Modulation of Expression of PVYNTN RNA-Dependent RNA Polymerase (NIb) and Heat Shock Cognate Host Protein HSC70 in Susceptible and Hypersensitive Potato Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Virus Inoculation, and DAS-ELISA Test
2.2. Isolation of RNA and Genomic DNA (gDNA) for Expression Analysis of Plant PVYNTN-NIb and StHsc70-8 in PVYNTN-Infected Potato Plants
2.3. Analysis of Expression of PVYNTN-NIb and HSC70-8 in Potato Plants Using qPCR
2.4. Immunofluorescence Localization of NIb and HSC70 in Susceptible and Hypersensitive Potato Leaves
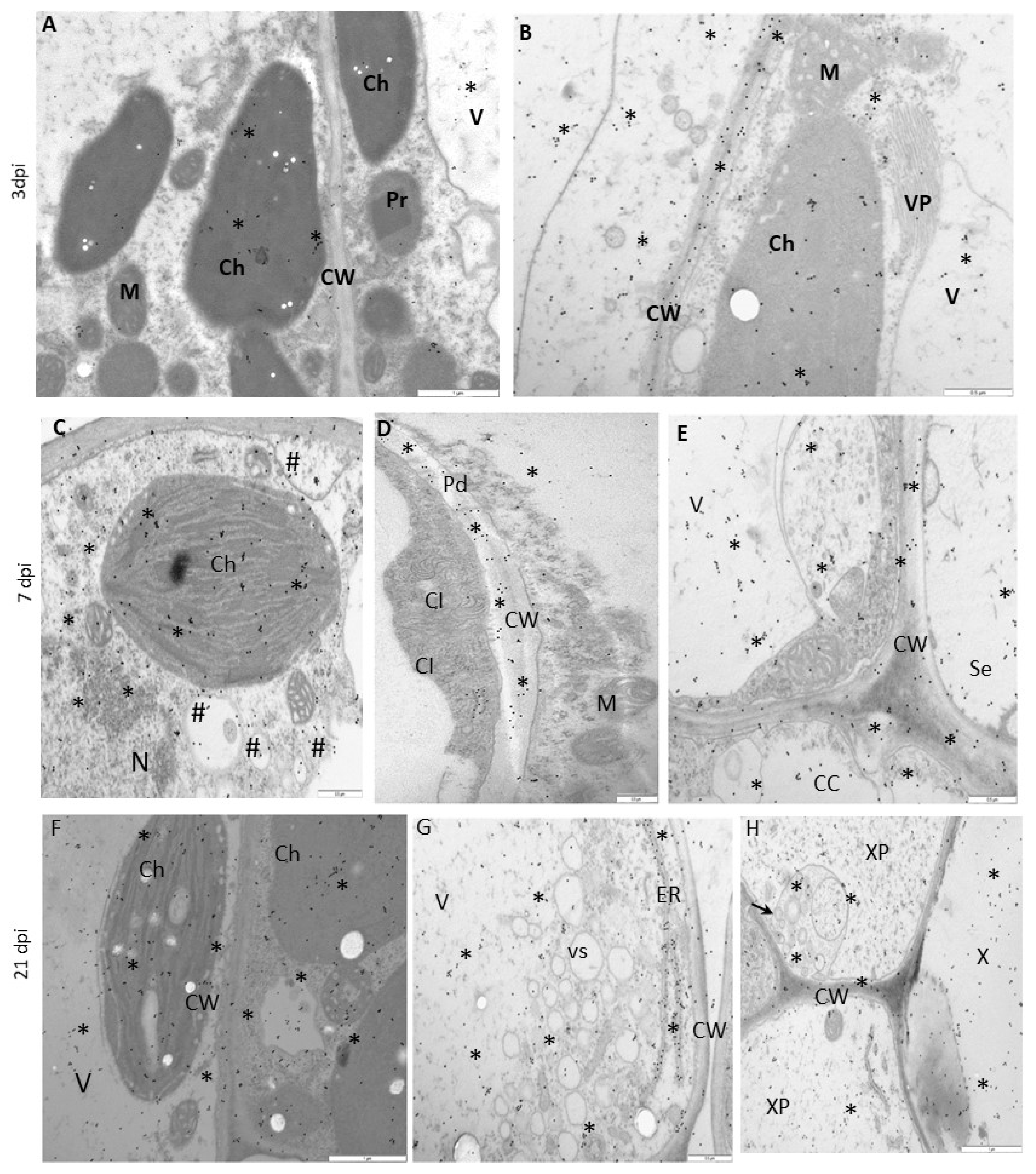
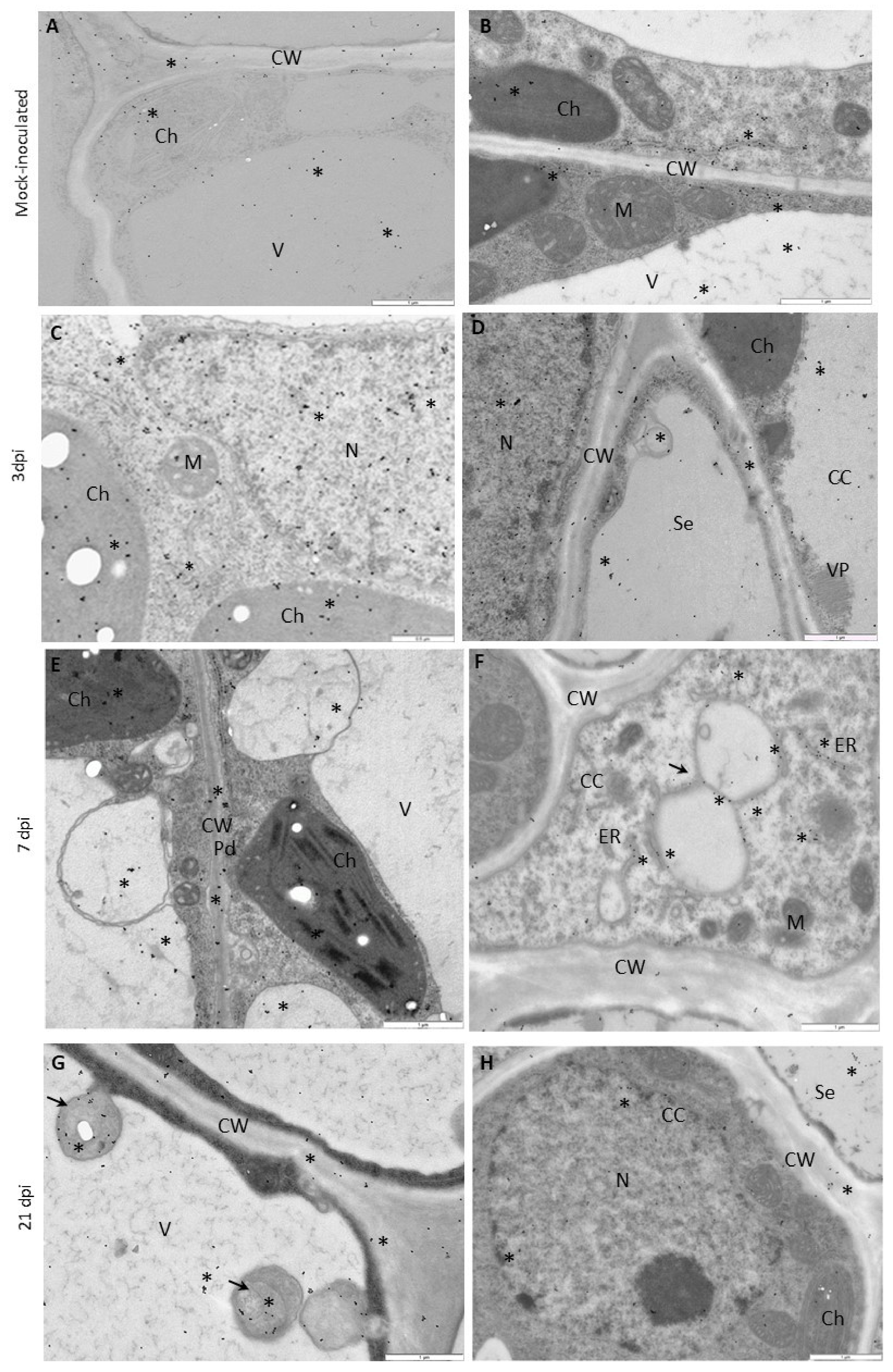
2.5. Electron Microscopy, Immunogold Localization, and Statistical Quantification
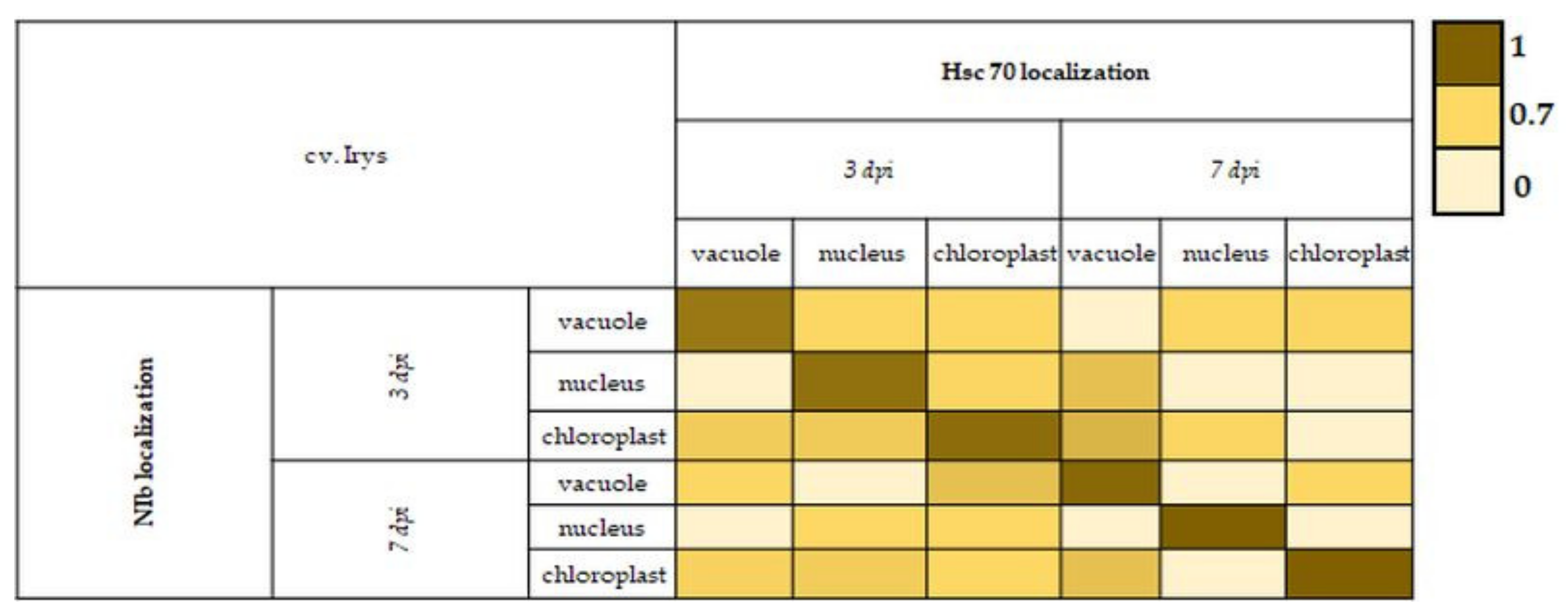
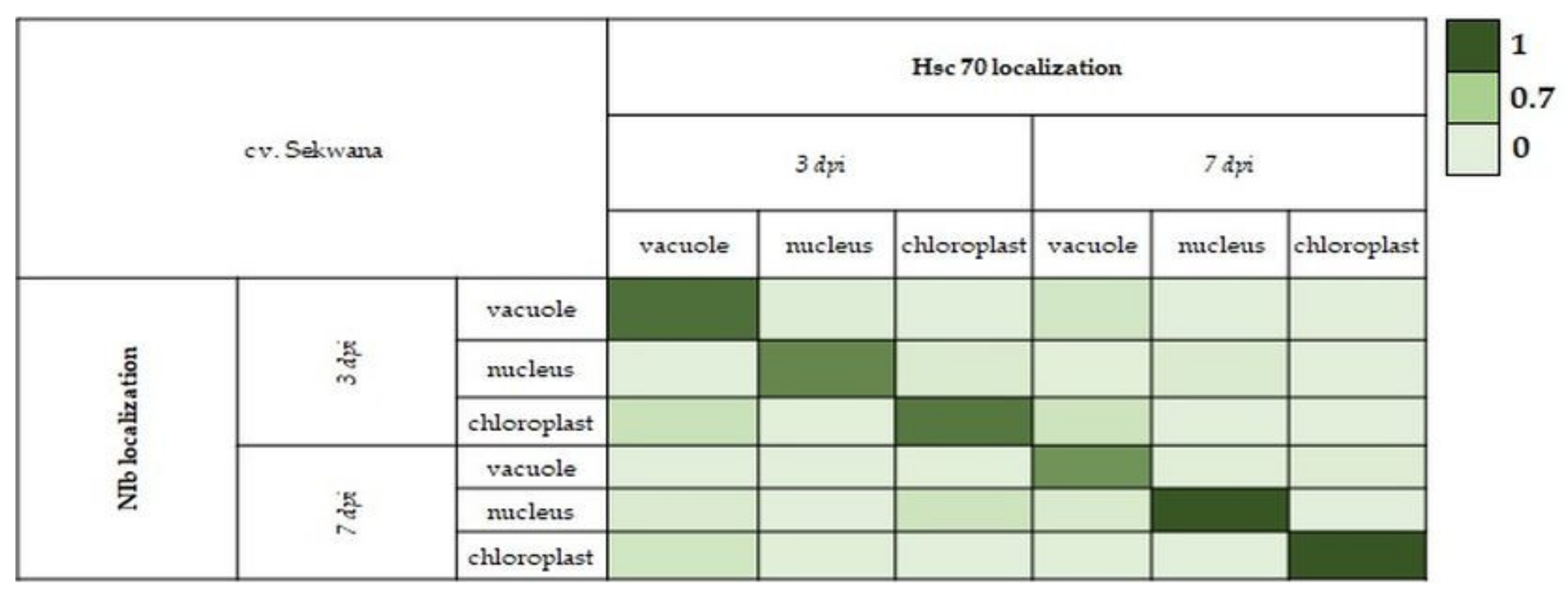
2.6. Pearson Correlation Coefficient for NIb and HSC70 in Selected Cell Compartments
2.7. Double-Immunogold Localization and Quantification of NIb and HSC70
3. Results
3.1. PVYNTN Concentration in Susceptible and Hypersensitive Potato Leaves
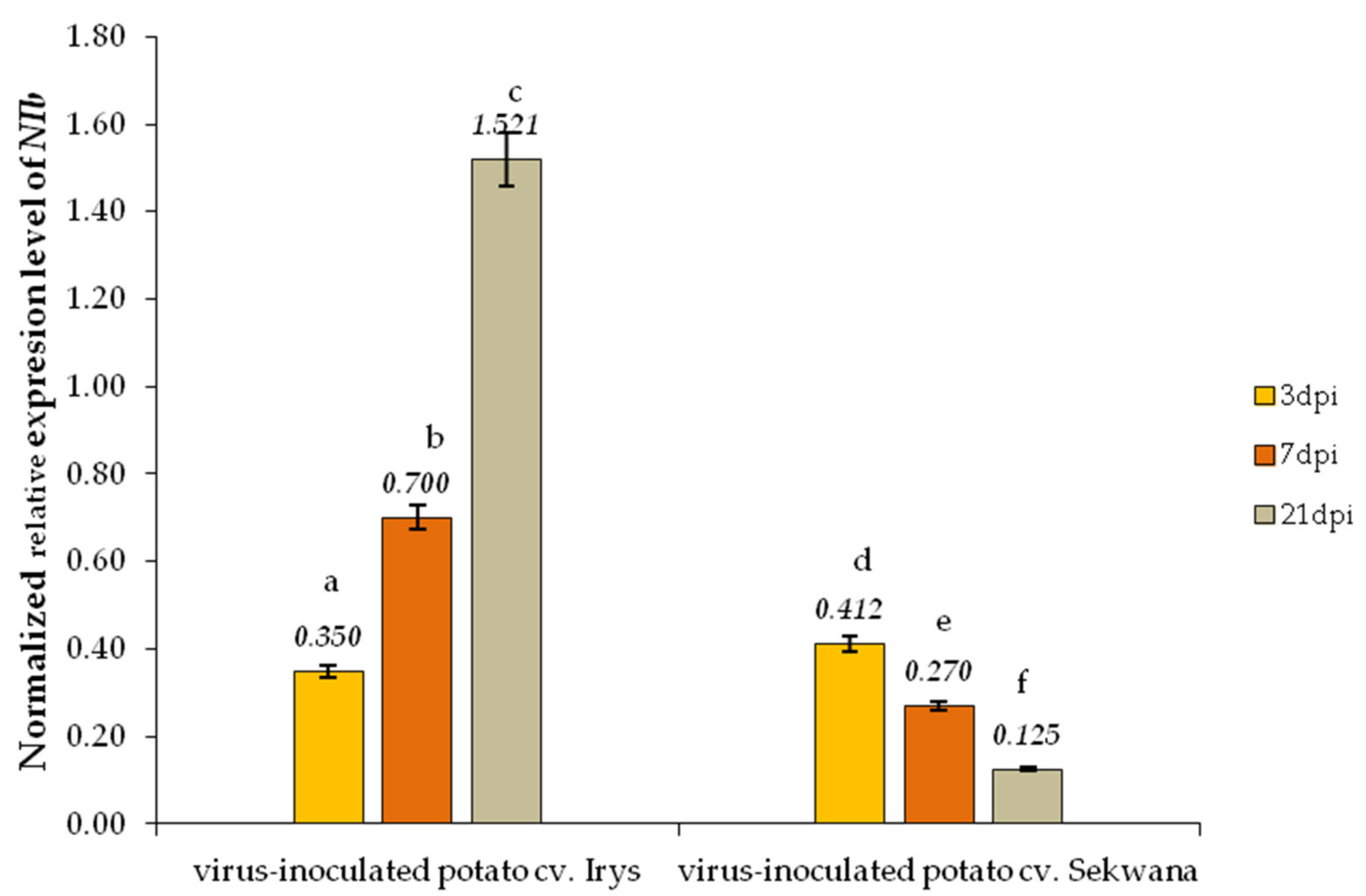
3.2. Relative Expression of PVYNTN-NIb and StHsc70-8 Genes during PVYNTN Infection
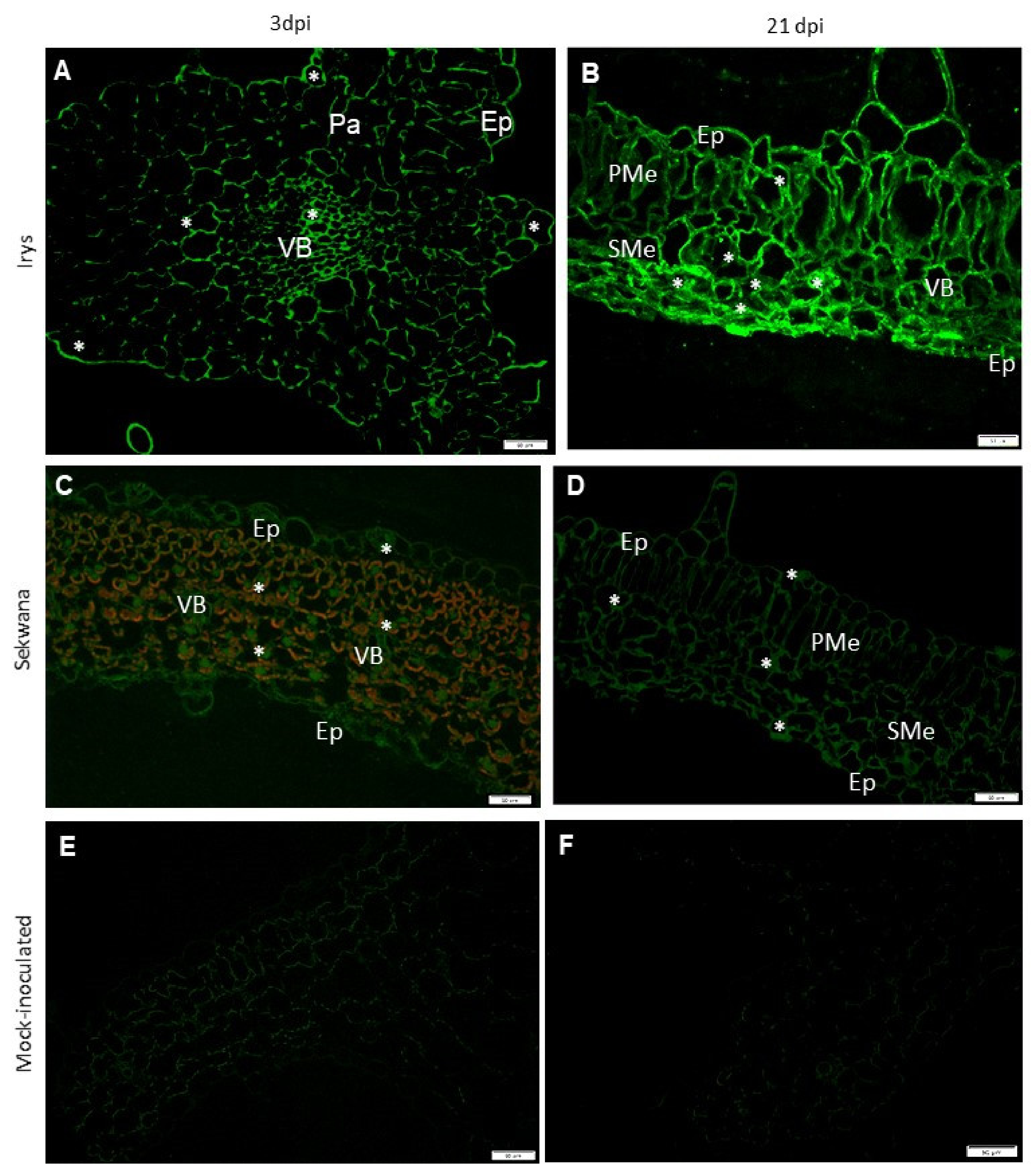
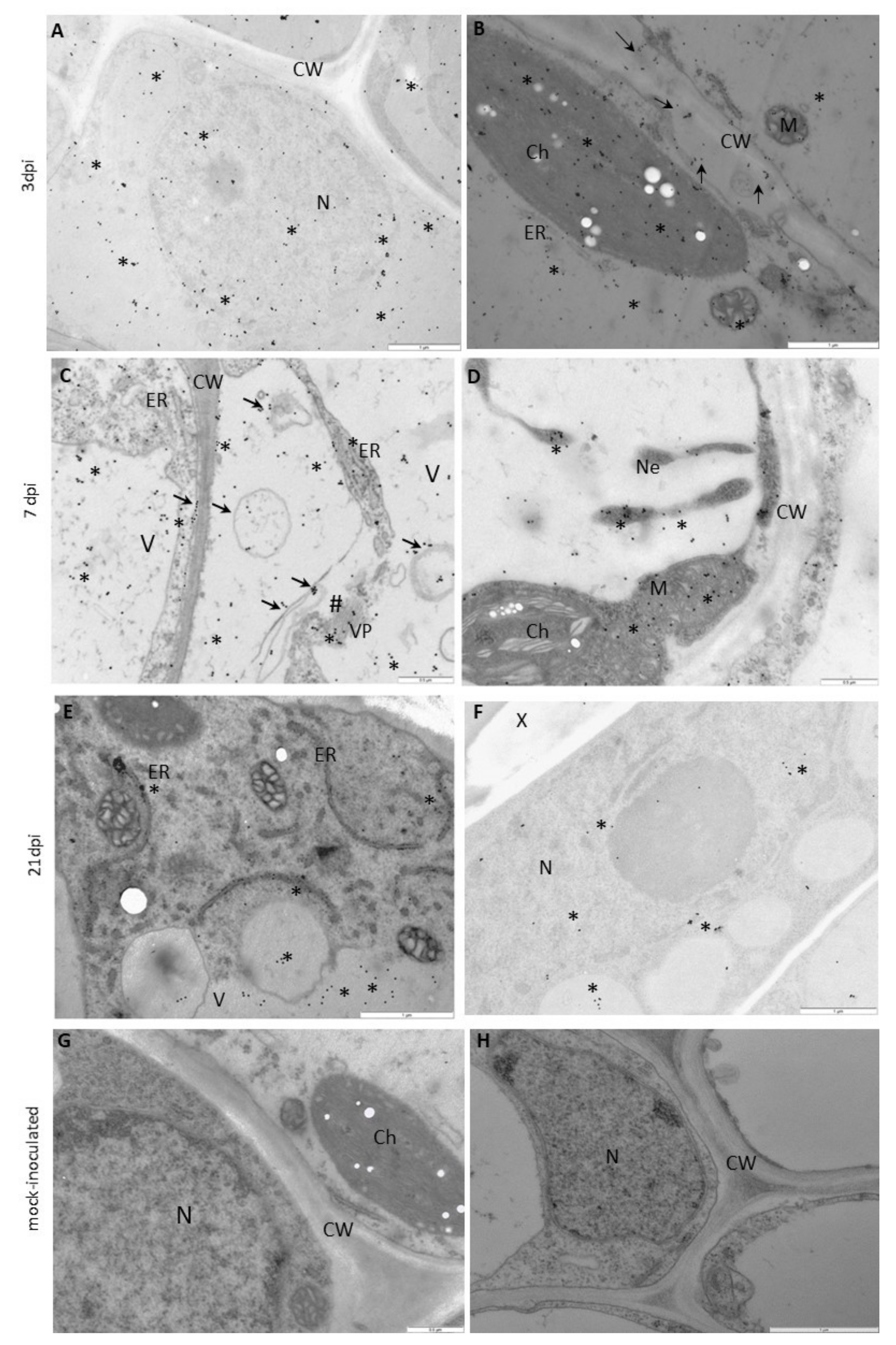
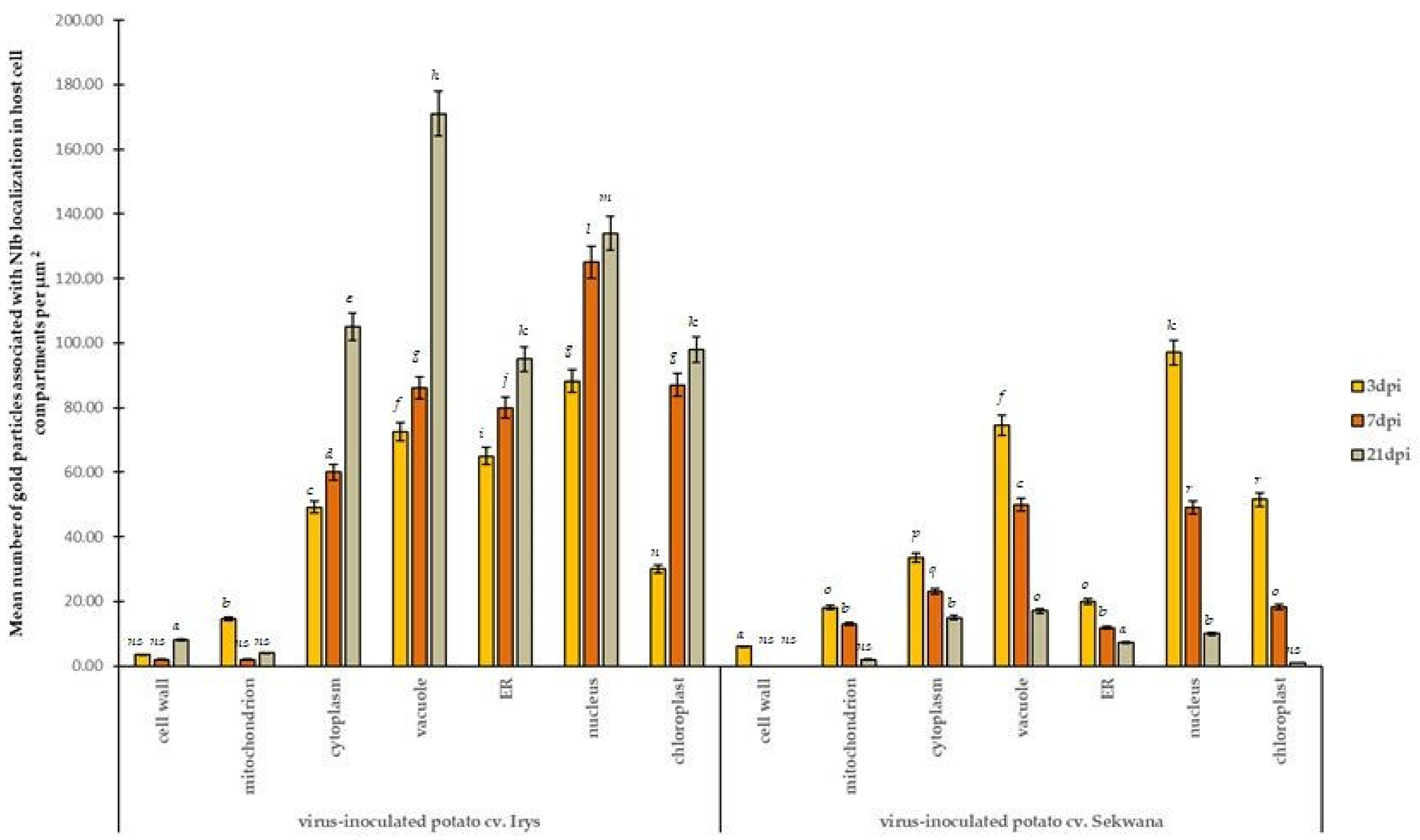
3.3. PVYNTN-NIb Localization Patterns Differ Significantly in Susceptible versus Hypersensitive Leaves
3.4. Deposition of HSC70 Proteins in Susceptible and Hypersensitive Plants
3.5. Strong Correlation between PVY-NIb and HSC70 in Chloroplasts, Vacuoles, and Nucleus during Hypersensitive and Susceptible Reactions
3.6. Colocalization of PVY-NIb and HSC70 in PVYNTN-Infected Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rybicki, E.P. A top ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef]
- Scholthof, K.B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant. Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Revers, F.; García, J.A. Molecular biology of potyviruses. Adv. Virus Res. 2015, 92, 101–199. [Google Scholar] [PubMed]
- Olspert, A.; Chung, B.Y.; Atkins, J.F.; Carr, J.P.; Firth, A.E. Transcriptional slippage in the positive-sense RNA virus family Potyviridae. EMBO Rep. 2015, 16, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, B.; Valli, A.; Mingot, A.; San León, D.; Baulcombe, D.; López-Moya, J.J.; García, J.A. RNA polymerase slippage as a mechanism for the production of frameshift gene products in plant viruses of the Potyviridae family. J. Virol. 2015, 89, 6965–6967. [Google Scholar] [CrossRef]
- Cordeo, T.; Mohamed, M.A.; López-Moya, J.J.; Daròs, J.A. A Recombinant Potato virus Y infectious clone Tagged with the Rosea1 Visual Marker (PVY-Ros1) facilitates the analysis of viral Infectivity and allows the production of large amounts of anthocyanins in plants. Front. Microbiol. 2017, 8, 611. [Google Scholar]
- Cui, H.; Wang, A. The biological impact of the hypervariable N-terminal region of potyviral genomes. Annu. Rev. Virol. 2019, 6, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Leal, R.; Xiong, X. Complementary functions of two recessive R-genes determine resistance durability of tobacco ‘Virgin A Mutant’ (VAM) to Potato virus Y. Virology 2008, 2, 275–283. [Google Scholar] [CrossRef]
- Martín, M.T.; García, J.A. Plum pox potyvirus RNA replication in a crude membrane fraction from infected Nicotiana clevelandii leaves. J. Gen. Virol. 1991, 72, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Schaad, M.C.; Jensen, P.E.; Carrington, J.C. Formation of plant RNA virus replication complexes on membranes: Role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997, 16, 4049–4059. [Google Scholar] [CrossRef]
- Léonard, S.; Viel, C.; Beauchemin, C.; Daigneault, N. Interaction of VPg-Pro of Turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J. Gen. Virol. 2004, 85, 1055–1063. [Google Scholar] [CrossRef]
- Riedel, D.; Lesemann, D.E.; Maiss, E. Ultrastructural localization of nonstructural and coat proteins of 19 potyviruses using antisera to bacterially expressed proteins of plum pox potyvirus. Arch. Virol. 1998, 143, 2133–2158. [Google Scholar] [CrossRef]
- Wang, X.; Ullah, Z.; Grumet, R. Interaction between Zucchini yellow mosaic potyvirus RNA-dependent RNA polymerase and host poly-(A) binding protein. Virology 2000, 275, 433–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thivierge, K.; Cotton, S.; Ufresne, P.J.D.; Mathieu, I.; Beauchemin, C.; Ide, C.; Fortin, M.G.; Laliberté, J.F. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology 2008, 377, 216–225. [Google Scholar] [CrossRef]
- Dufresne, P.J.; Thivierge, K.; Cotton, S.; Beauchemin, C.; Ide, C.; Ubalijoro, E.; Laliberté, J.F.; Fortin, M.G. Heat shock 70 protein interaction with Turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles. Virology 2008, 374, 217–227. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant. Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Huttner, S.; Strasser, R. Endoplasmic reticulum-associated degradation of glycoproteins in plants. Front. Plant. Sci. 2012, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Whitley, D.; Goldberg, S.P.; Jordan, W.D. Heat shock proteins: A review of the molecular chaperones. J. Vasc. Sur. 1999, 29, 748–751. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Tuteja, N. Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant. Signal. Behav. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.G.; Rafii, M.Y.; Martini, M.Y.; Yusuff, O.A.; Ismail, M.R.; Miah, G. Molecular analysis of Hsp70 mechanisms in plants and their function in response to stress. Biotechnol. Genet. Eng. Rev. 2017, 33, 26–39. [Google Scholar] [CrossRef]
- Anaraki, Z.E.; Hosseini, A.; Shariati, M. Transient silencing of heat shock proteins showed remarkable roles for HSP70 during adaptation to stress in plants. Environ. Exp. Bot. 2018, 155, 142–157. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Bolhassani, A.; Agi, E. Heat shock proteins in infection. Clin. Chim. Acta 2019, 498, 90–100. [Google Scholar] [CrossRef]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 genes conferring hypersensitive response to Potato virus Y (PVY) in cultivated potatoes: Mapping and marker-assisted selection validation for PVY resistance in potato breeding. Mol. Breed. 2014, 34, 267–271. [Google Scholar] [CrossRef]
- Zimoch-Guzowska, E.; Yin, Z.; Chrzanowska, M.; Flis, B. Sources and effectiveness of potato PVY resistance in IHAR’s breeding research. Am. J. Potato Res. 2013, 90, 21–27. [Google Scholar] [CrossRef]
- The European Cultivated Potato Database. Available online: https://www.europotato.org/quick_search.php (accessed on 21 August 2021).
- Szajko, K.; Chrzanowska, M.; Witek, K.; Strzelczyk-Żyta, D.; Zagórska, H.; Gebhart, C.; Hennig, J.; Marczewski, W. The novel gene Ny-1 on potato chromosome IX confers hypersensitive resistance to Potato virus Y and is an alternative to Ry genes in potato breeding for PVY resistance. Theor. Appl. Genes 2008, 116, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewska, G. Ultrastructural events during hypersensitive response of potato cv. Rywal infected with necrotic strains of Potato virus Y. Acta Physiol. Plant. 2010, 32, 635–644. [Google Scholar] [CrossRef]
- Otulak, K.; Kozieł, E.; Garbaczewska, G. Ultastructural impact of tobacco rattle virus on tobacco and pepper ovary and anther tissues. J. Phytopatol. 2016, 164, 226–241. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L.; Bujarski, J.J. The Expression of Potato expansin A3 (StEXPA3) and extensin4 (StEXT4) genes with distribution of StEXPAs and HRGPS-extensin changes as an effect of cell wall rebuilding in two types of PVYNTN–Solanum tuberosum Interactions. Viruses 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Bujarski, J.J. Spatiotemporal changes in xylan-1/xyloglucan and xyloglucan xyloglucosyl transferase (Xth-Xet5) as a step-in of ultrastructural cell wall remodelling in potato–Potato virus Y (PVYntn) hypersensitive and susceptible reaction. Int. J. Mol. Sci. 2018, 19, 2287. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, E.; Otulak-Kozieł, E.; Bujarski, J.J. Modifications in tissue and cell ultrastructure as elements of immunity-like reaction in Chenopodium quinoa against Prune dwarf Virus (PDV). Cells 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Bioreba Company Site. Available online: http://www.bioreba.ch/saas/CustomUpload/374O357O340O370O356O369O350O321O360O366O369O356O353O352O350O320O326O/Simple_ELISA_Data_Analysis.pdf (accessed on 24 May 2021).
- Otulak-Kozieł, K.; Kozieł, E.; Bujarski, J.J.; Frankowska-Łukawska, J.; Torres, M.A. Respiratory Burst Oxidase Homologs RBOHD and RBOHF as key modulating components of response in Turnip mosaic virus—Arabidopsis thaliana (L.) Heyhn System. Int. J. Mol. Sci. 2020, 21, 8510. [Google Scholar] [CrossRef]
- Liu, J.; Pang, X.; Cheng, Y.; Yin, Y.; Zhang, Q.; Su, W.; Hu, B.; Guo, Q.; Ha, S.; Zhang, J.; et al. The Hsp70 Gene Family in Solanum tuberosum: Genome-wide identification, phylogeny, and expression patterns. Sci. Rep. 2018, 8, 16628. [Google Scholar] [CrossRef]
- Luschin-Ebengreuth, N.; Zechmann, B. Compartment-specific investigations of antioxidants and hydrogen peroxide in leaves of Arabidopsis thaliana during dark-induced senescence. Acta Physiol. Plant. 2016, 38, 133. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Eghbali, M.; Ou, J.; Lu, R.; Toro, L.; Stefani, E. Quantitative determination of spatial protein-protein correlations in fluorescence confocal microscopy. Biophys. J. 2010, 98, 493–504. [Google Scholar] [CrossRef]
- Manders, E.M.; Stap, J.; Aten, J.A. Dynamics of three-dimensional replication patterns during the S-phase, analyzed by double labeling of DNA and confocal microscopy. J. Cell Sci. 1992, 103, 857–862. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak, K.; Lockhart, B.E.L.; Garbaczewska, G. Subcelullar localization of proteins associated with Prune dwarf virus replication. Eur. J. Plant. Pathol. 2017, 149, 653–668. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Ultrastructural analysis of Prune dwarf virus intercellular transport and pathogenesis. Int. J. Mol. Sci. 2018, 19, 2570. [Google Scholar] [CrossRef]
- Mayhew, T.M. Quantifying immunogold localization on electron microscopic thin sections: A compendium of new approaches for plant cell biologists. J. Exp. Bot. 2011, 62, 4101–4113. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Software Official Website. Available online: https://www.graphpad.com/quickcalcs/contingency1.cfm (accessed on 21 August 2021).
- Mayhew, T.M.; Lucocq, J.M. Multiple-labelling immunoEM using different sizes of colloidal gold: Alternative approaches to test for differential distribution and colocalization in subcellular structures. Histochem. Cell Biol. 2011, 135, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hafrén, A.; Hofius, D.; Rönnholm, G.; Sonnewald, U.; Mäkinen, K. HSP70 and Its cochaperone CPIP promote Potyvirus Infection in Nicotiana benthamiana by regulating viral coat protein functions. Plant. Cell 2010, 22, 523–535. [Google Scholar] [CrossRef]
- Lõhmus, A.; Hafrén, A.; Mäkinen, K. Coat protein regulation by CK2, CPIP, HSP70, and CHIP is required for Potato Virus A replication and coat protein accumulation. J. Virol. 2017, 91, 1–14. [Google Scholar] [CrossRef]
- Valli, A.A.; Gallo, A.; Rodamilans, B.; López-Moya, J.J.; García, A. The HCPro from the Potyviridae family: An enviable multitasking helper component that every virus would like to have. Mol. Plant. Pathol. 2018, 19, 744–763. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.C.; Volpon, L.; Rahardjo, A.K.; Osborne, M.J.; Culjkovic-Kraljacic, C.; Trahan, C.; Oeffinger, M.; Kwok, B.H.; Borden, K.L.B. Structural studies of the eIF4E-VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc. Natl. Acad. Sci. USA 2019, 116, 24056–24065. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Julio, E.; Candresse, T.; Cotucheau, J.; Decorps, C.; Volpatti, R.; Moury, B.; Glais, L.; Dorlhac de Borne, F.; Decroocq, V.; et al. NtTPN1: A RPP8-like R gene required for Potato virus Y-induced veinal necrosis in tobacco. Plant. J. 2018, 95, 700–714. [Google Scholar] [CrossRef]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gou, X.; He, K.; Xi, D.; Du, J.; Lin, H.; Li, J. BAK1 and BKK1 in Arabidopsis thaliana confer reduced susceptibility to Turnip crinkle virus. Eur. J. Plant. Pathol. 2010, 127, 149–156. [Google Scholar] [CrossRef]
- Korner, C.J.; Klauser, D.; Niehl, A.; Dominguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant. Microbe Interact. 2013, 26, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Wyrsch, I.; Boller, T.; Heinlein, M. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol. 2016, 211, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Heinlein, M. Perception of double-stranded RNA in plant antiviral immunity. Mol. Plant. Phytol. 2019, 20, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, J.P.T. Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato. Breed. Sci. 2015, 65, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Otulak-Kozieł, K.; Kozieł, E.; Valverde, R.A. The Respiratory burst oxidase homolog D (RbohD) cell and tissue distribution in potato–Potato virus Y (PVYNTN) hypersensitive and susceptible reactions. Int. J. Mol. Sci. 2019, 20, 2741. [Google Scholar] [CrossRef]
- Valkonen, J.P.T.; Gebhardt, C.H.; Zimnoch-Guzowska, E.; Watanabe, K.N. Resistance to Potato virus Y in potato. In Potato virus Y: Biodiversity, Pathogenicity, Epidemiology and Management, 1st ed.; Lacomme, C., Glais, L., Bellstedt, D., Dupuis, B., Karasev, A., Jacquot, E., Eds.; Springer International Publishing: Germany, Heidelberg, 2017; pp. 207–241. [Google Scholar]
- Adams, M.J.; Antoniw, J.F.; Fauquet, C.M. Molecular criteria for genus and species discrimination within the family Potyviridae. Arch. Virol. 2005, 150, 459–479. [Google Scholar] [CrossRef]
- Li, X.H.; Valdez, P.; Olvera, R.E.; Carrington, J.C. Functions of the tobacco etch virus RNA polymerase (NIb): Subcellular transport and protein-protein interaction with VPg/proteinase (NIa). J. Virol. 1997, 71, 1598–1607. [Google Scholar] [CrossRef]
- Mine, A.; Okuno, T. Composition of plant virus RNA replicase complexes. Curr. Opin. Virol. 2012, 2, 669–675. [Google Scholar] [CrossRef]
- Wei, T.; Huang, T.S.; McNeil, J.; Laliberte, J.F.; Hong, J.; Nelson, R.S.; Wang, A. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 2010, 84, 799–809. [Google Scholar] [CrossRef]
- Ferrer-Orta, C.; Arias, A.; Escarmis, C.; Verdaguer, N. A comparison of viral RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 2006, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bruenn, J.A. A structural and primary sequence comparison of the viral RNA-dependent RNA polymerases. Nucleic Acids Res. 2003, 31, 1821–1829. [Google Scholar] [CrossRef]
- Li, X.H.; Carrington, J.C. Complementation of tobacco etch potyvirus mutants by active RNA polymerase expressed in transgenic cells. Proc. Natl. Acad. Sci. USA 1995, 92, 457–461. [Google Scholar] [CrossRef]
- Shatskaya, G.S.; Drutsa, V.L.; Koroleva, O.N.; Osterman, I.A.; Dmitrieva, T.M. Investigation of activity of recombinant mengovirus RNA-dependent RNA polymerase and its mutants. Biochemistry 2013, 78, 96–101. [Google Scholar] [CrossRef]
- Zheng, L.; Wayper, P.J.; Gibbs, A.J.; Fourment, M.; Rodoni, B.C.; Gibbs, M.J. Accumulating variation at conserved sites in potyvirus genomes is driven by species discovery and affects degenerate primer design. PLoS ONE 2008, 3, e1586. [Google Scholar] [CrossRef]
- Vives-Adrian, L.; Lujan, C.; Oliva, B.; van der Linden, L.; Selisko, B.; Coutard, B.; Canard, B.; van Kuppeveld, F.J.; Ferrer-Orta, C.; Verdaguer, N. The crystal structure of a cardiovirus RNA-dependent RNA polymerase reveals an unusual conformation of the polymerase active site. J. Virol. 2014, 88, 5595–5607. [Google Scholar] [CrossRef]
- Grangeon, R.; Cotton, S.; Laliberte, J.F. A model for the biogenesis of turnip mosaic virus replication factories. Commun. Integr. Biol. 2010, 3, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Eskelin, K.; Lõhmus, A.; Mäkinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef]
- Wu, X.; Valli, A.; García, J.A.; Zhou, X.; Cheng, X. The tug-of-war between plants and viruses: Great progress and many remaining questions. Viruses 2019, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Janzac, B.F.; Fabre, M.F.; Palloix, A.; Moury, B. Phenotype and spectrum of action of the Pvr4 resistance in pepper against potyviruses, and selection for virulent variants. Plant. Pathol. 2009, 58, 443–449. [Google Scholar] [CrossRef]
- Janzac, B.; Montarry, J.; Palloix, A.; Navaud, O.; Moury, B. A point mutation in the polymerase of Potato virus Y confers virulence toward the Pvr4 resistance of pepper and a high competitiveness cost in susceptible cultivar. Mol. Plant. Microbe. Interact. 2010, 23, 823–830. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, H.Y.; Choi, E.H.; Park, E.; Kim, J.H.; Moon, K.B.; Kim, H.S.; Choi, D. The coiled-coil and leucine-rich repeat domain of the potyvirus resistance protein Pvr4 has a distinct role in signaling and pathogen recognition. Mol. Plant. Microbe Interact. 2018, 31, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Fellers, J.P.; Tremblay, D.; Handest, M.F.; Lommel, S.A. The Potato virus Y MSNR NIb-replicase is the elicitor of a veinal necrosis-hypersensitive response in root knot nematode resistant tobacco. Mol. Plant. Pathol. 2002, 3, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, C.; Li, Y.; Wu, G.; Hou, X.; Zhou, X.; Wang, A. Beclin1 restricts RNA virus infection in plants through suppression and degradation of the viral polymerase. Nat. Commun. 2018, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, P.; Ge, L.; Chang, Z.; Cheng, X.; Zhou, X.; Wang, A.; Li, F. Nuclear exportin 1 facilitates turnip mosaic virus infection by exporting the sumoylated viral replicase and by repressing plant immunity. New Phytol. 2021, 232, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewska, G. The participation of plant cell organelles in compatible and incompatible Potato virus Y-tobacco and-potato plant interaction. Acta Physiol. Plant. 2014, 36, 85–99. [Google Scholar] [CrossRef]
- Wei, T.; Wang, A. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J. Virol. 2008, 82, 12252–12264. [Google Scholar] [CrossRef]
- Movahed, N.; Sun, J.; Vali, H.; Laliberte, J.F.; Zheng, H. A host ER fusogen is recruited by turnip mosaic virus for maturation of viral replication vesicles. Plant. Physiol. 2019, 179, 507–518. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.; Hou, X.; Sanfacon, H.; Wang, A. The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog. 2013, 9, e1003378. [Google Scholar] [CrossRef]
- Martín, M.T.; García, J.A.; Cervera, M.T.; Goldbach, R.W.; van Lent, J.W.M. Intracellular localization of three non-structural plum pox potyvirus proteins by immunogold labelling. Virus Res. 1992, 25, 201–211. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, R.; Bernards, M.; Wang, A. Recruitment of arabidopsis RNA helicase AtRH9 to the viral replication complex by viral replicase to promote turnip mosaic virus replication. Sci. Rep. 2016, 6, 30297. [Google Scholar] [CrossRef]
- Park, S.H.; Li, F.; Renaud, J.; Shen, W.; Li, Y.; Guo, L.; Cui, H.; Sumarah, M.; Wang, A. NbEXPA1, an alpha-expansin, is plasmodesmata-specific and a novel host factor for potyviral infection. Plant. J. 2017, 92, 846–861. [Google Scholar] [CrossRef]
- Rodriguez-Peña, R.; Mounadi, K.E.; Garcia-Ruiz, H. Changes in subcellular localization of host proteins induced by plant viruses. Viruses 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, C.Y.; Yang, J.; Chen, J.P.; Zhang, H.M. Interaction of hsp20 with a viral rdrp changes its sub-cellular localization and distribution pattern in plants. Sci. Rep. 2015, 5, 14016. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with Potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Hýsková, V.; Bělonožníková, K.; Doričová, V.; Kavan, D.; Gillarová, S.; Henke, S.; Ryšlavá, H.; Čeřovská, N. Effects of heat treatment on metabolism of tobacco plants infected with Potato virus Y. Plant. Biol. 2021, 23, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, T.; Wu, X.; Hong, Y.; Fan, Z.; Li, H. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Mol. Plant. Pathol. 2008, 9, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Boevink, P.; Oparka, K.J. Virus-host interactions during movement processes. Plant. Physiol. 2005, 138, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Saitoh, H.; Ito, A.; Fujisawa, S.; Kamoun, S.; Katou, S.; Yoshioka, H.; Terauchi, R. Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana. Mol. Plant. Pathol. 2003, 4, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, R.; Moshe, A.; Ghanim, M.; Czosnek, H. Recruitment of the host plant heat shock protein 70 by Tomato yellow leaf curl virus coat protein is required for virus infection. PLoS ONE 2013, 8, e70280. [Google Scholar] [CrossRef]
- Turner, K.A.; Sit, T.L.; Callaway, A.S.; Allen, N.S.; Lommel, S.A. Red clover necrotic mosaic virus replication proteins accumulate at the endoplasmic reticulum. Virology 2004, 320, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Liu, M.; Zhang, R.; Zhang, X.; Gao, Z.; Zhao, X.; Xu, K.; Li, D.; Zhang, Y. Hsc70-2 is required for Beet black scorch virus infection through interaction with replication and capsid proteins. Sci. Rep. 2018, 8, 4526. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Chen, I.H.; Tsai, C.H. Host factors in the infection cycle of bamboo mosaic virus. Front. Microbiol. 2017, 8, 437. [Google Scholar] [CrossRef] [PubMed]





| Genes | Gene ID | Forward Primer | Reverse Primer | Temperature of Primer Annealing (°C) | Concentration in Reaction (μM) | Product Length (bp) |
|---|---|---|---|---|---|---|
| Investigated | ||||||
| StHsc70-8 | XM_006350698.2 | 5′-AGGGATGCCAAGATGGACAA-3′ | 3′-AACAGCTCATCTGGGTTGA-5′ | 58 | 0.5 | 144 |
| Nib | KX356068.1 | 5′-CTCATCATCAGAAGCACATACA-3′ | 3′-GCATAGCAAGGACAACCAT-5′ | 58 | 0.4 | 190 |
| Reference | ||||||
| StEF1a | AB061263 | 5′-GGTGATGCTGGTATGGTTAAG-3′ | 3′-GGTCCTTCTTGTCAACATTCTT-5′ | 58 | 0.5 | 148 |
| Program | Parameters |
|---|---|
| Preliminary denaturation | 95 °C for 5 min |
| Amplification (35 cycles) | 95 °C for 10 s 58 °C for 10 s 72 °C for 20 s * |
| Melting curve | 65–95 °C; 0.1 °C/s |
| Program | Parameters |
|---|---|
| Preliminary denaturation | 95 °C for 10 min |
| Amplification (35 cycles) | 95 °C for 10 s 58 °C for 10 s * |
| Sample | Mean OD450nm | Presence (+)/Absence of the Virus (−) |
|---|---|---|
| Buffer | 0.0000 | − |
| Mock-inoculated potato cv. Irys (3 dpi) | 0.0403 | − |
| Mock-inoculated potato cv. Sekwana (3 dpi) | 0.0399 | − |
| PVYNTN-inoculated potato cv. Irys (3 dpi) | 0.7383 | + |
| PVYNTN-inoculated potato cv. Sekwana (3 dpi) | 0.5839 | + |
| Mock-inoculated potato cv. Irys (7 dpi) | 0.0408 | − |
| Mock-inoculated potato cv. Sekwana (7 dpi) | 0.0404 | − |
| PVYNTN-inoculated potato cv. Irys (7 dpi) | 1.0498 | + |
| PVYNTN-inoculated potato cv. Sekwana (7 dpi) | 0.8504 | + |
| Mock-inoculated potato cv. Irys (21 dpi) | 0.0540 | − |
| Mock-inoculated potato cv. Sekwana (21 dpi) | 0.0551 | − |
| PVYNTN-inoculated potato cv. Irys (21 dpi) | 2.913 | + |
| PVYNTN-inoculated potato cv. Sekwana (21 dpi) | 0.3061 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozieł, E.; Surowiecki, P.; Przewodowska, A.; Bujarski, J.J.; Otulak-Kozieł, K. Modulation of Expression of PVYNTN RNA-Dependent RNA Polymerase (NIb) and Heat Shock Cognate Host Protein HSC70 in Susceptible and Hypersensitive Potato Cultivars. Vaccines 2021, 9, 1254. https://doi.org/10.3390/vaccines9111254
Kozieł E, Surowiecki P, Przewodowska A, Bujarski JJ, Otulak-Kozieł K. Modulation of Expression of PVYNTN RNA-Dependent RNA Polymerase (NIb) and Heat Shock Cognate Host Protein HSC70 in Susceptible and Hypersensitive Potato Cultivars. Vaccines. 2021; 9(11):1254. https://doi.org/10.3390/vaccines9111254
Chicago/Turabian StyleKozieł, Edmund, Przemysław Surowiecki, Agnieszka Przewodowska, Józef J. Bujarski, and Katarzyna Otulak-Kozieł. 2021. "Modulation of Expression of PVYNTN RNA-Dependent RNA Polymerase (NIb) and Heat Shock Cognate Host Protein HSC70 in Susceptible and Hypersensitive Potato Cultivars" Vaccines 9, no. 11: 1254. https://doi.org/10.3390/vaccines9111254
APA StyleKozieł, E., Surowiecki, P., Przewodowska, A., Bujarski, J. J., & Otulak-Kozieł, K. (2021). Modulation of Expression of PVYNTN RNA-Dependent RNA Polymerase (NIb) and Heat Shock Cognate Host Protein HSC70 in Susceptible and Hypersensitive Potato Cultivars. Vaccines, 9(11), 1254. https://doi.org/10.3390/vaccines9111254









