Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Conditions
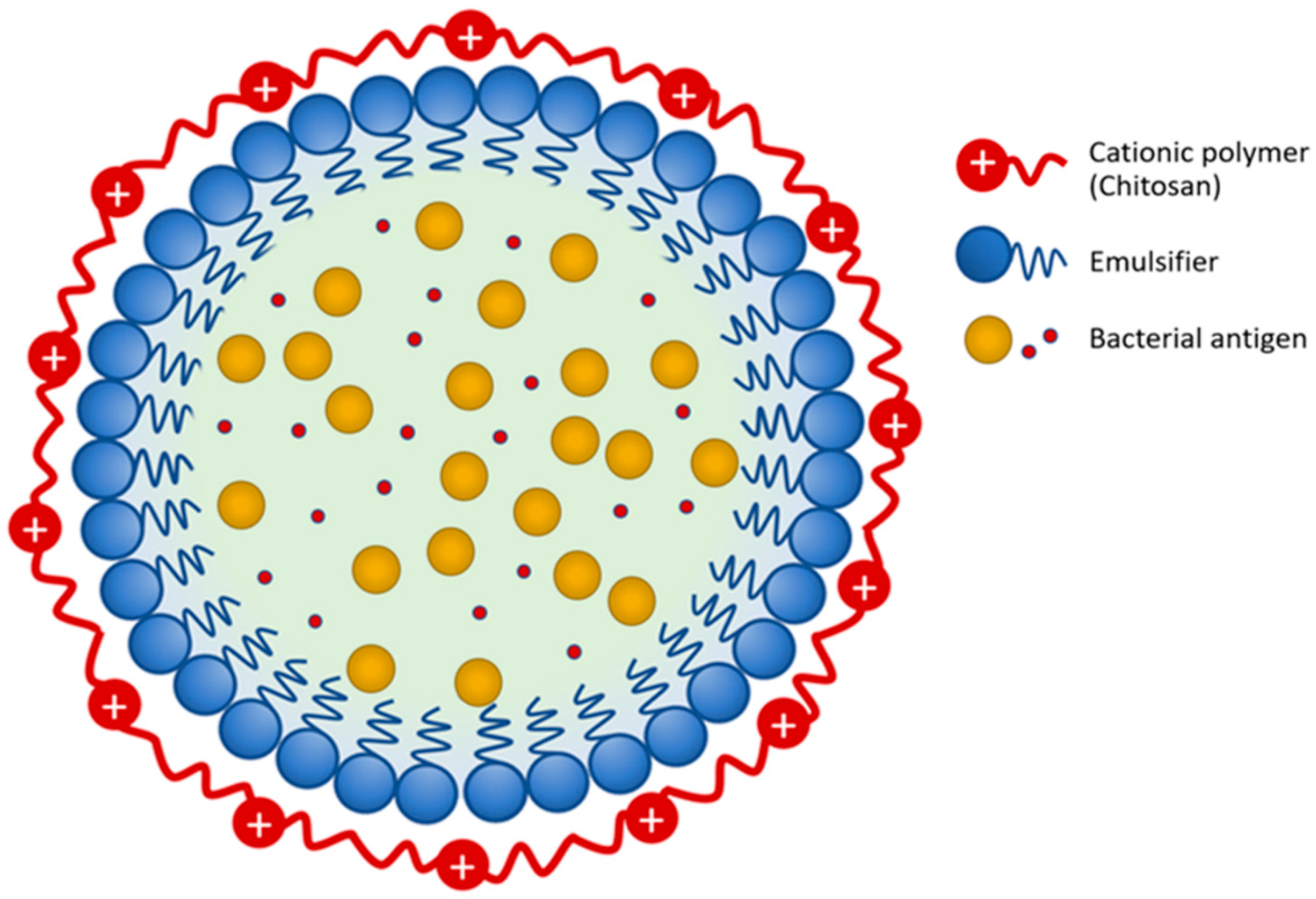
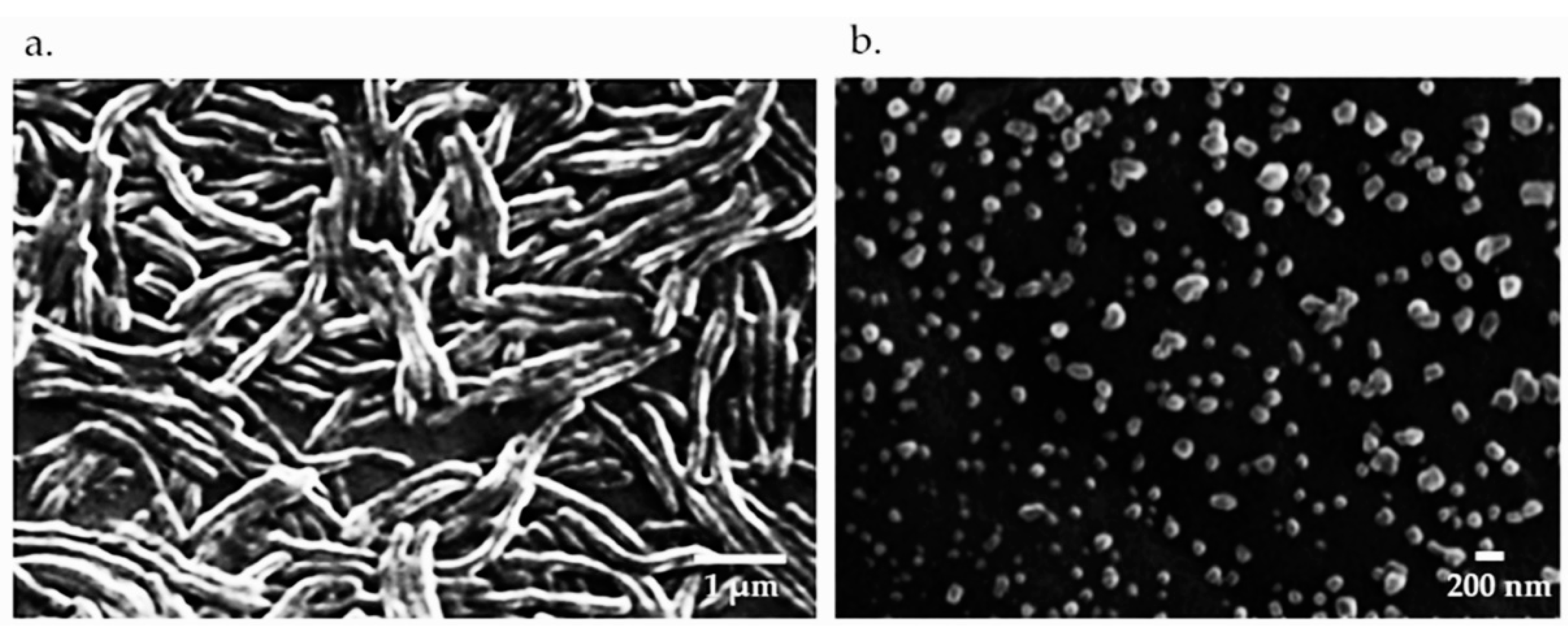
2.2. Bacteria and Vaccine Preparation
2.3. Vaccination and Vaccine Efficacy Test
2.4. Serum Bactericidal Activity (SBA)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Gene Expression Determined by RT-qPCR
2.7. Statistical Analysis
3. Results
3.1. Vaccine Efficacy
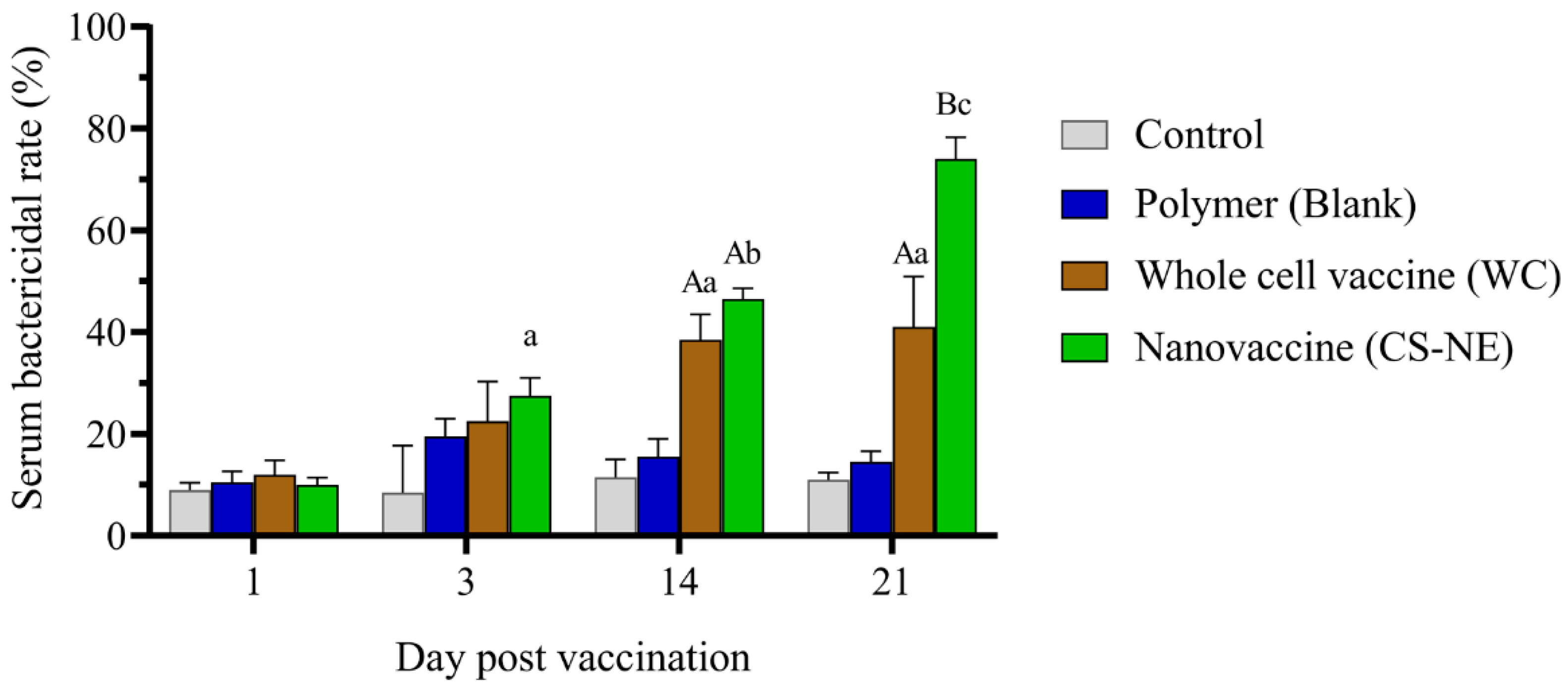
3.2. Serum Bactericidal Activity (SBA)
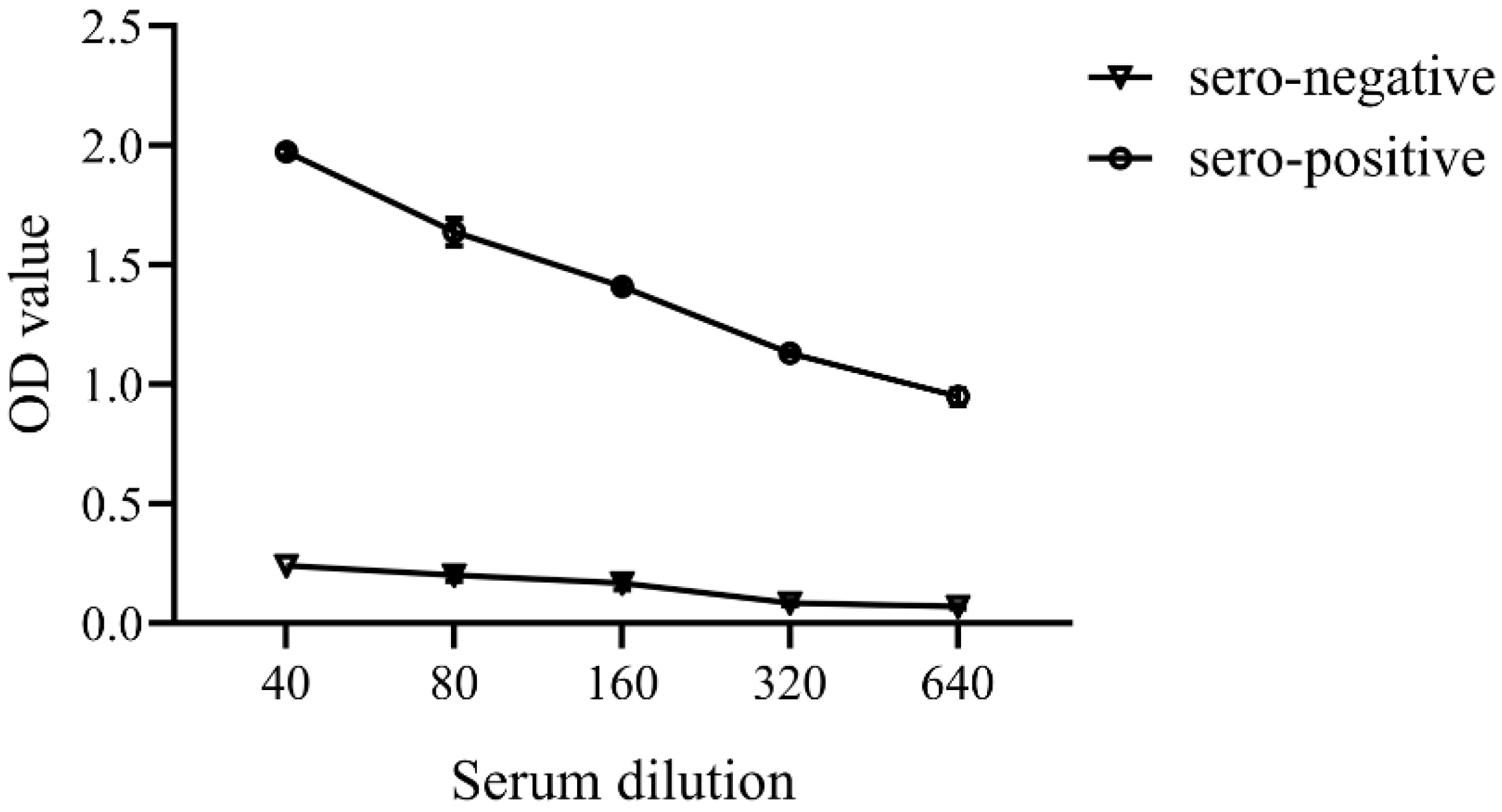
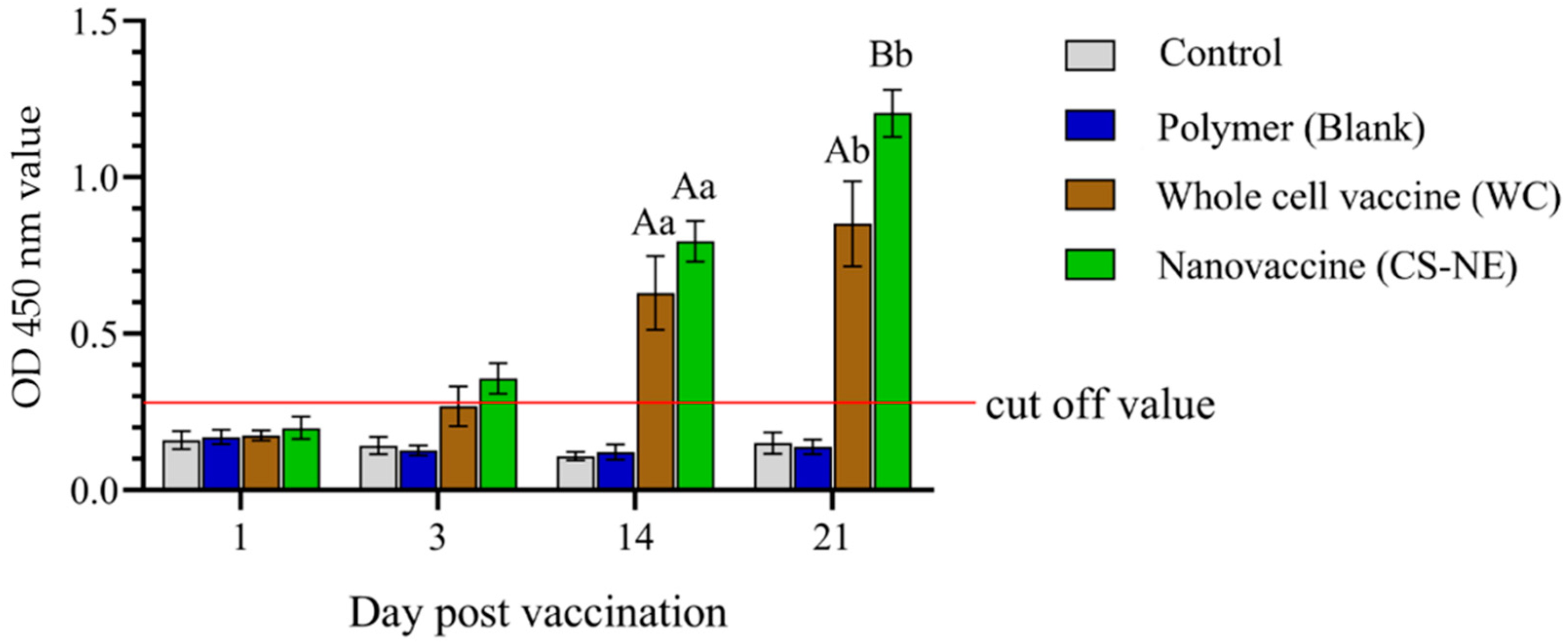
3.3. Enzyme-Linked Immunosorbent Assay (ELISA)
3.4. Gene Expression with RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- LaFrentz, B.R.; García, J.C.; Waldbieser, G.C.; Evenhuis, J.P.; Loch, T.P.; Liles, M.R.; Wong, F.S.; Chang, S.F. Identification of Four Distinct Phylogenetic Groups in Flavobacterium columnare with Fish Host Associations. Front. Microbiol. 2018, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.T.; LaFrentz, B.; Pirarat, N.; Rodkhum, C. Phenotypic characterization and genetic diversity of Flavobacterium columnare isolated from red tilapia, Oreochromis sp., in Thailand. J. Fish Dis. 2014, 38, 901–913. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; LaFrentz, B.R. Growth and survival of the fish pathogenic bacterium, Flavobacterium columnare, in tilapia mucus and porcine gastric mucin. FEMS Microbiol. Lett. 2015, 362, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune re-sponse of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish. Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef]
- Laanto, E.; Penttinen, R.K.; Bamford, J.K.; Sundberg, L.-R. Comparing the different morphotypes of a fish pathogen—Implications for key virulence factors in Flavobacterium columnare. BMC Microbiol. 2014, 14, 170. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, M.; Olson, P.; Nakatani, R. Some factors in susceptibility of juvenile rainbow trout and chinook salmon to Chondrococcus columnaris. J. Fish Res. Board Can. 1971, 28, 1739–1743. [Google Scholar] [CrossRef]
- Moore, A.A.; Eimers, M.E.; Cardella, M.A. Attempts to ControlFlexibacter columnarisEpizootics in Pond-Reared Channel Catfish by Vaccination. J. Aquat. Anim. Health 1990, 2, 109–111. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Shelby, R.A.; Klesius, P.H. Development of an indirect ELISA to detect humoral response to Flavobacterium columnare infection of channel catfish, Ictalurus punctatus. J. Appl. Aquac. 2003, 14, 43–52. [Google Scholar] [CrossRef]
- Mano, N.; Inui, T.; Arai, D.; Hirose, H.; Deguchi, Y. Immune Response in the Skin of Eel against Cytophaga columnaris. Fish Pathol. 1996, 31, 65–70. [Google Scholar] [CrossRef]
- Liewes, E.; Van Dam, R.; Vos-Maas, M.; Bootsma, R. Presence of antigen sensitized leukocytes in carp (Cyprinus carpio L) follow-ing bath immunization against Flexibacter columnaris. Vet. Immunol. Immunopathol. 1982, 3, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, L.; LaPatra, S.; Cain, K. Systemic and mucosal antibody response in tilapia, Oreochromis niloticus (L.), following im-munization with Flavobacterium columnare. J. Fish Dis. 2004, 27, 573–581. [Google Scholar] [CrossRef]
- Leal, C.; Carvalho-Castro, G.; Sacchetin, P.; Lopes, C.; Moraes, A.; Figueiredo, H. Oral and parenteral vaccines against Flavobacterium columnare: Evaluation of humoral immune response by ELISA and in vivo efficiency in Nile tilapia (Oreochromis niloticus). Aquac. Int. 2010, 18, 657–666. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Yostawornkul, J.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Enhanced efficacy of immersion vac-cination in tilapia against columnaris disease by chitosan-coated “pathogen-like” mucoadhesive nanovaccines. Fish Shellfish. Immunol. 2019, 95, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kitiyodom, S.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Rodkhum, C.; Pirarat, N.; Yata, T. The potential of mucoadhesive polymer in enhancing efficacy of direct immersion vaccination against Flavobacterium columnare infection in tilapia. Fish Shellfish. Immunol. 2018, 86, 635–640. [Google Scholar] [CrossRef]
- Austin, B. Developments in vaccination against fish bacterial disease. In Infectious Disease in Aquaculture; Woodhead Publishing: Sawston, UK, 2012; pp. 218–243. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, G.; Zhang, Y.; Yuan, J.; An, L. Generation of Biotechnology-Derived Flavobacterium columnare Ghosts by PhiX174 GeneE-Mediated Inactivation and the Potential as Vaccine Candidates against Infection in Grass Carp. J. Biomed. Biotechnol. 2012, 2012, 760730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowther, J.R. Validation of Diagnostic Tests for Infectious Diseases, The ELISA Guidebook; Humana Press: Totowa, NJ, USA, 2001; pp. 301–345. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and im-munity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Vet. Sci. Res. J. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT meth-od. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zhang, C.; Lian, A.; Xu, Y.; Jiang, Q. Signal Transduction Mechanisms for Glucagon-Induced Somatolactin Secretion and Gene Expression in Nile Tilapia (Oreochromis niloticus) Pituitary Cells. Front. Endocrinol. 2021, 11, 629077. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, W.; Hu, G.; Qiu, L.; Chen, J. Transcriptome Analysis of Juvenile Tilapia (Oreochromis niloticus) Blood, Fed with Different Concentrations of Resveratrol. Front. Physiol. 2020, 11, 600730. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2018, 86, 260–268. [Google Scholar] [CrossRef]
- Mohammed, H.; Olivares-Fuster, O.; LaFrentz, S.; Arias, C.R. New attenuated vaccine against columnaris disease in fish: Choos-ing the right parental strain is critical for vaccine efficacy. Vaccine 2013, 31, 5276–5280. [Google Scholar] [CrossRef] [PubMed]
- Ristow, S.; De Avila, J.; Lapatra, S.E.; Lauda, K. Detection and characterization of rainbow trout antibody against infectious hem-atopoietic necrosis virus. Dis. Aquat. Org. 1993, 15, 109–114. [Google Scholar] [CrossRef]
- Romestand, B.; Dragesco, A.; Breuil, G.; Coste, F.; Bouix, G. An ELISA technique for rapid diagnosis of vibriosis in sea bass Dicentrarchus labrax. Dis. Aquat. Org. 1993, 15, 137–143. [Google Scholar] [CrossRef]
- Xu, D.H.; Klesius, P. Protective effect of cutaneous antibody produced by channel catfish, Ictalurus punctatus (Rafinesque), im-mune to Ichthyophthirius multifiliis Fouquet on cohabited non-immune catfish. J. Fish Dis. 2003, 26, 287–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Arias, C.R.; Shoemaker, C.; Klesius, P.H. Comparison of lipopolysaccharide and protein profiles between Flavobacterium columnare strains from different genomovars. J. Fish Dis. 2006, 29, 657–663. [Google Scholar] [CrossRef]
- Lange, M.D.; Abernathy, J.; Farmer, B.D. Evaluation of a Recombinant Flavobacterium columnare DnaK Protein Vaccine as a Means of Protection against Columnaris Disease in Channel Catfish (Ictalurus punctatus). Front. Immunol. 2019, 10, 1175. [Google Scholar] [CrossRef]
- Rossi, O.; Molesti, E.; Saul, A.; Giannelli, C.; Micoli, F.; Necchi, F. Intra-Laboratory Evaluation of Luminescence Based High-Throughput Serum Bactericidal Assay (L-SBA) to Determine Bactericidal Activity of Human Sera against Shigella. High-Throughput 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Biller-Takahashi, J.; Takahashi, L.; Pilarski, F.; Sebastião, F.; Urbinati, E. Serum bactericidal activity as indicator of innate immunity in pacu Piaractus mesopotamicus (Holmberg, 1887). Arq. Bras. Med. Vet. Zootec. 2013, 65, 1745–1751. [Google Scholar] [CrossRef] [Green Version]
- Borrow, R.; Carlone, G.; Rosenstein, N.; Blake, M.; Feavers, I.; Martin, D.; Zollinger, W.; Robbins, J.; Aaberge, I.; Granoff, D.M.; et al. Neisseria meningitidis group B correlates of protection and assay standardization—International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine 2006, 24, 5093–5107. [Google Scholar] [CrossRef]
- Ndungo, E.; Pasetti, M.F. Functional antibodies as immunological endpoints to evaluate protective immunity against Shigella. Hum. Vaccines Immunother. 2019, 16, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Necchi, F.; Saul, A.; Rondini, S. Development of a high-throughput method to evaluate serum bactericidal activity using bacte-rial ATP measurement as survival readout. PLoS ONE 2017, 12, e0172163. [Google Scholar] [CrossRef] [PubMed]
- Munang′Andu, H.M.; Evensen, Ø. Correlates of protective immunity for fish vaccines. Fish Shellfish. Immunol. 2018, 85, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [Green Version]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug de-livery. J Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Balm, P.H.M.; Van Lieshout, E.; Lokate, J.; Bonga, S.E.W. Bacterial lipopolysaccharide (LPS) and interleukin 1 (IL-1) exert multiple physiological effects in the tilapia Oreochromis mossambicus (Teleostei). J. Comp. Physiol. B 1995, 165, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Taneichi, M.; Kasai, M.; Kakiuchi, T.; Uchida, T. Liposome-Coupled Antigens Are Internalized by Antigen-Presenting Cells via Pinocytosis and Cross-Presented to CD8+ T Cells. PLoS ONE 2010, 5, e15225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welker, T.L.; Shoemaker, C.A.; Arias, C.R.; Klesius, P.H. Transmission and detection of Flavobacterium columnare in channel catfish Ictalurus punctatus. Dis. Aquat. Org. 2005, 63, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongsri, P.; Meng, K.; Liu, X.; Wu, Z.; Yin, G.; Wang, Q.; Liu, M.; Xu, Z. The predominant role of mucosal immunoglobulin IgT in the gills of rainbow trout (Oncorhynchus mykiss) after infection with Flavobacterium columnare. Fish Shellfish. Immunol. 2020, 99, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Jouneau, L.; Pham, H.-P.; Bouchez, O.; Giudicelli, V.; Lefranc, M.-P.; Quillet, E.; Benmansour, A.; Cazals, F.; Six, A.; et al. Teleost Fish Mount Complex Clonal IgM and IgT Responses in Spleen upon Systemic Viral Infection. PLoS Pathog. 2013, 9, e1003098. [Google Scholar] [CrossRef] [PubMed]


| Gene | Target | Sequence Forward/Reverse (5′-3′) | Product (bp) | Reference |
|---|---|---|---|---|
| β-actin F β-actin R | Housekeeping gene | AAGGACCTGTACGCCAACAC ACATCTGCTGGAAGGTGGAC | 196 | [18] |
| TNFα F TNFα R | Inflammation related gene | CTCACAGATAGCGGCATCAA CCTGGGCTCTCTCTGTGTTC | 190 | [18] |
| MHC Iiβ F MHC Iiβ R | Adaptive immune-related gene | TCAGCACAGCAGATGGATTC GCCTGCTTCACTCCAAACTC | 175 | [18] |
| IL-1β F IL-1 β R | Adaptive immune-related gene | AAGATGAATTGTGGAGCTGTGTT AAAAGCATCGACAGTATGTGAAAT | 175 | [4] |
| IgM-F IgM-R | Adaptive immune-related gene | TGGTACTGGGGGTCAAACAT TAAGCGATCCATTCCAGTCC | 156 | [18] |
| IgT-F IgT-R | Adaptive immune-related gene | AGACACACCAGAGTGATTTCAT AGACACACCAGAGTGATTTCATCAG | 78 | [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitiyodom, S.; Yata, T.; Thompson, K.D.; Costa, J.; Elumalai, P.; Katagiri, T.; Temisak, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge. Vaccines 2021, 9, 1253. https://doi.org/10.3390/vaccines9111253
Kitiyodom S, Yata T, Thompson KD, Costa J, Elumalai P, Katagiri T, Temisak S, Namdee K, Rodkhum C, Pirarat N. Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge. Vaccines. 2021; 9(11):1253. https://doi.org/10.3390/vaccines9111253
Chicago/Turabian StyleKitiyodom, Sirikorn, Teerapong Yata, Kim D. Thompson, Janina Costa, Preetham Elumalai, Takayuki Katagiri, Sasithon Temisak, Katawut Namdee, Channarong Rodkhum, and Nopadon Pirarat. 2021. "Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge" Vaccines 9, no. 11: 1253. https://doi.org/10.3390/vaccines9111253
APA StyleKitiyodom, S., Yata, T., Thompson, K. D., Costa, J., Elumalai, P., Katagiri, T., Temisak, S., Namdee, K., Rodkhum, C., & Pirarat, N. (2021). Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium columnare Challenge. Vaccines, 9(11), 1253. https://doi.org/10.3390/vaccines9111253








