virol. 2012, 87, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, D.; Behrends, U.; Feederle, R.; Delecluse, H.-J.; Mautner, J. Standardized and Highly Efficient Expansion of Epstein-Barr Virus-Specific CD4 + T Cells by Using Virus-Like Particles. J. Virol. 2008, 82, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Bollard, C.M.; Gottschalk, S.; Torrano, V.; Diouf, O.; Ku, S.; Hazrat, Y.; Carrum, G.; Ramos, C.; Fayad, L.; Shpall, E.J.; et al. Sustained Complete Responses in Patients with Lymphoma Receiving Autologous Cytotoxic T Lymphocytes Targeting Epstein-Barr Virus Latent Membrane Proteins. J. Clin. Oncol. 2014, 32, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, S.; Rooney, C.M. Adoptive T-Cell Immunotherapy. Curr. Top. Microbiol. Immunol. 2015, 391, 427–454. [Google Scholar] [CrossRef]
- Bollard, C.M.; Tripic, T.; Cruz, C.R.; Dotti, G.; Gottschalk, S.; Torrano, V.; Dakhova, O.; Carrum, G.; Ramos, C.A.; Liu, H.; et al. Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients with Relapsed Hodgkin Lymphoma. J. Clin. Oncol. 2018, 36, 1128–1139. [Google Scholar] [CrossRef]
- Bencun, M.; Klinke, O.; Hotz-Wagenblatt, A.; Klaus, S.; Tsai, M.-H.; Poirey, R.; Delecluse, H.-J. Translational profiling of B cells infected with the Epstein-Barr virus reveals 5′ leader ribosome recruitment through upstream open reading frames. Nucleic Acids Res. 2018, 46, 2802–2819. [Google Scholar] [CrossRef]
- Bogedain, C.; Wolf, H.; Modrow, S.; Stuber, G.; Jilg, W. Specific cytotoxic T lymphocytes recognize the immediate-early transactivator Zta of Epstein-Barr virus. J. Virol. 1995, 69, 4872–4879. [Google Scholar] [CrossRef]
- Woodberry, T.; Suscovich, T.J.; Henry, L.M.; Davis, J.K.; Frahm, N.; Walker, B.D.; Scadden, D.T.; Wang, F.; Brander, C. Differential Targeting and Shifts in the Immunodominance of Epstein-Barr Virus–Specific CD8 and CD4 T Cell Responses during Acute and Persistent Infection. J. Infect. Dis. 2005, 192, 1513–1524. [Google Scholar] [CrossRef]
- Jondal, M. Antibody-dependent cellular cytotoxicity (ADCC) against Epstein-Barr virus-determined membrane antigens. I. Reactivity in sera from normal persons and from patients with acute infectious mononucleosis. Clin. Exp. Immunol. 1976, 25, 1–5. [Google Scholar] [PubMed]
- Strnad, B.C.; Schuster, T.; Klein, R.; Hopkins, R.F., 3rd; Witmer, T.; Neubauer, R.H.; Rabin, H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J. Virol. 1982, 41, 258–264. [Google Scholar] [CrossRef]
- Khyatti, M.; Patel, P.C.; Stefanescu, I.; Menezes, J. Epstein-Barr virus (EBV) glycoprotein gp350 expressed on transfected cells resistant to natural killer cell activity serves as a target antigen for EBV-specific antibody-dependent cellular cytotoxicity. J. Virol. 1991, 65, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- López-Montañés, M.; Alari-Pahissa, E.; Sintes, J.; Rodriguez, J.E.M.; Muntasell, A.; López-Botet, M. Antibody-Dependent NK Cell Activation Differentially Targets EBV-Infected Cells in Lytic Cycle and Bystander B Lymphocytes Bound to Viral Antigen–Containing Particles. J. Immunol. 2017, 199, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, S.; Steinberg, S.P.; LaRussa, P.S.; Shapiro, E.D.; Gershon, A.A. Risk of Herpes Zoster in Adults Immunized with Varicella Vaccine. J. Infect. Dis. 2008, 197 (Suppl. S2), S196–S199. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Berline, J.W.; Palefsky, J.M. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 2003, 9, 307–314. [Google Scholar] [CrossRef]
- Xiao, J.; Palefsky, J.M.; Herrera, R.; Berline, J.; Tugizov, S.M. EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells. Virology 2009, 388, 335–343. [Google Scholar] [CrossRef]
- Ohga, S.; Nomura, A.; Takada, H.; Hara, T. Immunological aspects of Epstein-Barr virus infection. Crit. Rev. Oncol. Hematol. 2002, 44, 203–215. [Google Scholar] [CrossRef]
- Ogembo, J.; Kannan, L.; Ghiran, I.; Nicholson-Weller, A.; Finberg, R.W.; Tsokos, G.C.; Fingeroth, J.D. Human Complement Receptor Type 1/CD35 Is an Epstein-Barr Virus Receptor. Cell Rep. 2013, 3, 371–385. [Google Scholar] [CrossRef]
- Sathiyamoorthy, K.; Hu, Y.X.; Möhl, B.S.; Chen, J.; Longnecker, R.; Jardetzky, T.S. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat. Commun. 2016, 7, 13557. [Google Scholar] [CrossRef]
- Sathiyamoorthy, K.; Jiang, J.; Hu, Y.X.; Rowe, C.L.; Möhl, B.S.; Chen, J.; Jiang, W.; Mellins, E.D.; Longnecker, R.; Zhou, Z.H.; et al. Assembly and Architecture of the EBV B Cell Entry Triggering Complex. PLoS Pathog. 2014, 10, e1004309. [Google Scholar] [CrossRef]
- Qualtiere, L.F.; Chase, R.; Pearson, G.R. Purification and biologic characterization of a major Epstein Barr virus-induced membrane glycoprotein. J. Immunol. 1982, 129, 814–818. [Google Scholar] [PubMed]
- Khanna, R.; Sherritt, M.; Burrows, S.R. EBV structural antigens, gp350 and gp85, as targets for ex vivo virus-specific CTL during acute infectious mononucleosis: Potential use of gp350/gp85 CTL epitopes for vaccine design. J. Immunol. 1999, 162, 3063–3069. [Google Scholar] [PubMed]
- Sokal, E.M.; Hoppenbrouwers, K.; Vandermeulen, C.; Moutschen, M.; Leonard, P.; Moreels, A.; Haumont, M.; Bollen, A.; Smets, F.; Denis, M. Recombinant gp350 vaccine for infectious mononucleosis: A phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 2007, 196, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Hettich, E.; Janz, A.; Zeidler, R.; Pich, D.; Hellebrand, E.; Weissflog, B.; Moosmann, A.; Hammerschmidt, W. Genetic design of an optimized packaging cell line for gene vectors transducing human B cells. Gene Ther. 2006, 13, 844–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granato, M.; Feederle, R.; Farina, A.; Gonnella, R.; Santarelli, R.; Hub, B.; Faggioni, A.; Delecluse, H.-J. Deletion of Epstein-Barr Virus BFLF2 Leads to Impaired Viral DNA Packaging and Primary Egress as Well as to the Production of Defective Viral Particles. J. Virol. 2008, 82, 4042–4051. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.E.; Hu, J.; Alfieri, C. Construction and Characterization of a Humanized Anti-Epstein-Barr Virus gp350 Antibody with Neutralizing Activity in Cell Culture. Cancers 2018, 10, 112. [Google Scholar] [CrossRef]
- Tosoni-Pittoni, E.; Joab, I.; Nicolas, J.C.; Perricaudet, M. Complete characterization of the gene coding for the Epstein-Barr virus major membrane antigen gp 220/340 and selective expression of a secreted form of gp 220. Biochem. Biophys. Res. Commun. 1989, 158, 676–684. [Google Scholar] [CrossRef]
- Cui, X.; Cao, Z.; Chen, Q.; Arjunaraja, S.; Snow, A.; Snapper, C.M. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 2016, 34, 4050–4055. [Google Scholar] [CrossRef]
- Bu, W.; Joyce, M.G.; Nguyen, H.; Banh, D.; Aguilar, F.; Tariq, Z.; Yap, M.L.; Tsujimura, Y.; Gillespie, R.A.; Tsybovsky, Y.; et al. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity 2019, 50, 1305–1316.e6. [Google Scholar] [CrossRef]
- Escalante, G.M.; Foley, J.; Mutsvunguma, L.Z.; Rodriguez, E.; Mulama, D.H.; Muniraju, M.; Ye, P.; Barasa, A.K.; Ogembo, J.G. A Pentavalent Epstein-Barr Virus-Like Particle Vaccine Elicits High Titers of Neutralizing Antibodies against Epstein-Barr Virus Infection in Immunized Rabbits. Vaccines 2020, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Dewey, F.; Klein, G.; Henle, G. Relation Between Neutralization of Epstein-Barr Virus and Antibodies to Cell-Membrane Antigens Induced by the Virus2. J. Natl. Cancer Inst. 1970, 45, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Mackett, M.; Arrand, J.R. Recombinant vaccinia virus induces neutralising antibodies in rabbits against Epstein-Barr virus membrane antigen gp340. EMBO J. 1985, 4, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.A.; Randle, B.J.; Finerty, S.; Kirkwood, J.K. Not all potently neutralizing, vaccine-induced antibodies to Epstein-Barr virus ensure protection of susceptible experimental animals. Clin. Exp. Immunol. 1986, 63, 485–490. [Google Scholar]
- Morgan, A.J.; Mackett, M.; Finerty, S.; Arrand, J.R.; Scullion, F.T.; Epstein, M.A. Recombinant vaccinia virus expressing epstein-barr virus glycoprotein gp340 protects cottontop tamarins against EB virus-induced malignant lymphomas. J. Med. Virol. 1988, 25, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jackman, W.T.; Mann, K.A.; Hoffmann, H.J.; Spaete, R.R. Expression of Epstein-Barr virus gp350 as a single chain glycoprotein for an EBV subunit vaccine. Vaccine 1999, 17, 660–668. [Google Scholar] [CrossRef]
- Servat, E.; Ro, B.W.; Cayatte, C.; Gemmell, L.; Barton, C.; Rao, E.; Lin, R.; Zuo, F.; Woo, J.C.; Hayes, G.M. Identification of the critical attribute(s) of EBV gp350 antigen required for elicitation of a neutralizing antibody response in vivo. Vaccine 2015, 33, 6771–6777. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Huang, T.M.; Ruan, L.; Miao, Y.H.; Lu, H.; Chu, C.M.; Motz, M.; Wolf, H. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev. Biol. Stand. 1995, 84, 171–177. [Google Scholar]
- Casey, C.G.; Iskander, J.K.; Roper, M.H.; Mast, E.E.; Wen, X.J.; Torok, T.J.; Chapman, L.E.; Swerdlow, D.L.; Morgan, J.; Heffelfinger, J.D.; et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA 2005, 294, 2734–2743. [Google Scholar] [CrossRef]
- Rees, L.; Tizard, E.J.; Morgan, A.J.; Cubitt, W.D.; Finerty, S.; Oyewole-Eletu, T.A.; Owen, K.; Royed, C.; Stevens, S.J.; Shroff, R.C.; et al. A Phase I Trial of Epstein-Barr Virus Gp350 Vaccine for Children with Chronic Kidney Disease Awaiting Transplantation. Transplantation 2009, 88, 1025–1029. [Google Scholar] [CrossRef]
- Nemerow, G.R.; Houghten, R.A.; Moore, M.D.; Cooper, N.R. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2). Cell 1989, 56, 369–377. [Google Scholar] [CrossRef]
- Hoffman, G.J.; Lazarowitz, S.G.; Hayward, S.D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc. Natl. Acad. Sci. USA 1980, 77, 2979–2983. [Google Scholar] [CrossRef]
- Tanner, J.E.; Coinçon, M.; Leblond, V.; Hu, J.; Fang, J.M.; Sygusch, J.; Alfieri, C. Peptides Designed to Spatially Depict the Epstein-Barr Virus Major Virion Glycoprotein gp350 Neutralization Epitope Elicit Antibodies That Block Virus-Neutralizing Antibody 72A1 Interaction with the Native gp350 Molecule. J. Virol. 2015, 89, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, H.; Liu, Z.; Le, Z.; Nie, T.; Qiao, D.; Su, Y.; Mai, H.; Chen, Y.; Liu, L. Therapeutic nanovaccines sensitize EBV-associated tumors to checkpoint blockade therapy. Biomaterials 2020, 255, 120158. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, S.; Shin, H.; Kim, T.; Yu, B.; Kim, J.; Yoo, D.; Jon, S. Sequential and Timely Combination of a Cancer Nanovaccine with Immune Checkpoint Blockade Effectively Inhibits Tumor Growth and Relapse. Angew. Chem. Int. Ed. 2020, 59, 14628–14638. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Burrows, S.R.; Kurilla, M.G.; Jacob, C.A.; Misko, I.S.; Sculley, T.B.; Kieff, E.; Moss, D.J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: Implications for vaccine development. J. Exp. Med. 1992, 176, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Burrows, S.R.; Sculley, T.B.; Misko, I.S.; Schmidt, C.; Moss, D.J. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J. Exp. Med. 1990, 171, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.J.; Kurilla, M.G.; Brooks, J.M.; Thomas, W.A.; Rowe, M.; Kieff, E.; Rickinson, A.B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): Implications for the immune control of EBV-positive malignancies. J. Exp. Med. 1992, 176, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Burrows, S.R.; Neisig, A.; Neefjes, J.; Moss, D.J.; Silins, S.L. Hierarchy of Epstein-Barr virus-specific cytotoxic T-cell responses in individuals carrying different subtypes of an HLA allele: Implications for epitope-based antiviral vaccines. J. Virol. 1997, 71, 7429–7435. [Google Scholar] [CrossRef]
- Rickinson, A.B.; Moss, D.J. Human Cytotoxic T Lymphocyte Responses to Epstein-Barr Virus Infection. Annu. Rev. Immunol. 1997, 15, 405–431. [Google Scholar] [CrossRef]
- Tellam, J.; Connolly, G.; Green, K.J.; Miles, J.; Moss, D.J.; Burrows, S.R.; Khanna, R. Endogenous Presentation of CD8+ T Cell Epitopes from Epstein-Barr Virus–encoded Nuclear Antigen 1. J. Exp. Med. 2004, 199, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, J.; Sherritt, M.; Thomson, S.; Tellam, J.; Cooper, L.; Connolly, G.; Bharadwaj, M.; Khanna, R. Therapeutic LMP1 polyepitope vaccine for EBV-associated Hodgkin disease and nasopharyngeal carcinoma. Blood 2003, 101, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yao, K.; Wang, B.; Chen, Y.; Zhou, F.; Guo, Y.; Xu, J.; Shi, H. Immunotherapy of Epstein-Barr Virus Associated Malignancies Using Mycobacterial HSP70 and LMP2A356–364 Epitope Fusion Protein. Cell. Mol. Immunol. 2009, 6, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Q.; Zhou, J.; Ma, D.; Xiao, X.; Wang, D.W. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Mol. Cancer Ther. 2009, 8, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, S.; Xue, X.; Lu, L.; Zhu, S.; Li, W.; Chen, X.; Zhong, X.; Jiang, P.; Sename, T.S.; et al. Chimerically fused antigen rich of overlapped epitopes from latent membrane protein 2 (LMP2) of Epstein-Barr virus as a potential vaccine and diagnostic agent. Cell. Mol. Immunol. 2015, 13, 492–501. [Google Scholar] [CrossRef]
- Liu, G.; Yao, K.; Wang, B.; Zhou, F.; Chen, Y.; Li, L.; Chi, J.; Peng, G. Reconstituted complexes of mycobacterial HSP70 and EBV LMP2A-derived peptides elicit peptide-specific cytotoxic T lymphocyte responses and anti-tumor immunity. Vaccine 2011, 29, 7414–7423. [Google Scholar] [CrossRef]
- Silveira, E.; Fogg, M.H.; Leskowitz, R.M.; Ertl, H.C.; Wiseman, R.W.; O’Connor, D.; Lieberman, P.; Wang, F.; Villinger, F. Therapeutic Vaccination against the Rhesus Lymphocryptovirus EBNA-1 Homologue, rhEBNA-1, Elicits T Cell Responses to Novel Epitopes in Rhesus Macaques. J. Virol. 2013, 87, 13904–13910. [Google Scholar] [CrossRef][Green Version]
- Ogembo, J.G.; Muraswki, M.R.; McGinnes, L.W.; Parcharidou, A.; Sutiwisesak, R.; Tison, T.; Avendano, J.; Agnani, D.; Finberg, R.W.; Morrison, T.G.; et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J. Transl. Med. 2015, 13, 50. [Google Scholar] [CrossRef]
- Li, F.; Song, D.; Lu, Y.; Zhu, H.; Chen, Z.; He, X. Delayed-type Hypersensitivity (DTH) Immune Response Related With EBV-DNA in Nasopharyngeal Carcinoma Treated with Autologous Dendritic Cell Vaccination After Radiotherapy. J. Immunother. 2013, 36, 208–214. [Google Scholar] [CrossRef]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.; Hill, A.; Dorrell, L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef]
- Small, J.C.; Ertl, H.C. Viruses—From pathogens to vaccine carriers. Curr. Opin. Virol. 2011, 1, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; di Pilato, M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef]
- Parrino, J.; McCurdy, L.H.; Larkin, B.D.; Gordon, I.J.; Rucker, S.E.; Enama, M.E.; Koup, R.A.; Roederer, M.; Bailer, R.T.; Moodie, Z.; et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax® challenge in vaccinia-naïve and vaccinia-immune individuals. Vaccine 2006, 25, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Tsang, J.; Beagley, L.; Chua, D.; Lee, V.; Li, V.; Moss, D.J.; Coman, W.; Chan, K.H.; Nicholls, J.M.; et al. Effective Treatment of Metastatic Forms of Epstein-Barr Virus–Associated Nasopharyngeal Carcinoma with a Novel Adenovirus-Based Adoptive Immunotherapy. Cancer Res. 2012, 72, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Hammerschmidt, W. Structure and role of the terminal repeats of Epstein-Barr virus in processing and packaging of virion DNA. J. Virol. 1995, 69, 3147–3155. [Google Scholar] [CrossRef]
- Feederle, R.; Shannon-Lowe, C.; Baldwin, G.; Delecluse, H.J. Defective Infectious Particles and Rare Packaged Genomes Produced by Cells Carrying Terminal-Repeat-Negative Epstein-Barr Virus. J. Virol. 2005, 79, 7641–7647. [Google Scholar] [CrossRef] [PubMed]
- Ruiss, R.; Jochum, S.; Wanner, G.; Reisbach, G.; Hammerschmidt, W.; Zeidler, R. A Virus-Like Particle-Based Epstein-Barr Virus Vaccine. J. Virol. 2011, 85, 13105–13113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Ding, M.; Song, S.; Kang, Y.; Yu, Y.; Xu, M.; Xiang, T.; Gao, L.; Feng, Q.; et al. A novel vaccine candidate based on chimeric virus-like particle displaying multiple conserved epitope peptides induced neutralizing antibodies against EBV infection. Theranostics 2020, 10, 5704–5718. [Google Scholar] [CrossRef]
- Ngo, M.C.; Ando, J.; Leen, A.M.; Ennamuri, S.; Lapteva, N.; Vera, J.F.; Min-Venditti, A.; Mims, M.P.; Heslop, H.E.; Bollard, C.M.; et al. Complementation of Antigen-presenting Cells to Generate T Lymphocytes with Broad Target Specificity. J. Immunother. 2014, 37, 193–203. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Chen, H.; Rao, P.; Xue, X.; Chen, S.; Zhu, S.; Zhang, L. Immune response of mice to a latency membrane protein 2 multiepitope antigen of Epstein-Barr virus applied as DNA vaccine and/or peptide vaccine. Acta Virol. 2013, 57, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiang, C.; Zhang, Y.; Govande, A.; Trudeau, S.J.; Chen, F.; Fry, C.J.; Puri, R.; Wolinsky, E.; Schineller, M.; et al. MYC Controls the Epstein-Barr Virus Lytic Switch. Mol. Cell 2020, 78, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Van Diemen, F.R.; Kruse, E.M.; Hooykaas, M.J.; Bruggeling, C.E.; Schurch, A.C.; van Ham, P.M.; Imhof, S.M.; Nijhuis, M.; Wiertz, E.J.; Lebbink, R.J. CRISPR/Cas9-Mediated Genome Editing of Herpesviruses Limits Productive and Latent Infections. PLoS Pathog. 2016, 12, e1005701. [Google Scholar] [CrossRef]
- Noh, K.-W.; Park, J.; Kang, M.-S. Targeted disruption of EBNA1 in EBV-infected cells attenuated cell growth. BMB Rep. 2016, 49, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, X.-C.; Kang, Y.-F.; Zeng, M.-S. The Status and Prospects of Epstein-Barr Virus Prophylactic Vaccine Development. Front. Immunol. 2021, 12, 677027. [Google Scholar] [CrossRef] [PubMed]
- Younger, D.S.; Younger, A.P.; Guttmacher, S. Childhood Vaccination: Implications for Global and Domestic Public Health. Neurol. Clin. 2016, 34, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Van Wijhe, M.; McDonald, S.A.; de Melker, H.E.; Postma, M.J.; Wallinga, J. Effect of vaccination programmes on mortality burden among children and young adults in the Netherlands during the 20th century: A historical analysis. Lancet Infect Dis. 2016, 16, 592–598. [Google Scholar] [CrossRef]
- Rühl, J.; Leung, C.S.; Münz, C. Vaccination against the Epstein-Barr virus. Cell. Mol. Life Sci. 2020, 77, 4315–4324. [Google Scholar] [CrossRef]
- Liu, H.; Gemmell, L.; Lin, R.; Zuo, F.; Balfour, H.H., Jr.; Woo, J.C.; Hayes, G.M. Development of an Improved Epstein-Barr Virus (EBV) Neutralizing Antibody Assay to Facilitate Development of a Prophylactic gp350-Subunit EBV Vaccine. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020016. [Google Scholar] [CrossRef]
| Vaccine | Year | Target Group | Immune Response | Result | Reference |
|---|---|---|---|---|---|
| Live recombinant virus gp350 vaccinia | 1995 | Nine children that were both EBV-seropositive and vaccinia-virus-seronegative | Vaccination boosted EBV-neutralizing antibody titers, but the target group was too small to prove efficacy | No vaccine efficacy | [169,170] |
| Recombinant gp350 vaccine produced in Chinese hamster ovary cells | 2007 | EBV-seropositive and seronegative adults | ELISA antibody titers to gp350 were detected; higher efficacy with MPL adjuvant | No vaccine efficacy | [105] |
| 2007 | EBV-seronegative adults | ELISA antibody titers to gp350 were detected; neutralizing titers developed in 50–60% of persons; higher efficacy with alum adjuvant | One serious adverse event occurred; no vaccine efficacy | [105] | |
| Recombinant gp350 vaccine | 2007 | 181 EBV-negative teenagers/adult subjects | Neutralizing antibodies were detected; vaccine efficacy to prevent infectious mononucleosis by 78%, but no prevention of EBV infection | No serious adverse events were reported; prospects for prevention of Hodgkin’s lymphoma or MS. No further reports | [155] |
| Recombinant gp350 vaccine | 2009 | 16 pediatric renal-transplant EBV-seronegative candidates | Poorly immunogenic, probably due to a low dose and weak adjuvant | The trial could not assess protection from PTLD | [171] |
| EBV peptide vaccine | 2008 | 14 HLA B*801 EBV-seronegative young adults | A CD8+ T cell peptide vaccine was immunogenic with a hint of efficacy | No serious adverse events occurred; CD8+ T cell peptide vaccine: HLA restricted | [125,178] |
| EBV-specific HLA-A2-restricted DC vaccine | 2013 | 16 human leukocyte antigen-A2 (HLA-A2)-positive NPC patients | Th1-specific immune responses were elicited, particularly in DTH test-positive individuals | The vaccine was well-tolerated; this vaccination is a promising treatment for EBV-related NPCs | [190] |
| Adenovirus ΔLMP1–LMP2 transduced DC vaccine | 2012 | 16 metastatic NPC patients | Modified DC induced a low-level immune response | The potency of the current vaccine was too low for significant benefits in patients with extensive disease | [91] |
| AdE1-LMPpoly vaccine | 2012 | 24 EBV-positive nasopharyngeal carcinoma (NPC) | Highly efficient in expanding antigen-specific T cells from patients with advanced recurrent or metastatic NPC disease, and these expanded T cells displayed high levels of functional capacity, as assessed by IFN-γ expression | The AdE1-LMPpoly vaccine was safe and well-tolerated and may offer clinical benefits to patients with NPC | [196] |
| Ankara vaccinia recombinant vector expressing EBNA-1 and LMP2 | 2013 | 18 EBV-positive NPC | CD4+ T cell responses to one or two vaccine antigens | MVA-EL was both safe and immunogenic. However, therapeutic efficacy has not yet been assessed. The highest dose is to be examined in phase II studies for clinical benefits | [92] |
| 2014 | 16 EBV-positive NPC | CD4+ T cell responses to one or two vaccine antigens | MVA-EL was safe and immunogenic across diverse ethnicities and thus suitable for use in trials against different EBV-positive cancers globally | [93] | |
| Adenoviral vaccine of EBV-LMP2 (rAd5-EBV-LMP2) | 2016 | 24 patients with advanced regional NPC | Failed to significantly affect peripheral CD8+ T cells | The rAd5-EBV-LMP2 vaccine was safe and well-tolerated, but has no vaccine efficacy | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Zhang, B.; Li, Y.; Zhu, W.; Akihisa, T.; Li, W.; Kikuchi, T.; Liu, W.; Feng, F.; Zhang, J. Prophylactic and Therapeutic EBV Vaccines: Major Scientific Obstacles, Historical Progress, and Future Direction. Vaccines 2021, 9, 1290. https://doi.org/10.3390/vaccines9111290
Cai J, Zhang B, Li Y, Zhu W, Akihisa T, Li W, Kikuchi T, Liu W, Feng F, Zhang J. Prophylactic and Therapeutic EBV Vaccines: Major Scientific Obstacles, Historical Progress, and Future Direction. Vaccines. 2021; 9(11):1290. https://doi.org/10.3390/vaccines9111290
Chicago/Turabian StyleCai, Jing, Bodou Zhang, Yuqi Li, Wanfang Zhu, Toshihiro Akihisa, Wei Li, Takashi Kikuchi, Wenyuan Liu, Feng Feng, and Jie Zhang. 2021. "Prophylactic and Therapeutic EBV Vaccines: Major Scientific Obstacles, Historical Progress, and Future Direction" Vaccines 9, no. 11: 1290. https://doi.org/10.3390/vaccines9111290
APA StyleCai, J., Zhang, B., Li, Y., Zhu, W., Akihisa, T., Li, W., Kikuchi, T., Liu, W., Feng, F., & Zhang, J. (2021). Prophylactic and Therapeutic EBV Vaccines: Major Scientific Obstacles, Historical Progress, and Future Direction. Vaccines, 9(11), 1290. https://doi.org/10.3390/vaccines9111290







