The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
Exclusion Criteria
3. Results
3.1. The Human Microbiota
3.2. Surveying the Skin Microbiome
3.3. Composition and Diversity of the Human Skin Microbiome
3.4. Skin Microbiota–Immune System Crosstalk
3.5. Cutaneous Dysbiosis
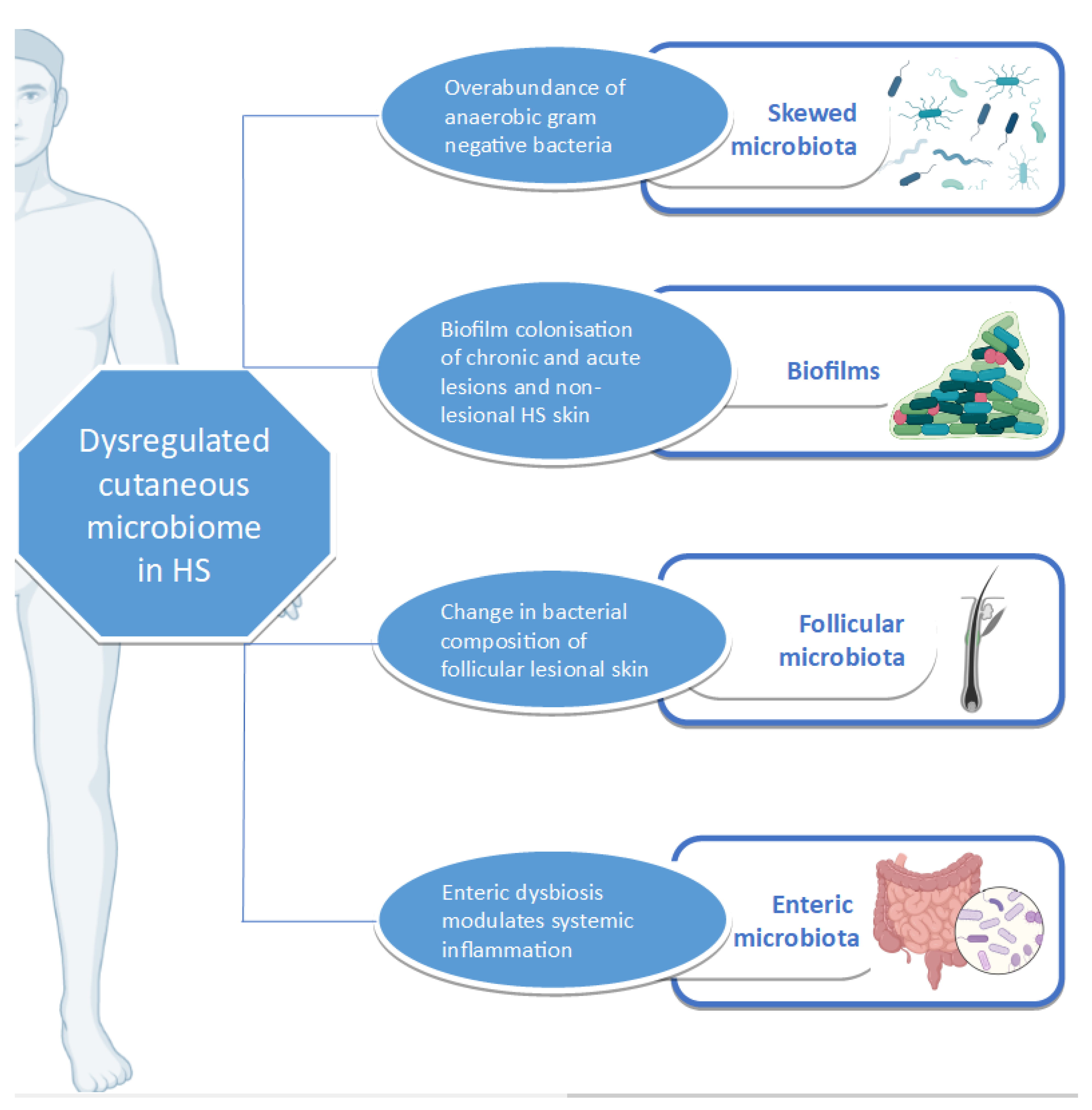
3.6. Cutaneous Dysbiosis in Hidradenitis Suppurativa
3.7. Beyond the Skin Microbiome
3.8. Targeting the Microbiome as a Treatment Modality
3.8.1. Antibiotics
3.8.2. Enteric Microbiome and Diet
3.9. The Microbiome—A Potential HS Biomarker?
3.9.1. Prognostic Biomarker
3.9.2. Risk Biomarker
3.9.3. Pharmacodynamic Biomarker
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matusiak, Ł.; Bieniek, A.; Szepietowski, J.C. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J. Am. Acad. Dermatol. 2010, 62, 706–708. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Benhadou, F.; Byrd, A.S.; Chandran, N.S.; Giamarellos-Bourboulis, E.J.; Fabbrocini, G.; Frew, J.W.; Fujita, H.; González-López, M.A.; Guillem, P.; et al. What causes hidradenitis suppurativa?—15 years after. Exp. Dermatol. 2020, 29, 1154–1170. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K.; Krahl, D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int. J. Dermatol. 2005, 44, 535–540. [Google Scholar] [CrossRef]
- Scheinfeld, N. Hidradenitis should not be renamed acne inversa. Dermatol. Online J. 2006, 12, 6. [Google Scholar]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Pothmann, A.; Illing, T.; Wiegand, C.; Hartmann, A.A.; Elsner, P. The Microbiome and Atopic Dermatitis: A Review. Am. J. Clin. Dermatol. 2019, 20, 749–761. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: Dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Treuren, W.V.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Huang, Y.J.; Boushey, H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015, 135, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.T.; Hug, L.A.; Thomas, B.C.; Sharon, I.; Castelle, C.J.; Singh, A.; Wilkins, M.J.; Wrighton, K.C.; Williams, K.H.; Banfield, J.F. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature 2015, 523, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Eloe-Fadrosh, E.A.; Ivanova, N.N.; Woyke, T.; Kyrpides, N.C. Metagenomics uncovers gaps in amplicon-based detection of microbial diversity. Nat. Microbiol. 2016, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Ward, D.V.; Pasolli, E.; Tolio, T.; Zolfo, M.; Asnicar, F.; Truong, D.T.; Tett, A.; Morrow, A.L.; Segata, N. Strain-level microbial epidemiology and population genomics from shotgun metagenomics. Nat. Methods 2016, 13, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, F.P.; Lu, J.; Salzberg, S.L. A review of methods and databases for metagenomic classification and assembly. Brief. Bioinform. 2017, 20, 1125–1136. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R. Microbial genomics, transcriptomics and proteomics: New discoveries in decomposition research using complementary methods. Appl. Microbiol. Biotechnol. 2014, 98, 1531–1537. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular “omics” for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Blakeley-Ruiz, J.A.; Erickson, A.R.; Cantarel, B.L.; Xiong, W.; Adams, R.; Jansson, J.K.; Fraser, C.M.; Hettich, R.L. Metaproteomics reveals persistent and phylum-redundant metabolic functional stability in adult human gut microbiomes of Crohn’s remission patients despite temporal variations in microbial taxa, genomes, and proteomes. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Vogtmann, E.; Ahlquist, D.A.; Devens, M.E.; Kisiel, J.B.; Taylor, W.R.; White, B.A.; Hale, V.L.; Sung, J.; Chia, N.; et al. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. MBio 2020, 11, e03186-19. [Google Scholar] [CrossRef] [Green Version]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clooney, A.G.; Fouhy, F.; Sleator, R.D.; O’ Driscoll, A.; Stanton, C.; Cotter, P.D.; Claesson, M.J. Comparing Apples and Oranges?: Next Generation Sequencing and Its Impact on Microbiome Analysis. PLoS ONE 2016, 11, e0148028. [Google Scholar] [CrossRef]
- Bjerre, R.D.; Hugerth, L.W.; Boulund, F.; Seifert, M.; Johansen, J.D.; Engstrand, L. Effects of sampling strategy and DNA extraction on human skin microbiome investigations. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Ogai, K.; Nagase, S.; Mukai, K.; Iuchi, T.; Mori, Y.; Matsue, M.; Sugitani, K.; Sugama, J.; Okamoto, S. A Comparison of Techniques for Collecting Skin Microbiome Samples: Swabbing Versus Tape-Stripping. Front. Microbiol. 2018, 9, 2362. [Google Scholar] [CrossRef] [PubMed]
- Manus, M.B.; Kuthyar, S.; Perroni-Marañón, A.G.; Mora, A.N.D.L.; Amato, K.R. Comparing different sample collection and storage methods for field-based skin microbiome research. Am. J. Hum. Biol. 2021, e23584. [Google Scholar] [CrossRef]
- Kong, H.H.; Andersson, B.; Clavel, T.; Common, J.E.; Jackson, S.A.; Olson, N.D.; Segre, J.A.; Traidl-Hoffmann, C. Performing Skin Microbiome Research: A Method to the Madness. J. Investig. Dermatol. 2017, 137, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Gribbon, E.M.; Cunliffe, W.J.; Holland, K.T. Interaction of Propionibacterium acnes with skin lipids in vitro. J. Gen. Microbiol. 1993, 139, 1745–1751. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [Green Version]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. MBio 2015, 6, e01578-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Byrd, A.L.; Park, M.; NISC Comparative Sequencing Program; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341. [Google Scholar] [CrossRef] [Green Version]
- Marples, R.R.; Downing, D.T.; Kligman, A.M. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J. Investig. Dermatol. 1971, 56, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.L.; Wertz, P.W. Effects of Endogenous Lipids on the Skin Microbiome. In Skin Microbiome Handbook; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2020; pp. 217–235. ISBN 978-1-119-59305-8. [Google Scholar]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [Green Version]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.-M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, H.; Henne, A.; Hoster, F.; Liesegang, H.; Wiezer, A.; Strittmatter, A.; Hujer, S.; Dürre, P.; Gottschalk, G. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 2004, 305, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, H.B.; Kilian, M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS ONE 2010, 5, e12277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.-I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.-H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Chng, K.R.; Tay, A.S.L.; Li, C.; Ng, A.H.Q.; Wang, J.; Suri, B.K.; Matta, S.A.; McGovern, N.; Janela, B.; Wong, X.F.C.C.; et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Hanski, I.; Hertzen, L.V.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riverain-Gillet, É.; Guet-Revillet, H.; Jais, J.-P.; Ungeheuer, M.-N.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Nassif, A.; Join-Lambert, O. The Surface Microbiome of Clinically Unaffected Skinfolds in Hidradenitis Suppurativa: A Cross-Sectional Culture-Based and 16S rRNA Gene Amplicon Sequencing Study in 60 Patients. J. Investig. Dermatol. 2020, 140, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. The Follicular Skin Microbiome in Patients With Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017, 153, 897–905. [Google Scholar] [CrossRef]
- Ring, H.C.; Sigsgaard, V.; Thorsen, J.; Fuursted, K.; Fabricius, S.; Saunte, D.M.; Jemec, G.B. The microbiome of tunnels in hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Theut Riis, P.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. Moderate to severe hidradenitis suppurativa patients do not have an altered bacterial composition in peripheral blood compared to healthy controls. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Hispán, P.; Murcia, O.; Gonzalez-Villanueva, I.; Francés, R.; Giménez, P.; Riquelme, J.; Betlloch, I.; Pascual, J.C. Identification of bacterial DNA in the peripheral blood of patients with active hidradenitis suppurativa. Arch. Dermatol. Res. 2020, 312, 159–163. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Fuursted, K.; Bjarnsholt, T.; Bay, L.; Saunte, D.M.; Thomsen, S.F.; Jemec, G.B. Probiotics in Hidradenitis Suppurativa: A potential treatment option? Clin. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Naik, H.B.; Jo, J.-H.; Paul, M.; Kong, H.H. Skin Microbiota Perturbations Are Distinct and Disease Severity-Dependent in Hidradenitis Suppurativa. J. Invest. Dermatol. 2020, 140, 922–925. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ring, H.C.; Thorsen, J.; Jørgensen, A.H.; Bay, L.; Bjarnsholt, T.; Fuursted, K.; Thomsen, S.F.; Jemec, G.B. Predictive Metagenomic Analysis Reveals a Role of Cutaneous Dysbiosis in the Development of Hidradenitis Suppurativa. J. Invest. Dermatol. 2020, 140, 1473–1476. [Google Scholar] [CrossRef]
- Guet-Revillet, H.; Coignard-Biehler, H.; Jais, J.-P.; Quesne, G.; Frapy, E.; Poirée, S.; Guern, A.-S.L.; Flèche-Matéos, A.L.; Hovnanian, A.; Consigny, P.-H.; et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg. Infect. Dis. 2014, 20, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Lapins, J.; Jarstrand, C.; Emtestam, L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br. J. Dermatol. 1999, 140, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahns, A.C.; Killasli, H.; Nosek, D.; Lundskog, B.; Lenngren, A.; Muratova, Z.; Emtestam, L.; Alexeyev, O.A. Microbiology of hidradenitis suppurativa (acne inversa): A histological study of 27 patients. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2014, 122, 804–809. [Google Scholar] [CrossRef]
- Dinh, K.M.; Erikstrup, L.T.; Andersen, R.K.; Andersen, P.S.; Mikkelsen, S.; Kjerulff, B.D.; Burgdorf, K.S.; Hansen, T.F.; Nielsen, K.R.; Hjalgrim, H.; et al. Cross-sectional study identifies lower risk of Staphylococcus aureus nasal colonization in Danish blood donors with hidradenitis suppurativa symptoms. Br. J. Dermatol. 2020, 183, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Caprio, R.D.; Cacciapuoti, S.; Caiazzo, G.; Fusco, A.; Tortorella, E.; Fabbrocini, G.; Balato, A. A new T helper 17 cytokine in hidradenitis suppurativa: Antimicrobial and proinflammatory role of interleukin-26. Br. J. Dermatol. 2019, 181, 1038–1045. [Google Scholar] [CrossRef]
- Larochette, V.; Miot, C.; Poli, C.; Beaumont, E.; Roingeard, P.; Fickenscher, H.; Jeannin, P.; Delneste, Y. IL-26, a Cytokine With Roles in Extracellular DNA-Induced Inflammation and Microbial Defense. Front. Immunol. 2019, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, V.K.; Jones, D.; McNish, S.; Bendall, M.L.; Crandall, K.A. Transcriptome patterns in hidradenitis suppurativa: Support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin. Exp. Dermatol. 2019, 44, 882–892. [Google Scholar] [CrossRef]
- Coates, M.; Mariottoni, P.; Corcoran, D.L.; Kirshner, H.F.; Jaleel, T.; Brown, D.A.; Brooks, S.R.; Murray, J.; Morasso, M.I.; MacLeod, A.S. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS ONE 2019, 14, e0216249. [Google Scholar] [CrossRef] [Green Version]
- Alatas, E.T.; Polat, A.K.; Kalayci, M.; Dogan, G.; Belli, A.A. Plasma dermcidin levels in acne patients, and the effect of isotretinoin treatment on dermcidin levels. Dermatol. Ther. 2019, 32, e13044. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.A.E.A.; Omar, S.S.; Collier, A.; El-Wafa, R.A.H.A.; Gomaa, N. The effect of continuous high versus low dose oral isotretinoin regimens on dermcidin expression in patients with moderate to severe acne vulgaris. Dermatol. Ther. 2018, 31, e12715. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; McKenzie, S.A.; Harview, C.L.; Truong, A.K.; Shi, V.Y.; Chen, L.; Grogan, T.R.; Bennett, R.G.; Hsiao, J.L. Isotretinoin in the treatment of hidradenitis suppurativa: A retrospective study. J. Dermatol. Treat. 2021, 32, 473–475. [Google Scholar] [CrossRef]
- Gallagher, C.G.; Kirthi, S.K.; Cotter, C.C.; Revuz, J.R.; Tobin, A.M.T. Could isotretinoin flare hidradenitis suppurativa? A case series. Clin. Exp. Dermatol. 2019, 44, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Gemert, M.J.V. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 1999, 40, 73–76. [Google Scholar] [CrossRef]
- Kravvas, G.; Veitch, D.; Al-Niaimi, F. The increasing relevance of biofilms in common dermatological conditions. J. Dermatol. Treat. 2018, 29, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Okoye, G.A.; Vlassova, N.; Olowoyeye, O.; Agostinho, A.; James, G.; Stewart, P.S.; Leung, S.; Lazarus, G. Bacterial biofilm in acute lesions of hidradenitis suppurativa. Br. J. Dermatol. 2017, 176, 241–243. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.K.; Ring, H.C.; Kallenbach, K.; Eriksen, J.O.; Jemec, G.B.E. Bacterial biofilm is associated with higher levels of regulatory T cells in unaffected hidradenitis suppurativa skin. Exp. Dermatol. 2019, 28, 312–316. [Google Scholar] [CrossRef]
- Ardon, C.B.; Prens, E.P.; Fuursted, K.; Ejaz, R.N.; Shailes, J.; Jenssen, H.; Jemec, G.B.E. Biofilm production and antibiotic susceptibility of Staphylococcus epidermidis strains from Hidradenitis Suppurativa lesions. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Nikolakis, G.; Liakou, A.I.; Bonovas, S.; Seltmann, H.; Bonitsis, N.; Join-Lambert, O.; Wild, T.; Karagiannidis, I.; Zolke-Fischer, S.; Langner, K.; et al. Bacterial Colonization in Hidradenitis Suppurativa/Acne Inversa: A Cross-sectional Study of 50 Patients and Review of the Literature. Acta Derm. Venereol. 2017, 97, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, A.J.; Hsiao, J.L.; Lowes, M.A.; Shi, V.Y. A Comparison of International Management Guidelines for Hidradenitis Suppurativa. Dermatology 2021, 237, 81–96. [Google Scholar] [CrossRef]
- Ardon, C.B.; Prens, E.P.; Tkadlec, J.; Fuursted, K.; Abourayale, S.; Jemec, G.B.E.; Jenssen, H. Virulent Staphylococcus lugdunensis with limited genetic diversity in hidradenitis suppurativa lesions. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, e248–e250. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Kallenbach, K.; Miller, I.M.; Prens, E.; Saunte, D.M.; Bjarnsholt, T.; Jemec, G.B.E. Normal Skin Microbiota is Altered in Pre-clinical Hidradenitis Suppurativa. Acta Derm. Venereol. 2017, 97, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Nikolakis, G.; Join-Lambert, O.; Karagiannidis, I.; Guet-Revillet, H.; Zouboulis, C.C.; Nassif, A. Bacteriology of hidradenitis suppurativa/acne inversa: A review. J. Am. Acad. Dermatol. 2015, 73, S12–S18. [Google Scholar] [CrossRef]
- Maraki, S.; Evangelou, G.; Stafylaki, D.; Scoulica, E. Actinotignum schaalii subcutaneous abscesses in a patient with hidradenitis suppurativa: Case report and literature review. Anaerobe 2017, 43, 43–46. [Google Scholar] [CrossRef]
- Snyder, A.; Aleisa, A.; Lewis, J.; Mazza-McCrann, J.; Forcucci, J.A. Cryptic deep dermatophytosis in a renal transplant recipient with hidradenitis suppurativa. JAAD Case Rep. 2021, 9, 86–89. [Google Scholar] [CrossRef]
- Nielsen, V.W.; Jørgensen, A.-H.R.; Thomsen, S.F. Fatal outcome of malignant transformation of hidradenitis suppurativa: A case report and literature review. Clin. Case Rep. 2020, 8, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Juviler, P.G.; Patel, A.P.; Qi, Y. Infiltrative squamous cell carcinoma in hidradenitis suppurativa: A case report for early surgical intervention. Int. J. Surg. Case Rep. 2019, 55, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Nedomansky, J.; Weiss, D.; Willinger, B.; Nickl, S.; Steininger, C. Acne inversa complicated by Actinomyces neuii. Infection 2016, 44, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; Chen, X.; Peng, C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front. Microbiol. 2020, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and the gastrointestinal system. Australas. J. Dermatol. 2020, 61, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Mallonee, C.J.; Stanisic, D.; Homme, R.P.; George, A.K.; Singh, M.; Tyagi, S.C. Hidradenitis Suppurativa and 1-Carbon Metabolism: Role of Gut Microbiome, Matrix Metalloproteinases, and Hyperhomocysteinemia. Front. Immunol. 2020, 11, 1730. [Google Scholar] [CrossRef]
- Lam, S.Y.; Radjabzadeh, D.; Eppinga, H.; Nossent, Y.R.A.; Zee, H.H.V.D.; Kraaij, R.; Konstantinov, S.R.; Fuhler, G.M.; Prens, E.P.; Thio, H.B.; et al. A microbiome study to explore the gut-skin axis in hidradenitis suppurativa. J. Dermatol. Sci. 2021, 101, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Kam, S.; Collard, M.; Lam, J.; Alani, R.M. Gut Microbiome Perturbations in Patients with Hidradenitis Suppurativa: A Case Series. J. Invest. Dermatol. 2021, 141, 225–228. [Google Scholar] [CrossRef]
- Ott, S.J.; Schreiber, S. Reduced microbial diversity in inflammatory bowel diseases. Gut 2006, 55, 1207. [Google Scholar]
- Eppinga, H.; Weiland, C.J.S.; Thio, H.B.; Woude, C.J.V.D.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium prausnitzii in Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J. Crohns Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.-C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Kromann, C.B.; Ibler, K.S.; Kristiansen, V.B.; Jemec, G.B.E. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm. Venereol. 2014, 94, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High Fat Diet Alters Gut Microbiota and the Expression of Paneth Cell-Antimicrobial Peptides Preceding Changes of Circulating Inflammatory Cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef] [PubMed]
- Haskin, A.; Fischer, A.H.; Okoye, G.A. Prevalence of Firmicutes in Lesions of Hidradenitis Suppurativa in Obese Patients. JAMA Dermatol. 2016, 152, 1276–1278. [Google Scholar] [CrossRef] [Green Version]
- Gildner, T.E. Links between metabolic syndrome and the microbiome. Evol. Med. Public Health 2020, 2020, 45–46. [Google Scholar] [CrossRef]
- Sartorius, K.; Lapins, J.; Jalal, S.; Emtestam, L.; Hedberg, M. Bacteraemia in patients with hidradenitis suppurativa undergoing carbon dioxide laser surgery: Detection and quantification of bacteria by lysis-filtration. Dermatology 2006, 213, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Assan, F.; Gottlieb, J.; Tubach, F.; Lebbah, S.; Guigue, N.; Hickman, G.; Pape, E.; Madrange, M.; Delaporte, E.; Sendid, B.; et al. Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J. Allergy Clin. Immunol. 2020, 146, 452–455. [Google Scholar] [CrossRef] [Green Version]
- Kvehaugen, A.S.; Aasbrenn, M.; Farup, P.G. Anti-Saccharomyces cerevisiae antibodies (ASCA) are associated with body fat mass and systemic inflammation, but not with dietary yeast consumption: A cross-sectional study. BMC Obes. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Straalen, K.R.V. Anti-Saccharomyces cerevisiae antibodies could reflect distinct phenotypes in hidradenitis suppurativa. J. Allergy Clin. Immunol. 2020, 146, 461. [Google Scholar] [CrossRef]
- Murdoch, T.B.; Xu, W.; Stempak, J.M.; Landers, C.; Targan, S.R.; Rotter, J.I.; Silverberg, M.S. Pattern Recognition Receptor and Autophagy Gene Variants Are Associated With Development of Antimicrobial Antibodies in Crohn’s Disease. Inflamm. Bowel Dis. 2012, 18, 1743–1748. [Google Scholar] [CrossRef] [Green Version]
- Duchatelet, S.; Join-Lambert, O.; Delage, M.; Miskinyte, S.; Guet-Revillet, H.; Lam, T.; Coignard-Biehler, H.; Ungeheuer, M.-N.; Chatenoud, L.; Lortholary, O.; et al. Remission of chronic acne fulminans and severe hidradenitis suppurativa with targeted antibiotherapy. JAAD Case Rep. 2019, 5, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Join-Lambert, O.; Duchatelet, S.; Delage, M.; Miskinyte, S.; Coignard, H.; Lemarchand, N.; Alemy-Carreau, M.; Lortholary, O.; Nassif, X.; Hovnanian, A.; et al. Remission of refractory pyoderma gangrenosum, severe acne, and hidradenitis suppurativa (PASH) syndrome using targeted antibiotic therapy in 4 patients. J. Am. Acad. Dermatol. 2015, 73, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.C.; Alvarez-chinchilla, P.; Garcia, F.; Poveda, I. Intralesional triamcinolone for fistulous tracts in hidradenitis suppurativa: An uncontrolled prospective trial with clinical and ultrasonographic follow- up. Exp. Dermatol. 2019, 28 (Suppl. S2), 5–55. [Google Scholar]
- Bettoli, V.; Manfredini, M.; Massoli, L.; Carillo, C.; Barozzi, A.; Amendolagine, G.; Ruina, G.; Musmeci, D.; Libanore, M.; Curtolo, A.; et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 930–936. [Google Scholar] [CrossRef]
- Leiphart, P.; Ma, H.; Naik, H.B.; Kirby, J.S. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J. Am. Acad. Dermatol. 2019, 80, 821–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, J.M. The anti-inflammatory effects of tetracyclines. Cutis 2005, 75, 6–11. [Google Scholar] [PubMed]
- Mintoff, D.; Benhadou, F.; Pace, N.P.; Frew, J.W. Metabolic syndrome and hidradenitis suppurativa: Epidemiological, molecular, and therapeutic aspects. Int. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Costa, A.N. da Drug repurposing through drug–gene interaction profiles for hidradenitis suppurativa/acne inversa treatment. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e251–e254. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannistrà, C.; Finocchi, V.; Trivisonno, A.; Tambasco, D. New perspectives in the treatment of hidradenitis suppurativa: Surgery and brewer’s yeast-exclusion diet. Surgery 2013, 154, 1126–1130. [Google Scholar] [CrossRef]
- Aboud, C.; Zamaria, N.; Cannistrà, C. Treatment of hidradenitis suppurativa: Surgery and yeast (Saccharomyces cerevisiae)-exclusion diet. Results after 6 years. Surgery 2020, 167, 1012–1015. [Google Scholar] [CrossRef]
- Boer, J. Does obesity cause a distinct phenotype of hidradenitis suppurativa? J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, e195–e196. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.-I.; Mitran, M.-I.; Mitran, C.-I.; Matei, C.; Georgescu, S.-R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018, 5823684. [Google Scholar] [CrossRef] [Green Version]
- Reiger, M.; Traidl-Hoffmann, C.; Neumann, A.U. The skin microbiome as a clinical biomarker in atopic eczema: Promises, navigation, and pitfalls. J. Allergy Clin. Immunol. 2020, 145, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Corazza, M.; Borghi, A.; Bettoli, V.; Pora, R.; Bononi, I.; Mazzoni, E.; Mazzola, E.; Saraceni, S.; Maritati, M.; Contini, C. Irrelevance of Panton-Valentine leukocidin in hidradenitis suppurativa: Results from a pilot, observational study. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 77–83. [Google Scholar] [CrossRef]
- Stergianou, D.; Tzanetakou, V.; Argyropoulou, M.; Kanni, T.; Bagos, P.G.; Giamarellos-Bourboulis, E.J. Staphylococcus aureus Carriage in Hidradenitis Suppurativa: Impact on Response to Adalimumab. Dermatology 2021, 237, 372–377. [Google Scholar] [CrossRef]
- Naik, H.B.; Piguet, V. Standardizing Hidradenitis Suppurativa Skin Microbiome Research: The Methods Matter. J. Invest. Dermatol. 2020, 140, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.W.; Hawkes, J.E.; Krueger, J.G. Topical, systemic and biologic therapies in hidradenitis suppurativa: Pathogenic insights by examining therapeutic mechanisms. Ther. Adv. Chronic Dis. 2019, 10, 2040622319830646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assan, F.; Bachelez, H. Reply. J. Allergy Clin. Immunol. 2020, 146, 461–462. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Specimen | Findings | Comments |
|---|---|---|---|
| Riverain-Gillet et al. [63] | Skin surface of non-lesional skin swab | Fourteen bacterial taxa statistically discriminated HS from healthy control skin were predominantly Prevotella but also Actinomyces, Campyobacter ureolyticus, and Mobiluncus. | Bacterial richness is similar between HS skin and skin of healthy controls. Evenness is significantly higher in HS skin samples. The microbiome of clinically unaffected intertriginous skin in HS patients is characterized by an anaerobic shift contrasting with a decreased abundance of aerobic skin commensals. |
| H C Ring et al. [64] | Lesional HS skin biopsy | Dominated by Corynebacterium spp., Prophyromonas, and Peptoniphilus spp. | No difference in richness between three groups. |
| Non-lesional HS skin biopsy | Dominated by Acinetobacter and Moraxella spp. | ||
| Healthy control skin biopsy | Equally distributed microbiome. | ||
| H C Ring et al. [65] | Tunnels | Dominated by anaerobic species (Prophyromonas spp and Prevotella spp.). | |
| H C Ring et al. [66] | Consistent levels of various bacteria such as Acinetobacter and Moraxella, which do not differ significantly between control and HS blood. | Source of bacteria is likely from skin of the antecubital fossa discovered due to high sensitivity of NGS. | |
| Hispan et al. [67] | Presence of bacterial DNA was significantly higher in HS group vs. control. Dominant species was E. coli. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintoff, D.; Borg, I.; Pace, N.P. The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review. Vaccines 2021, 9, 1076. https://doi.org/10.3390/vaccines9101076
Mintoff D, Borg I, Pace NP. The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review. Vaccines. 2021; 9(10):1076. https://doi.org/10.3390/vaccines9101076
Chicago/Turabian StyleMintoff, Dillon, Isabella Borg, and Nikolai Paul Pace. 2021. "The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review" Vaccines 9, no. 10: 1076. https://doi.org/10.3390/vaccines9101076
APA StyleMintoff, D., Borg, I., & Pace, N. P. (2021). The Clinical Relevance of the Microbiome in Hidradenitis Suppurativa: A Systematic Review. Vaccines, 9(10), 1076. https://doi.org/10.3390/vaccines9101076






