Factors Influencing Vaccine Hesitancy in China: A Qualitative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Sampling Strategy
2.4. Data Analyses
3. Results
3.1. Trust in Vaccine Safety
3.2. Access to Professional Advice
3.3. Vaccine Price
3.4. Vaccine Effectiveness
3.5. Other Factors
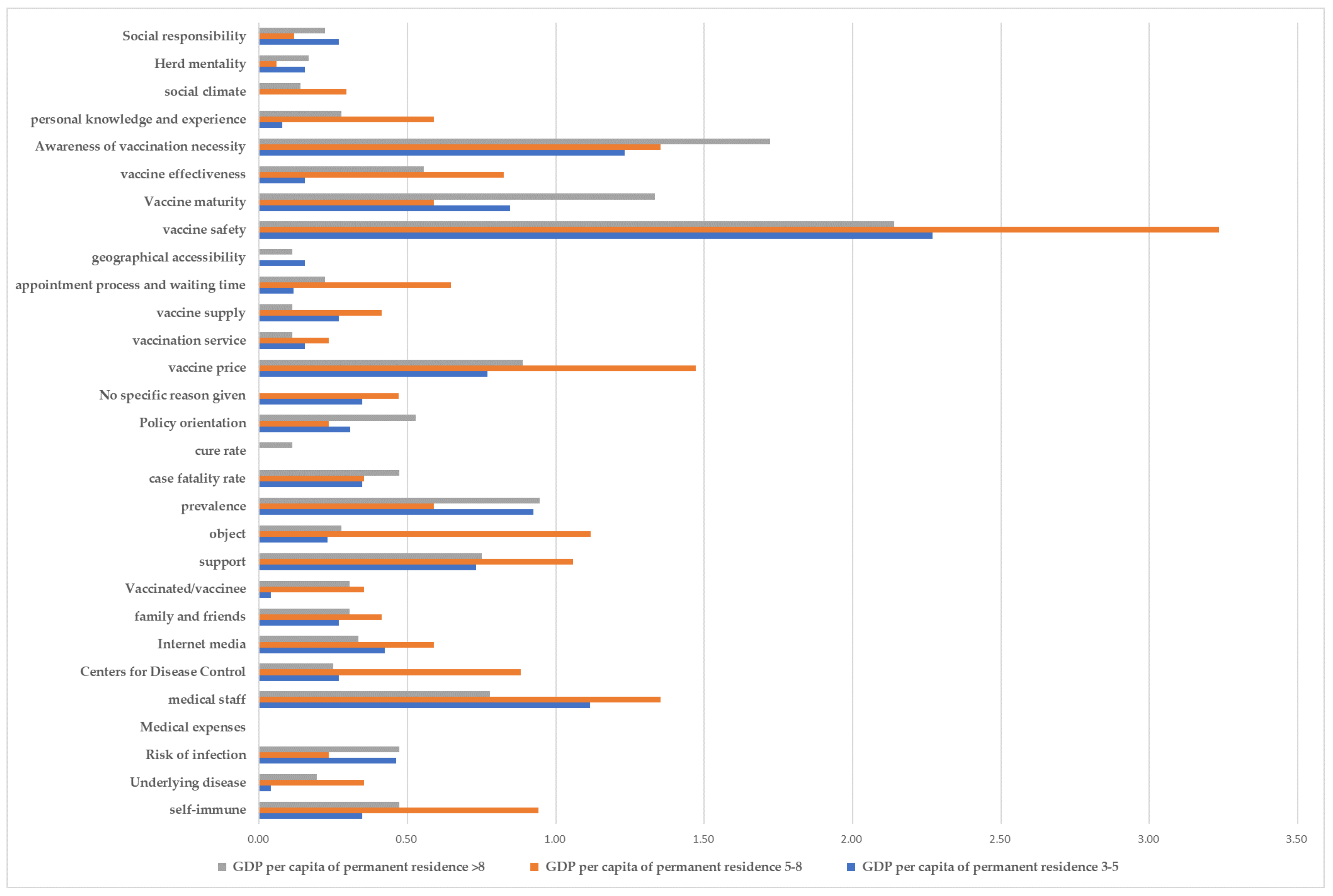
3.6. Regional Differences
4. Discussion
4.1. Ensure Vaccine Safety and Effectiveness
4.2. Reduce the Price of Self-Funded Vaccines and Provide Free Vaccination to High Risk Groups
4.3. Strengthen the Publicity Role of Medical Staff
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 September 2021).
- Rehm, K.E.; Connor, R.F.; Jones, G.J.B.; Yimbu, K.; Mannie, M.D.; Roper, R.L. Vaccinia virus decreases major histocompatibility complex (MHC) class II antigen presentation, T-cell priming, and peptide association with MHC class II. Immunology 2009, 128, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Sallam, M.; Dababseh, D.; Eid, H.; Al-Mahzoum, K.; Al-Haidar, A.; Taim, D.; Yaseen, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: A study in jordan and kuwait among other arab countries. Vaccines 2021, 9, 42. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Wong, L.P.; Wong, P.F.; AbuBakar, S. Vaccine hesitancy and the resurgence of vaccine preventable diseases: The way forward for Malaysia, a Southeast Asian country. Hum. Vaccines Immunother. 2020, 16, 1511–1520. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature 2021, 594, 161–162. [Google Scholar] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Long, S.; Fu, X.; Zhang, X.; Zhao, S.; Xiu, S.; Wang, X.; Lu, B.; Jin, H. Non-epi vaccine hesitancy among chinese adults: A cross-sectional study. Vaccines 2021, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Zhang, T.T.; Shi, G.F.; Cheng, F.M.; Zheng, Y.M.; Tung, T.H.; Chen, H.X. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev. Vaccines 2021, 20, 891–898. [Google Scholar] [CrossRef]
- Liu, X.J.; Mesch, G.S. The adoption of preventive behaviors during the COVID-19 pandemic in China and Israel. Int. J. Environ. Res. Public Health 2020, 17, 7170. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.F.; Masters, D.; Massey, G. Enablers and barriers to COVID-19 vaccine uptake: An international study of perceptions and intentions. Vaccine 2021, 39, 5116–5128. [Google Scholar] [CrossRef]
- Sanders, C. Application of Colaizzi’s method: Interpretation of an auditable decision trail by a novice researcher. Contemp. Nurse 2003, 14, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.Z.; Zude, X.; Xiaochao, L.; Youfeng, M.; Laiyun, S.; Yuping, Z.; Zhihong, X.; Jianwu, W.; Aihua, L. China Statistical Yearbook, 2020; China Statistical Press: Beijing, China, 2020. [Google Scholar]
- Wagner, A.L.; Masters, N.B.; Domek, G.J.; Mathew, J.L.; Sun, X.; Asturias, E.J.; Ren, J.; Huang, Z.; Contreras-Roldan, I.L.; Gebremeskel, B.; et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines 2019, 7, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Giammanco, G.M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; et al. COVID-19 vaccine and death: Causality algorithm according to the who eligibility diagnosis. Diagnostics 2021, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Edler, C.; Klein, A.; Schröder, A.S.; Sperhake, J.P.; Ondruschka, B. Deaths associated with newly launched SARS-CoV-2 vaccination (Comirnaty®). Leg. Med. 2021, 51, 101895. [Google Scholar] [CrossRef]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; Salerno, M.; et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica 2021, 106, 2291–2293. [Google Scholar] [PubMed]
- Rodeghiero, F.; Balduini, C.L. A new enemy is emerging in the fight against the SARS-CoV-2 pandemic. Haematologica 2021, 106, 2040. [Google Scholar]
- Larson, H.J.; de Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, A.R.; Jones, N.S. The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; Alicandro, G.; Scarpino, V. Attitudes towards influenza vaccine and a potential COVID-19 vaccine in Italy and differences across occupational groups, September 2020. Med. Lav. 2020, 111, 445–448. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Srivastav, A.; Razzaghi, H.; Williams, W.; Lindley, M.C.; Jorgensen, C.; Abad, N.; Singleton, J.A. COVID-19 Vaccination Intent, Perceptions, and Reasons for Not Vaccinating Among Groups Prioritized for Early Vaccination—United States, September and December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 217–222. [Google Scholar] [CrossRef]
- Zhang, P.X.; Yin, Z.D.; Qiu, Y.X. Current vaccine hesitancy situation and health education strategies in immunization programmes. Chin. J. Health Educ. 2020, 36, 925–928. [Google Scholar]
- Schneider, J.; Sottmann, L.; Greinacher, A.; Hagen, M.; Kasper, H.-U.; Kuhnen, C.; Schlepper, S.; Schmidt, S.; Schulz, R.; Thiele, T.; et al. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int. J. Legal Med. 2021, 135, 2335–2345. [Google Scholar] [PubMed]
- Bahri, P.; Rägo, L. CIOMS Guide To Vaccine Safety Communication—Executive summary. Vaccine 2018, 37, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Guo, X.; Li, J. Analysis on pneumonia vaccination willness and its influential factors among the elderly people in Shanghai City. Chin. J. Dis. Control Prev. 2015, 19, 975–978. [Google Scholar]
- Black, C.L.; Yue, X.; Ball, S.W.; Fink, R.V.; de Perio, M.A.; Laney, A.S.; Williams, W.W.; Graitcer, S.B.; Fiebelkorn, A.P.; Lu, P.-J.; et al. Influenza Vaccination Coverage Among Health Care Personnel—United States, 2017–2018 Influenza Season. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1050–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.X. A study on parental hesitation attitudes and influence factors about childhood vaccination in 260 cases. Fudan Univ. J. Med. Sci. 2021, 48, 136–139. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and receptivity for covid-19 vaccines: A rapid systematic review. Vaccines 2021, 9, 16. [Google Scholar]
- Wang, H.F.; Lai, R.P.; Xie, D.S. Effect evaluation of comprehensive intervention techniques on influenza vaccination in patients with type 2 diabetes. Prev. Med. 2019, 31, 930–932. [Google Scholar]
- French, J.; Deshpande, S.; Evans, W.; Obregon, R. Key guidelines in developing a pre-emptive COVID-19 vaccination uptake promotion strategy. Int. J. Environ. Res. Public Health 2020, 17, 5893. [Google Scholar] [CrossRef] [PubMed]
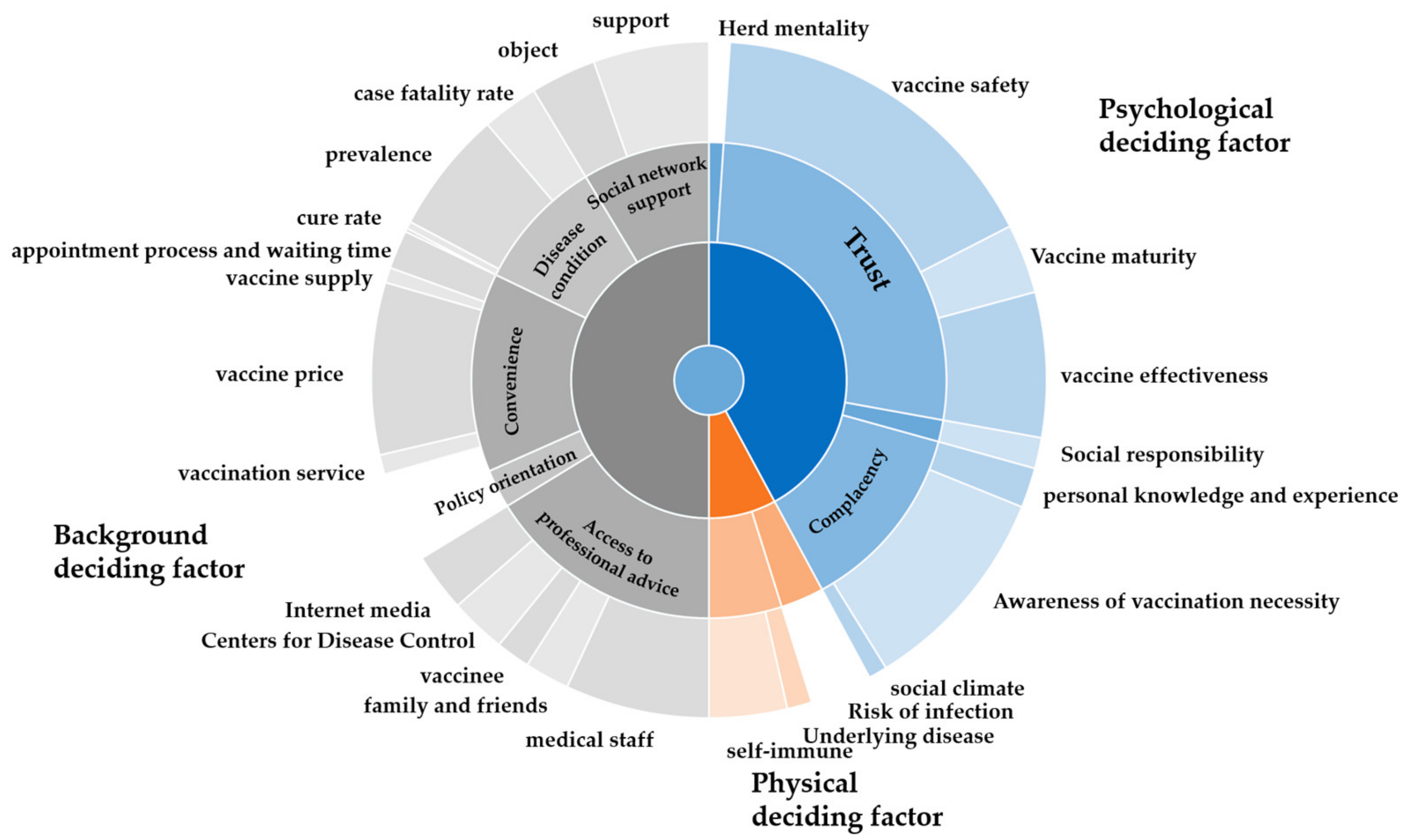
| Theme | Sub-Theme |
|---|---|
| Background deciding factor | Disease condition |
| case fatality rate; prevalence; cure rate | |
| Social network support | |
| object/support | |
| Convenience | |
| vaccination service; vaccine supply; vaccine price | |
| geographical accessibility | |
| appointment process and waiting time | |
| Policy orientation | |
| Access to professional advice | |
| Centers for Disease Control; medical staff | |
| family and friends; Internet media | |
| vaccinee | |
| Physical deciding factor | Risk of infection |
| Physical conditions | |
| Underlying disease; self-immune | |
| Medical expenses | |
| Psychological deciding factor | Herd mentality |
| Social responsibility | |
| Trust | |
| vaccine safety; Vaccine maturity; vaccine effectiveness | |
| Complacency | |
| personal knowledge and experience | |
| Awareness of vaccination necessity | |
| social climate |
| Demographic Characteristics | Healthcare Workers | Adults Aged 18–59 | Older People Over 60 | Parents of Children Aged 0–6 |
|---|---|---|---|---|
| Gender | - | - | - | - |
| Male | 4 (26.7) | 13 (38.2) | 11 (52.4) | 6 (27.3) |
| Female | 11 (73.3) | 21 (61.8) | 10 (47.6) | 16 (72.7) |
| GDP per capita of permanent residence (RMB 10,000) | - | - | - | - |
| 3–5 | 4 (26.7) | 13 (38.2) | 7 (33.3) | 6 (27.3) |
| 5–8 | 2 (13.3) | 14 (41.2) | 3 (14.3) | 8 (36.4) |
| >8 | 9 (60.0) | 7 (20.6) | 11 (52.4) | 8 (36.4) |
| Educational level | - | - | - | - |
| elementary school or below | 0 (0.0) | 0 (0.0) | 3(14.3) | 0 (0.0) |
| Junior high school | 0 (0.0) | 3 (8.8) | 7(33.3) | 0 (0.0) |
| High school graduate or equivalent | 1 (6.7) | 4 (11.8) | 5 (23.8) | 3 (72.7) |
| College or equivalent | 13 (86.7) | 24 (70.6) | 6 (28.6) | 16 (72.7) |
| Master’s Diploma or above | 1 (6.7) | 3 (8.8) | 0 (0.0) | 3 (72.7) |
| Annual household income (RMB 10,000) | - | - | - | - |
| <5 | 2 (13.3) | 5 (14.7) | 3 (14.3) | 2 (9.1) |
| 5–10 | 1 (6.7) | 15 (44.1) | 10 (47.6) | 8 (36.4) |
| 11–15 | 4 (26.7) | 6 (17.6) | 1 (4.8) | 5 (22.7) |
| >16 | 8 (53.3) | 8 (23.5) | 7 (33.3) | 7 (31.8) |
| Occupation | - | - | - | - |
| Government agencies and institutions | 4 (11.8) | 0 (0.0) | 6 (27.3) | |
| Business/enterprise | 2 (5.9) | 1 (4.8) | 8 (36.4) | |
| Production staff/worker | 4 (11.8) | 0 (0.0) | 4 (18.2) | |
| Full-time student | 23 (67.6) | 0 (0.0) | 0 (0.0) | |
| Retired | 0 (0.0) | 19 (90.5) | 0 (0.0) | |
| Else | 1 (2.9) | 1 (4.8) | 3 (13.6) | |
| None | 0 (0.0) | 0 (0.0) | 1 (4.5) | |
| Number of children | - | |||
| 1 | 12 (54.5) | |||
| 2 | 10 (45.5) | |||
| Has the child played in the last year influenza vaccine | - | |||
| Yes | 10 (45.5) | |||
| No | 12 (54.5) |
| Themes | Sub-Themes | Frequency (%) 1 |
|---|---|---|
| Background deciding factor | case fatality rate | 32 (34.8) |
| prevalence | 68 (73.9) | |
| vaccine price | 77 (83.7) | |
| appointment process and waiting time | 22 (23.9) | |
| vaccine supply | 18 (19.6) | |
| Physical deciding factor | risk of infection | 33 (35.9) |
| self-immune | 42 (45.7) | |
| Psychological deciding factor | social responsibility | 17 (18.5) |
| vaccine safety | 191 (>100) | |
| vaccine effectiveness | 80 (87.0) | |
| awareness of vaccination necessity | 117 (>100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ji, Q.; Dong, S.; Zhao, S.; Li, X.; Zhu, Q.; Long, S.; Zhang, J.; Jin, H. Factors Influencing Vaccine Hesitancy in China: A Qualitative Study. Vaccines 2021, 9, 1291. https://doi.org/10.3390/vaccines9111291
Wang J, Ji Q, Dong S, Zhao S, Li X, Zhu Q, Long S, Zhang J, Jin H. Factors Influencing Vaccine Hesitancy in China: A Qualitative Study. Vaccines. 2021; 9(11):1291. https://doi.org/10.3390/vaccines9111291
Chicago/Turabian StyleWang, Jianli, Qianqian Ji, Shuheng Dong, Shuangyu Zhao, Xinchen Li, Qiuqi Zhu, Sigui Long, Jingjing Zhang, and Hui Jin. 2021. "Factors Influencing Vaccine Hesitancy in China: A Qualitative Study" Vaccines 9, no. 11: 1291. https://doi.org/10.3390/vaccines9111291
APA StyleWang, J., Ji, Q., Dong, S., Zhao, S., Li, X., Zhu, Q., Long, S., Zhang, J., & Jin, H. (2021). Factors Influencing Vaccine Hesitancy in China: A Qualitative Study. Vaccines, 9(11), 1291. https://doi.org/10.3390/vaccines9111291





