Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems
Abstract
1. Introduction
2. Mucosal Immune System
2.1. Comparison between the Parenteral Route; Local vs. Systemic Immunity
2.2. Induction of Effective Immune Responses at Sites Distant from the Site of Administration
2.3. Sublingual Delivery of Antigens as an Alternative for Allergy Treatment
3. Antigen Delivery Systems and Commonly Used Mucosal Adjuvants
3.1. Films
3.2. Microneedle Array
3.3. Mucosal Adjuvants
3.3.1. Bacterial Enterotoxins
3.3.2. Toll-like Receptor Agonist
3.3.3. Polymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MALT | mucosa-associated lymphoid tissue |
| NALT | nasopharynx-associated lymphoid tissue |
| BALT | bronchus-associated lymphoid tissue |
| GALT | gut-associated lymphoid tissue |
| CMIS | common mucosal immune system |
| SIV | simian immunodeficiency virus |
| Ig | immunoglobulin |
| HIV | human immunodeficiency virus |
| OVA | ovalbumin |
| ODF | orally dissolving films |
| MHC | major histocompatibility complex |
| SF | silk fibroin |
| PCL | polycaprolactone |
| Chitosan-PEO | chitosan-polyethylene oxide |
| PLGA-PEG | poly(lactic acid-co-glycolic acid and polyethylene glycol |
| pAPC | porcine antigen-presenting cells |
| CT | cholera toxin |
| PLA | polylactic acid |
| CMC | carboxymethyl cellulose |
| BSA | bovine serum albumin |
| MPC | mannose-PEG-cholesterol |
| PVP | polyvinylpyrrolidone |
| MLL | MPC/lipid A-liposome |
| proMMA | proMLL-filled microneedle array |
| CNS | central nervous system |
| LT | lethal toxin |
| mLT | mutant lethal toxin |
| dmLT | double mutant lethal toxin |
| AFC | antibody-forming cells |
| PEG | polyethylene glycol |
| HPMC | hydroxypropyl methylcellulose |
| DCs | Dendritic cells. |
References
- Davitt, C.J.H.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hashizume, T.; Kurita-Ochiai, T.; Yamamoto, M. Sublingual vaccination with outer membrane protein of Porphyromonas gingivalis and Flt3 ligand elicits protective immunity in the oral cavity. Biochem. Biophys. Res. Commun. 2009, 390, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Azegami, T. The mucosal immune system: From dentistry to vaccine development. Proc. Jpn. Acad. Ser. B 2015, 91, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mumper, R.J. Bilayer Films for Mucosal (Genetic) Immunization via the Buccal Route in Rabbits. Pharm. Res. 2002, 19, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Allon, A.; Roni, M.A.; Kouzi, S. Overview and Future Potential of Fast Dissolving Buccal Films as Drug Delivery System for Vaccines. J. Pharm. Pharm. Sci. 2019, 22, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, S.; Vertruyen, A.; Esposito, S.; Mckeith, D.D.; Klemola, T.; Biolek, J.; Bujnowski, T.; Desgrandchamps, D.; Cheng, S.; Skinner, J.; et al. Superior Relative Efficacy of Live Attenuated Influenza Vaccine Compared With Inactivated Influenza Vaccine in Young Children With Recurrent Respiratory Tract Infections. Pediatr. Infect. Dis. J. 2006, 25, 870–879. [Google Scholar] [CrossRef]
- Lundholm, P. Induction of mucosal IgA by a novel jet delivery technique for HIV-1 DNA. Vaccine 1999, 17, 2036–2042. [Google Scholar] [CrossRef]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and Preclinical Evaluation of a Novel Buccal Measles Vaccine. AAPS PharmSciTech 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Negri, D.R.M.; Riccomi, A.; Pinto, D.; Vendetti, S.; Rossi, A.; Cicconi, R.; Ruggiero, P.; Del Giudice, G.; De Magistris, M.T. Persistence of mucosal and systemic immune responses following sublingual immunization. Vaccine 2010, 28, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Gallichan, W.S.; Rosenthal, K.L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 1996, 184, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, C.; Luci, C.; Çuburu, N.; Cremel, M.; Bekri, S.; Vimeux, L.; Marañón, C.; Czerkinsky, C.; Hosmalin, A.; Anjuère, F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine 2010, 28, 5582–5590. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Pizza, M.; Douce, G.; Dougan, G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 1999, 20, 493–500. [Google Scholar] [CrossRef]
- Riese, P.; Sakthivel, P.; Trittel, S.; Guzmán, C.A. Intranasal formulations: Promising strategy to deliver vaccines. Expert Opin. Drug Deliv. 2014, 11, 1619–1634. [Google Scholar] [CrossRef]
- McGhee, J.R.; Fujihashi, K. Inside the Mucosal Immune System. PLoS Biol. 2012, 10, e1001397. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, K.; Bienenstock, J. Characteristics and Functions of Mucosa-Associated Lymphoid Tissue. In Handbook of Mucosal Immunology; Elsevier: Amsterdam, The Nederlands, 1994; pp. 141–149. [Google Scholar]
- Nyström, J.; Raghavan, S.; Svennerholm, A.-M. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes. Infect. 2006, 8, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Function of Mucosa-Associated Lymphoid Tissue in Antibody Formation. Immunol. Investig. 2010, 39, 303–355. [Google Scholar] [CrossRef]
- Gamazo, C.; Martín-Arbella, N.; Brotons-Canto, A.; Camacho, A.I.; Irache, J. Mimicking microbial strategies for the design of mucus-permeating nanoparticles for oral immunization. Eur. J. Pharm. Biopharm. 2015, 96, 454–463. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Pinzón Martín, S.; Seeberger, P.H.; Varón Silva, D. Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators During Infection and Targets for Diagnostics and Vaccines. Front. Chem. 2019, 7, 710. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicol. Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ruehl-Fehlert, C.; Parker, G.A.; Elmore, S.A.; Kuper, C.F. Immune System. In Fundamentals of Toxicologic Pathology; Elsevier: Amsterdam, The Nederlands, 2018; pp. 273–313. [Google Scholar]
- Rand, J.H.; Wu, X.-X.; Lin, E.Y.; Griffel, A.; Gialanella, P.; McKitrick, J.C. Annexin A5 Binds to Lipopolysaccharide and Reduces Its Endotoxin Activity. mBio 2012, 3, e00292-11. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Hase, K. Glycoprotein 2 (GP2): Grabbing the FimH+ bacteria into M cells for mucosal immunity. Gut Microbes 2010, 1, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gusti, V.; Saraswati, A.; Lo, D.D. Convergent and Divergent Development among M Cell Lineages in Mouse Mucosal Epithelium. J. Immunol. 2011, 187, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccines Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal. Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Bankvall, M.; Östberg, A.-K.; Jontell, M.; Wold, A.; Östman, S. The engagement of oral-associated lymphoid tissues during oral versus gastric antigen administration. Immunology 2016, 149, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Kiyono, H. New generation of mucosal adjuvants for the induction of protective immunity. Rev. Med. Virol. 2003, 13, 293–310. [Google Scholar] [CrossRef]
- Kurono, Y.; Yamamoto, M.; Fujihashi, K.; Kodama, S.; Suzuki, M.; Mogi, G.; McGhee, J.R. Nasal Immunization Induces Haemophilus influenzae—Specific Th1 and Th2 Responses with Mucosal IgA and Systemic IgG Antibodies for Protective Immunity. J. Infect. Dis. 1999, 180, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Russell, M.W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 1997, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Iwatani, K.; Iijima, H.; Kodama, S.; Yanagita, M.; Kiyono, H. Nasal immune system: Distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur. J. Immunol. 1997, 8, 3346–3353. [Google Scholar] [CrossRef]
- Kiyono, H.; Fukuyama, S. NALT-versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, I.M.; Derby, M.A.; Ahlers, J.D.; Kelsall, B.L.; Earl, P.; Moss, B.; Strober, W.; Berzofsky, J. AMucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 1998, 95, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Roan, N.R.; Gierahn, T.M.; Higgins, D.E.; Starnbach, M.N. Monitoring the T cell response to genital tract infection. Proc. Natl. Acad. Sci. USA 2006, 103, 12069–12074. [Google Scholar] [CrossRef]
- Bolton, D.L.; Song, K.; Wilson, R.L.; Kozlowski, P.A.; Tomaras, G.D.; Keele, B.F.; Lovingood, R.V.; Rao, S.; Roederer, M. Comparison of systemic and mucosal vaccination: Impact on intravenous and rectal SIV challenge. Mucosal. Immunol. 2012, 5, 41–52. [Google Scholar] [CrossRef]
- Pinczewski, J.; Zhao, J.; Malkevitch, N.; Patterson, L.J.; Aldrich, K.; Alvord, W.G.; Robert-Guroff, M. Enhanced Immunity and Protective Efficacy against SIV mac251 Intrarectal Challenge Following Ad-SIV Priming by Multiple Mucosal Routes and gp120 Boosting in MPL-SE. Viral Immunol. 2005, 18, 236–243. [Google Scholar] [CrossRef]
- Song, K.; Bolton, D.L.; Wei, C.-J.; Wilson, R.L.; Camp, J.V.; Bao, S.; Mattapallil, J.; Herzenberg, L.A.; Andrews, C.A.; Sadoff, J.C.; et al. Genetic immunization in the lung induces potent local and systemic immune responses. Proc. Natl. Acad. Sci. USA 2010, 107, 22213–22218. [Google Scholar] [CrossRef]
- Balmelli, C.; Demotz, S.; Acha-Orbea, H.; De Grandi, P.; Nardelli-Haefliger, D. Trachea, Lung, and Tracheobronchial Lymph Nodes Are the Major Sites Where Antigen-Presenting Cells Are Detected after Nasal Vaccination of Mice with Human Papillomavirus Type 16 Virus-Like Particles. J. Virol. 2002, 76, 12596–12602. [Google Scholar] [CrossRef] [PubMed]
- Nardellihaefliger, D.; Lurati, F.; Wirthner, D.; Spertini, F.; Schiller, J.; Lowy, D.; Ponci, F.; Grandi, P. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine 2005, 23, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Mestecky, J. Mucosal immunoglobulins. Immunol. Rev. 2005, 206, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Petäjä, T.; Pedersen, C.; Poder, A.; Strauss, G.I.; Catteau, G.; Thomas, F.; Lehtinen, M.; Descamps, D. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int. J. Cancer 2011, 129, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Murakami, T.; Hakamata, Y.; Ajiki, T.; Jinbu, Y.; Akasaka, Y.; Ohtsuki, M.; Nakagawa, H.; Kobayashi, E. Gene gun–mediated oral mucosal transfer of interleukin 12 cDNA coupled with an irradiated melanoma vaccine in a hamster model: Successful treatment of oral melanoma and distant skin lesion. Cancer Gene. Ther. 2001, 8, 705–712. [Google Scholar] [CrossRef]
- Effros, R.B.; Bennink, J.; Doherty, P.C. Characteristics of secondary cytotoxic T-cell responses in mice infected with influenza A viruses. Cell. Immunol. 1978, 36, 345–353. [Google Scholar] [CrossRef]
- Hill, A.B.; Blanden, R.V.; Parrish, C.R.; Müllbacher, A. Restimulated memory Tc cells have a higher apparent avidity of interaction with targets than primary virusimmune Tc cells as indicated by anti-CD 8 blocking. Immunol. Cell Biol. 1992, 70, 259–265. [Google Scholar] [CrossRef]
- Nugent, C.T.; Wolcott, R.M.; Chervenak, R.; Jennings, S.R. Analysis of the cytolytic T-lymphocyte response to herpes simplex virus type 1 glycoprotein B during primary and secondary infection. J. Virol. 1994, 68, 7644–7648. [Google Scholar] [CrossRef]
- Müllbacher, A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J. Exp. Med. 1994, 179, 317–321. [Google Scholar] [CrossRef]
- Mackay, C.R. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 1993, 5, 423–427. [Google Scholar] [CrossRef]
- Mestecky, J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.R.; Goldsmith, C.H.; Rosenthal, K.L.; Brais, L.J. T Lymphocytes in Genital Lymph Nodes Protect Mice from Intravaginal Infection with Herpes Simplex Virus Type 2. J. Infect. Dis. 1989, 159, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Picker, L.J. Control of lymphocyte homing. Curr. Opin. Immunol. 1994, 6, 394–406. [Google Scholar] [CrossRef]
- Seavey, M.M.; Mosmann, T.R. Estradiol-induced vaginal mucus inhibits antigen penetration and CD8+ T cell priming in response to intravaginal immunization. Vaccine 2009, 27, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Czerkinsky, C.; Çuburu, N.; Kweon, M.-N.; Anjuère, F.; Holmgren, J. Sublingual vaccination. Hum. Vaccines 2011, 7, 110–114. [Google Scholar] [CrossRef]
- Çuburu, N.; Kweon, M.-N.; Song, J.-H.; Hervouet, C.; Luci, C.; Sun, J.-B.; Hofman, P.; Holmgren, J.; Anjuère, F.; Czerkinsky, C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 2007, 25, 8598–8610. [Google Scholar] [CrossRef]
- Kweon, M.-N. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine 2011, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, D.; Zhang, X.; Mu, J.; Medina, M.F.; Xing, Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet. Vaccines Ther. 2008, 6, 1–9. [Google Scholar] [CrossRef]
- Lewis, D.J.M.; Huo, Z.; Barnett, S.; Kromann, I.; Giemza, R.; Galiza, E.; Woodrow, M. Transient Facial Nerve Paralysis (Bell’s Palsy) following Intranasal Delivery of a Genetically Detoxified Mutant of Escherichia coli Heat Labile Toxin. PLoS ONE 2009, 4, e6999. [Google Scholar] [CrossRef]
- Song, J.-H.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.-Y.; Park, S.-H.; Czerkinsky, C.; Kweon, M.-N. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kiyono, H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012, 2, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Scurati, S.; Puccinelli, P.; David, M.; Hilaire, C.; Capecce, M.; Marcucci, F.; Incorvaia, C. Development of a sublingual allergy vaccine for grass pollinosis. Drug Des. Dev. Ther. 2010, 4, 99–105. [Google Scholar] [CrossRef][Green Version]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Lockey, R.; Malling, H. Allergen immunotherapy: Therapeutic vaccines for allergic diseases A WHO position paper. J. Allergy Clin. Immunol. 1998, 102, 558–562. [Google Scholar] [CrossRef]
- Passalacqua, G.; Lombardi, C.; Canonica, G.W. Sublingual immunotherapy: An update. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 31–36. [Google Scholar] [CrossRef]
- Durham, S.R. Sublingual immunotherapy: What have we learnt from the ‘big trials’? Curr. Opin. Allergy Clin. Immunol. 2008, 8, 577–584. [Google Scholar] [CrossRef]
- Holt, P.G.; Vines, J.; Britten, D. Sublingual allergen administration. I. Selective suppression of IgE production in rats by high allergen doses. Clin. Exp. Allergy 1988, 18, 229–234. [Google Scholar] [CrossRef]
- Lou, H.; Liu, M.; Qu, W.; Hu, Z.; Brunson, E.; Johnson, J.; Almoazen, H. Evaluation of Chlorpheniramine Maleate microparticles in orally disintegrating film and orally disintegrating tablet for pediatrics. Drug Dev. Ind. Pharm. 2014, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Mašek, J.; Lubasová, D.; Lukáč, R.; Turánek-Knotigová, P.; Kulich, P.; Plocková, J.; Mašková, E.; Procházka, L.; Koudelka, Š.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles-important step towards effective mucosal vaccines. J. Control. Release 2017, 249, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.W.; Frijlink, H.W.; Hinrichs, W.L.J.; Visser, J.C. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics 2020, 12, 245. [Google Scholar] [CrossRef]
- Lal, M.; White, J.; Zhu, C. Preparing an Adjuvanted Thermoresponsive Gel Formulation for Sublingual Vaccination. In Vaccine Adjuvants; Fox, C.B., Ed.; Springer: New York, NY, USA, 2017; pp. 153–163. [Google Scholar]
- Ma, Y.; Tao, W.; Krebs, S.J.; Sutton, W.F.; Haigwood, N.; Gill, H.S. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm. Res. 2014, 31, 2393–2403. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Cha, H.-R.; Hwang, S.J.; Kim, D.-S.; Choi, Y.-J.; Kim, Y.-S.; Shin, Y.-R.; Nguyen, T.T.; Choi, S.-O.; Lee, J.M.; et al. Ovalbumin and cholera toxin delivery to buccal mucus for immunization using microneedles and comparison of immunological response to transmucosal delivery. Drug Deliv. Transl. Res. 2021, 11, 1390–1400. [Google Scholar] [CrossRef]
- Zhen, Y.; Wang, N.; Gao, Z.; Ma, X.; Wei, B.; Deng, Y.; Wang, T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 2015, 33, 4330–4340. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhen, Y.; Ma, X.; Wei, B.; Li, S.; Wang, N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf. B 2015, 126, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M. MucoJet: A novel noninvasive buccal mucosa immunization strategy. Ann. Transl. Med. 2018, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Cox, P.S.; Wertz, P.W.; Downing, D.T. The lipid composition of porcine epidermis and oral epithelium. Arch. Oral. Biol. 1986, 31, 741–747. [Google Scholar] [CrossRef]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.-C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Kruithof, A.C.; Zwier, R.; Dietz, E.; Bouwstra, J.A.; Lademann, J.; Meinke, M.C. Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo. J. Control. Release 2010, 147, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coating Formulations for Microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef]
- Zhao, X.; Coulman, S.A.; Hanna, S.J.; Wong, F.S.; Dayan, C.M.; Birchall, J.C. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J. Control. Release 2017, 265, 2–13. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Sekerdag, E.; Jiskoot, W.; Bouwstra, J. Impact-Insertion Applicator Improves Reliability of Skin Penetration by Solid Microneedle Arrays. AAPS J. 2014, 16, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Ellison, T.J.; Talbott, G.C.; Henderson, D.R. VaxiPatchTM, a novel vaccination system comprised of subunit antigens, adjuvants and microneedle skin delivery: An application to influenza B/Colorado/06/2017. Vaccine 2020, 38, 6839–6848. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef]
- Flynn, O.; Dillane, K.; Lanza, J.S.; Marshall, J.; Jin, J.; Silk, S.; Draper, S.; Moore, A. Low Adenovirus Vaccine Doses Administered to Skin Using Microneedle Patches Induce Better Functional Antibody Immunogenicity as Compared to Systemic Injection. Vaccines 2021, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Volunteers. Clin. J. Pain. 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Lycke, N.; Czerkinsky, C. Cholera toxin and cholera B subunit as oral—Mucosal adjuvant and antigen vector systems. Vaccine 1993, 11, 1179–1184. [Google Scholar] [CrossRef]
- Lycke, N. From toxin to adjuvant: Basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol. Lett. 2005, 97, 193–198. [Google Scholar] [CrossRef]
- Clements, J.D.; Hartzog, N.M.; Lyon, F.L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 1988, 6, 269–277. [Google Scholar] [CrossRef]
- Ottsjo, L.; Jeverstam, F.; Yrlid, L.; Wenzel, A.U.; Raghavan, S. Induction of mucosal immune responses against Helicobacter pylori infection after sublingual and intragastric route of immunization. Immunology 2016, 150, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [PubMed]
- Spanglert, B.D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 1992, 56, 622–647. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; DiLorenzo, S.C.; Walker, R.I. Safety and Immunogenicity of Oral Inactivated Whole-Cell Helicobacter pylori Vaccine with Adjuvant among Volunteers with or without Subclinical Infection. Infect. Immun. 2001, 69, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Michetti, P.; Kreiss, C.; Kotloff, K.L.; Porta, N.; Blanco, J.; Bachmann, D. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori–infected adults. Gastroenterology 1999, 116, 804–812. [Google Scholar] [CrossRef]
- Lundgren, A.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Hartford, M.; Holmgren, J.; Petzold, M.; Walker, R.; Svennerholm, A.M. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine 2014, 32, 7077–7084. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Weeratna, R.D.; Davis, H.L. The potential of oligodeoxynucleotides as mucosal and parenteral adjuvants. Vaccine 2001, 19, 2657–2660. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Davis, H.L. CpG DNA Is a Potent Enhancer of Systemic and Mucosal Immune Responses Against Hepatitis B Surface Antigen with Intranasal Administration to Mice. J. Immunol. 1998, 161, 4463–4466. [Google Scholar]
- Chang, E.; Kobayashi, R.; Hagiwara, M.; Komiya, M.; Kurita-Ochiai, T. Evaluation of suitable antigens and adjuvant concentration for sublingual immunization to prevent periodontal disease. Oral. Sci. Int. 2019, 16, 80–86. [Google Scholar] [CrossRef]
- Borchard, G.; Lueβen, H.L.; de Boer, A.G.; Verhoef, J.; Lehr, C.-M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- van der Lubben, I. Chitosan microparticles for oral vaccination: Preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 2001, 22, 687–694. [Google Scholar] [CrossRef]
- van der Lubben, I.M.; Konings, F.A.J.; Borchard, G.; Verhoef, J.C.; Junginger, H.E. In Vivo Uptake of Chitosan Microparticles by Murine Peyer’s Patches: Visualization Studies using Confocal Laser Scanning Microscopy and Immunohistochemistry. J. Drug Target. 2001, 9, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.; Mansouri, S.; McEntee, C.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Grégoire-Gélinas, P.; Cheng, A.P.; Mezheritsky, T.; Lavertu, M.; Sato, S.; Hoemann, C.D. Lysosomal rupture induced by structurally distinct chitosans either promotes a type 1 IFN response or activates the inflammasome in macrophages. Biomaterials 2017, 129, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.Y.; Sekiya, T.; Al Kobaisi, M.; Short, K.R.; Mainwaring, D.E.; Jackson, D.C. A single dose biodegradable vaccine depot that induces persistently high levels of antibody over a year. Biomaterials 2015, 53, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Zhan, X.; Tran, K.K.; Shen, H. Effect of the Poly (ethylene glycol) (PEG) Density on the Access and Uptake of Particles by Antigen-Presenting Cells (APCs) after Subcutaneous Administration. Mol. Pharm. 2012, 9, 3442–3451. [Google Scholar] [CrossRef]
- De Koker, S.; Cui, J.; Vanparijs, N.; Albertazzi, L.; Grooten, J.; Caruso, F.; De Geest, B.G. Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery. Angew. Chem. Int. Ed. 2016, 55, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Bajrovic, I.; Schafer, S.C.; Romanovicz, D.K.; Croyle, M.A. Novel technology for storage and distribution of live vaccines and other biological medicines at ambient temperature. Sci. Adv. 2020, 6, eaau4819. [Google Scholar] [CrossRef] [PubMed]
- Ubale, R.V.; D’souza, M.J.; Infield, D.T.; McCarty, N.A.; Zughaier, S.M. Formulation of meningococcal capsular polysaccharide vaccine-loaded microparticles with robust innate immune recognition. J. Microencapsul. 2013, 30, 28–41. [Google Scholar] [CrossRef] [PubMed]
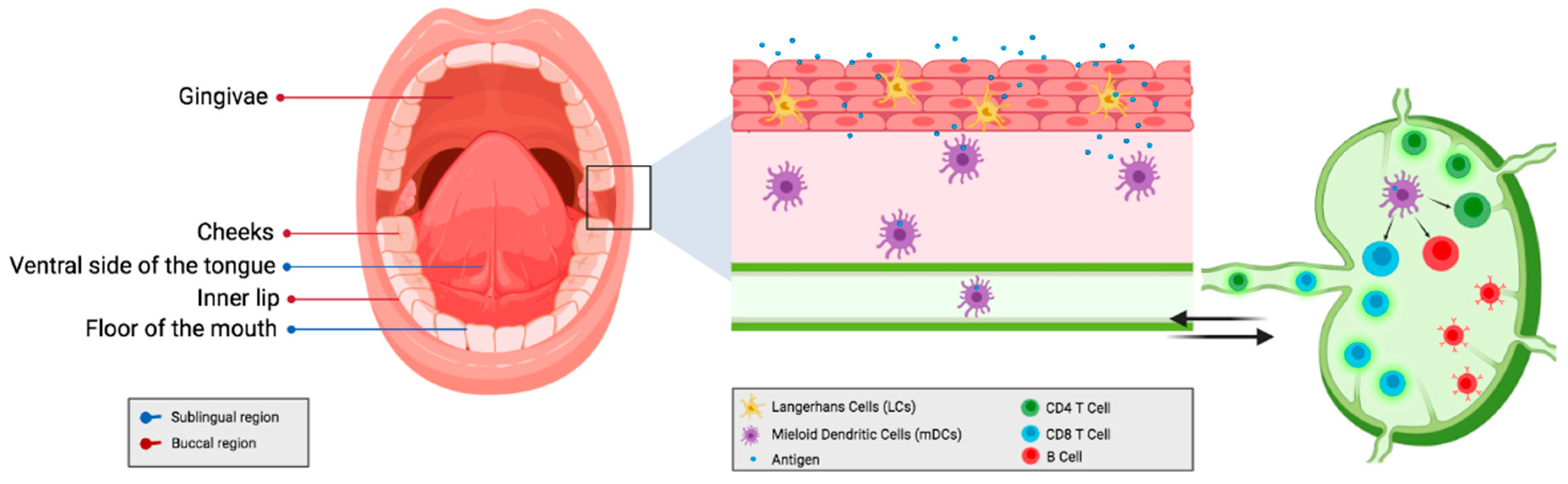

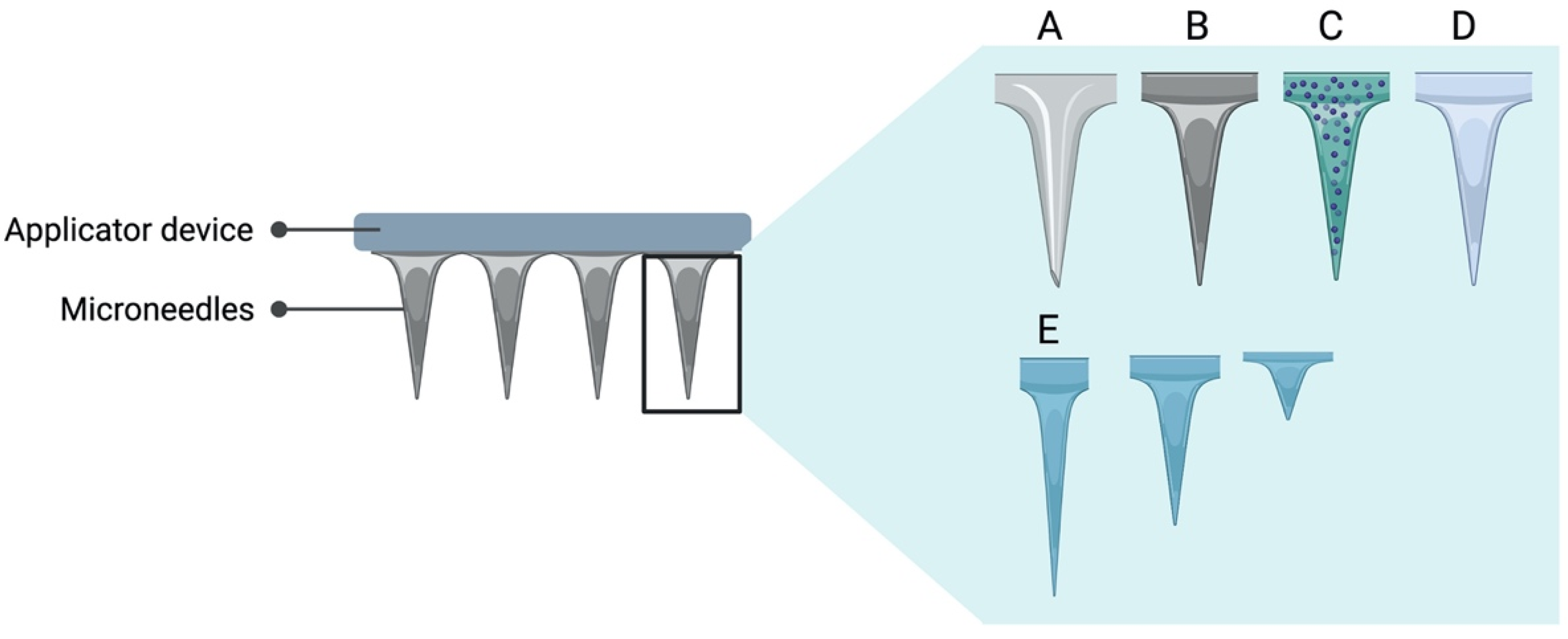
| Route of Administration | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Subcutaneous | Assured absorption | Pain at the puncture site | [5] |
| Induce systemic immune responses | Requires medical personnel | [6] | |
| Avoid first-pass effect | Does not induce effective mucosal immune responses | ||
| Intramuscular | Assured adsorption | Pain at the puncture site | [7] |
| Induce systemic immune responses Avoid first-pass effect | Requires medical personnel Does not induce effective mucosal immune responses Requires cold chain Expensive to prepare | [6] | |
| Oral | Painless | Degradation of vaccine by harsh stomach environment and high enzymatic levels | [8] |
| Induce systemic and mucosal immune responses | Taste issues | [6] | |
| Easy to administer | |||
| Buccal | Painless Induce systemic and mucosal immune responses | Salivary washout can dilute the vaccine Motion stress Taste issues | [5,9] |
| Easy to administer Mild pH values | [6] | ||
| Sublingual | Painless Induce systemic and mucosal immune responses Easy | Salivary washout can dilute the vaccine Motion stress Taste issues | [6,10] |
| Intranasal | Painless Induce systemic and mucosal immune responses Mild pH values | Retrograde passage to the CNS Short residence time Quick clearance of antigens | [7,11,12,13,14] |
| Administration Route | Dosage Form | Main Feature | Antigen/Model Antigen Used | Reference |
|---|---|---|---|---|
| Buccal | Film | Orally disintegrating film loaded with microparticulate vaccine | Live attenuated Measles microparticulate vaccine | [9] |
| Buccal | Film | Bilayer mucoadhesive film | β-galactosidase/plasmid DNA-expressing β-galactosidase | [5] |
| Buccal | Film | Electrospun nanofibrous reservoir multilayer film | Green fluorescent protein loaded nanoparticle and liposomes | [72] |
| Inner lip/ Tongue | Microneedle | Solid stainless steel coated microneedle array | HIV and Ovalbumin Antigens | [75] |
| Buccal | Microneedle | CMC coated solid PLA micromeedle array | Ovalbumin | [76] |
| Oral mucosa | Microneedle | Liposome loaded dissolving microneedle array | BSA | [77] |
| Adjuvant | Origin | Effect | Reference |
|---|---|---|---|
| Cholera Toxin | Vibrio cholera | Immunostimulatory | [92] |
| CTA1-DD | Vibrio cholera | Immunostimulatory | [93] |
| Heat-labile enterotoxin LT | Escherichia coli | Immunostimulatory | [94] |
| DmLT | Escherichia coli | Immunostimulatory | [95] |
| CpG DNA | Oligonucleotide DNA | Immunostimulatory | [96] |
| Chitosan | Cationic polysaccharide | Enhance adsorption of antigens | [97] |
| Polyethylene Glycol PEG | Polymeric | Mucoadhesion and mucopenetration | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trincado, V.; Gala, R.P.; Morales, J.O. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines 2021, 9, 1177. https://doi.org/10.3390/vaccines9101177
Trincado V, Gala RP, Morales JO. Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines. 2021; 9(10):1177. https://doi.org/10.3390/vaccines9101177
Chicago/Turabian StyleTrincado, Valeria, Rikhav P. Gala, and Javier O. Morales. 2021. "Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems" Vaccines 9, no. 10: 1177. https://doi.org/10.3390/vaccines9101177
APA StyleTrincado, V., Gala, R. P., & Morales, J. O. (2021). Buccal and Sublingual Vaccines: A Review on Oral Mucosal Immunization and Delivery Systems. Vaccines, 9(10), 1177. https://doi.org/10.3390/vaccines9101177







