Abstract
A 76 year-old female came to our observation one week after the vaccination with ChAdOx1 nCoV-19 AZD1222 for the onset of purpuric rash on her gluteal and legs regions associated with coxalgia and episodes of macrohaematuria. Henoch-Schönlein purpura (HSP) was diagnosed on the basis of the revised criteria developed by the European League Against Rheumatism, the Paediatric Rheumatology International Trials Organization, and the Paediatric Rheumatology European Society (EULAR/PRINTO/PRES). HSP is a common IgA-mediated small vessel vasculitis, typical of childhood, that affects several systems and is characterized by a tetrad of dermatological, abdominal, joint, and renal manifestations. The Etiology of HSP is not completely understood, but it was observed following upper respiratory tract infections, medications, vaccinations, and malignancies. HSP has previously been reported following immunization with various vaccines, mostly within 12 weeks post, suggesting a possible correlation. To our knowledge, this is the first report of the possible association between COVID-19 ChAdOx1 nCoV-19 AZD1222 and the onset of HSP in a previously healthy woman. No similar cases were reported amongst 23.848 participants in the ChAdOx1 nCoV-19 AZD1222 trial.
1. Introduction
Growing apprehension about the risks of vaccination have been associated with the current global health situation. In the safety evaluation of a vaccine, it is difficult to take into account interindividual factors, such as the genetic predisposition to immune hyperstimulation and autoimmunity. Immunologic phenomena and autoimmune disorders are usually the result of an interplay of factors, such as genetic predisposition, epigenetic modification, and environmental triggers such as infections and drugs [1]. It is now established that viruses, as well as vaccines, can induce a hyperstimulation of the immune system and produce an anti-autoantigen response through mechanisms such as molecular mimicry, epitope spreading, bystander activation, and polyclonal spreading [2]. Swelling and pain at the injection site, fatigue, fever, chills, nausea, headache, muscle soreness, and joint pain are common side effects of almost all COVID-19 vaccines. In addition, specific vaccines show unique adverse events such as neutropenia with the AstraZeneca/Oxford vaccine [3].
Henoch–Schönlein purpura (HSP), also known as IgA vasculitis, is a disorder that causes the small blood vessels in skin, joints, intestines, and kidneys to become inflamed and bleed [4,5]. HSP mostly occurs in children and is less common in adults, with an estimated annual incidence of 0.8–1.8/100.000 adult patients [6]. The tetrad of palpable purpura, arthralgia/arthritis, gastrointestinal involvement, and glomerulonephritis characterizes HSP, but not all the features are necessarily present and other organs can also be involved [7]. The most striking feature of this form of vasculitis is a purplish rash, typically on the lower legs and buttocks. The diagnosis is of exclusion without specific diagnostic tests [8] and clinically based on the revised criteria developed by the European League Against Rheumatism, the Paediatric Rheumatology International Trials Organization and the Paediatric Rheumatology European Society (EULAR/PRINTO/PRES) in 2008, which includes mandatory and supportive criteria (Table 1) [9].

Table 1.
European League Against Rheumatism, the Paediatric Rheumatology International Trials Organization and the Paediatric Rheumatology European Society (EULAR/PRINTO/PRES) 2008 (7).
To date, the etiology of HSP is not completely understood and seems to be associated with genetic and environmental factors, infections, medications, vaccinations or malignancies [10]. Many types of viruses and bacteria are believed to be associated with the pathogenesis of IgA vasculitis, as they have the capacity to induce abnormal IgA autoimmune reactions in the host. The COVID-19 pandemic is recognized to cause vasculitis-like syndromes, and several reports have described COVID-19 associated IgA vasculitis [11]. HSP has been previously reported following immunization with various vaccines, mostly within 12 weeks post vaccination [5]. The ChAdOx1 nCoV-19 vaccine (AZD1222) was developed at Oxford University and consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene [12]. The aim of this report is to highlight the possible association between COVID-19 ChAdOx1 nCoV-19 AZD1222 and first onset of HSP in a previously healthy adult. There were no cases reported amongst 23.848 participants in the ChAdOx1 nCoV-19 AZD1222 safety and immunogenicity trial [12].
2. Case Report
We report the case of a 76 year-old female who came to our observation for the new onset of purpuric rash on her gluteal and legs regions associated with coxalgia and episodes of macrohaematuria. She had no significant previous medical history and received long-term supplementation with Calcifediol 1.5 mg/10 mL 20 drops/week per os for hypovitaminosis D. Seven days before, she received the first dose of the Vaxzevria (ChAdOx1 nCoV-19 AZD1222) vaccine. Skin examination showed diffuse, non-whitening, maculopapular rash not itchy in both lower extremities, extending from the soles to the thigh and involving the buttocks (Figure 1).
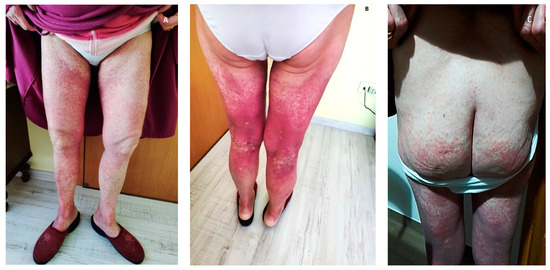
Figure 1.
(A–C) Diffuse, non-whitening, maculopapular rash in both lower extremities.
Her ankle joints were tender and painful and there was evidence of functional impotence of the limbs with difficulty in walking. Laboratory investigations revealed normal values for complete blood cell count, coagulation, kidney and liver function, autoimmune and neoplastic disease negative, but high values of CRP 40.85 mg/L and ESR 36 mm (Table 2). The urinalysis highlighted 72 red cells per high-power-field (HPF), and the urine culture report showed no evidence of growth. She did not have proteinuria. Instrumental examinations including chest radiograph, abdominal ultrasonography, and ecocolor-Doppler lower limb were normal. She did not undergo kidney and skin biopsy.

Table 2.
Blood Tests.
Positive criteria for vasculitic disease were the clinical picture of purpura, normal platelet count, and normal coagulation test results [13]. Furthermore, the patient fulfilled the EULAR/PRINTO/PRES criteria, as she had both the mandatory criterion, palpable purpura in the absence of thrombocytopaenia, and two of the supporting criteria, renal involvement in the form of haematuria and acute onset of arthralgia; the patient had the clinical signs and symptoms of HSP.
The trigger for the vasculitis was supposed to be ChAdOx1 nCoV-19 AZD1222 vaccination and, considering the renal and joint involvement, was proposed therapy with Paracetamol 1 g per os bis in die for seven days and Deflazacort 30 mg/die per os for ten days, then 15 mg/die per os for five days and, further, 7.5 mg/die per os for seven days and asked the patient to monitor signs and symptoms. After two weeks, signs resolved with normalization of laboratory parameters of ESR 10 mm, RCP 3.10, and urinalysis with no evidence of red cells. The arthritis also resolved. The patient, according to vaccination calendar, afterwards received the second dose, reporting only itching in the site of inoculum.
This work was conducted after receiving the patient’s informed consent to participate in the study and to publish this report and images, in compliance with the ethical standards in the field and the norms established by the Internal Review Board of University of L’Aquila (comitato etico di Ateneo D.R. n. 206/2013 and D.R. n. 46/2017).
3. Discussion and Conclusions
HSP has been observed in the setting of numerous infections and after the administration of several vaccines, including those against influenza, pneumococcal disease, rabies, hepatitis B virus, and meningococcal disease [14,15]. To date, several cases of HSP after SARS-CoV-2 infections are described in the literature [4,16,17,18,19,20,21,22,23,24,25,26,27,28]. Recently, Obeid et coll. reported a case of reactivation of IgA vasculitis occurring after COVID-19 vaccination and suggested a possible link between the reactivation of pre-existing IgA vasculitis observed after vaccination and the increase in anti-SARS-CoV-2 spike IgA. IgA vasculitis flares are reported following vaccinations and patients with IgA nephropathy show a stronger IgA response to intramuscular influenza vaccine respect to healthy controls. Furthermore, three cases of haematuria and IgA nephropathy flares following the second dose of mRNA COVID-19 vaccines have been reported in subjects with biopsy-proven IgA nephropathy, one patient following the BNT162b2 (BioNTech-Pfizer) vaccine, two of these after the mRNA-1273 vaccine [29].
To our knowledge, this is the first case described of HSP after the administration of a COVID-19 viral vector vaccine, which was likely the cause. Given the temporal association, this vaccine may have the potential to induce post-vaccination vasculitis, a rare adverse event. In the literature is described a case of HSV triggered by the Pfizer-BioNTech BNT16B2b2 mRNA vaccination [8]. It is possible that, after administration of the COVID-19 vaccine, immune complexes may form between vaccine antigens and native antibodies, thereby initiating a vasculitic process, as previously observed for other vaccinations [30]. IgA is driven in response to external stimuli and aberrant IgA responses underlie the pathogenesis of IgA vasculitis; IgA activates mannan-binding lectin and alternative complement pathways after creating IgA immunocomplexes and deposition on small vessels [11]. In the case reported, the problem of the booster dose was also addressed: should it be administered or not? Similar to other vaccines (e.g., influenza) [31], in the case of COVID-19 vaccination, cases are occurring of HSP after vaccination as well as pre-existing HSP exacerbated by the vaccine. Vaccinations could be associated with a variety of immunologic or immunologic-like adverse effects, but the precise pathogenetic mechanism remains unknown. Repeating the administration could potentially cause more severe immunologic reactions, but the antigen responsible for the reaction may not be the specific vaccine antigen (the spike protein in this case), furthermore, the product label with all vaccines and medications state that “hypersensitivity” to the product is a contraindication to its use.
There are currently no clear guidelines for the most appropriate management for patients with a history of vaccine-induced vasculitis and revaccination [32]. Using common sense and the standards of health professionals, after discussing with our patient the potential benefits and risks, it was decided to give the booster, and on this second occasion she had no reactions whatsoever. In the case described, we could not exclude the possibility that the occurrence of vasculitis following the COVID-19 vaccination was coincidental. As mass vaccination campaigns start worldwide, particularly in younger patients where HSP is more frequently observed, physicians should be aware of this complication, but should continue to encourage vaccination efforts given the well documented safety profile and efficacy of the ChAdOx1 nCoV-19 AZD1222 viral vector vaccine. Vaccination with COVID-19 vaccines is the only way today to eliminate, or at least control, COVID-19, and vaccine hesitancy is the potential nemesis [33]. Several rare but serious adverse reactions have been reported in the post marketing surveillance of COVID-19 vaccines. Monitoring the safety of COVID-19 vaccines is essential to improve safety profiles and enhance public trust. The correct information about these potential harms and the use of COVID-19 vaccines is essential [34]. People should be educated about their individual benefits and risks associated with COVID-19 vaccination, and we must ensure that vaccine recipients are aware of risks and that they should seek care if they experience concerning symptoms [3,35].
Author Contributions
Conceptualization, M.M.S., M.R., L.M.M., L.G. and M.D.M.; methodology, M.M.S., M.R., L.M.M., L.G. and M.D.M.; investigation, M.M.S., M.R., L.M.M., L.G. and M.D.M.; data curation, M.M.S., M.R., L.M.M., L.G. and M.D.M.; writing—original draft preparation, M.M.S., M.R., L.M.M., L.G. and M.D.M.; writing—review and editing, M.M.S., M.R., L.M.M., L.G. and M.D.M.; visualization, M.M.S., M.R., L.M.M., L.G. and M.D.M.; supervision, M.M.S., M.R., L.M.M., L.G. and M.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The work was conducted, in compliance with the ethical standards in the field and the norms established by the Internal Review Board of University of L’Aquila (comitato etico di Ateneo D.R. n. 206/2013 and D.R. n. 46/2017).
Informed Consent Statement
The work was conducted after receiving the patient’s informed consent to participate in the study and to publish this report and images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Omer, S.B.; Salmon, D.A.; Orenstein, W.A.; deHart, M.P.; Halsey, N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N. Engl. J. Med. 2009, 360, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Shoenfeld, Y. All Magic Comes with a Price: A Case of Henoch-Schönlein Purpura Post-influenza Vaccination. Isr Med. Assoc. J. 2020, 22, 648–649. [Google Scholar] [PubMed]
- Shrestha, S.; Khatri, J.; Shakya, S.; Danekhu, K.; Khatiwada, A.P.; Sah, R.; Kc, B.; Paudyal, V.; Khanal, S.; Rodriguez-Morales, A.J. Adverse events related to COVID-19 vaccines: The need to strengthen pharmacovigilance monitoring systems. Drugs Ther. Perspect. 2021, 37, 376–382. [Google Scholar] [CrossRef] [PubMed]
- AlGhoozi, D.A.; AlKhayyat, H.M. A child with Henoch-Schonlein purpura secondary to a COVID-19 infection. BMJ Case Rep. 2021, 14, e239910. [Google Scholar] [CrossRef]
- Felicetti, P.; Trotta, F.; Bonetto, C. Spontaneous reports of vasculitis as an adverse event following immunization: A descriptive analysis across three international databases. Vaccine 2016, 34, 6634–6640. [Google Scholar] [CrossRef]
- Piram, M.; Mahr, A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schonlein): Current state of knowledge. Curr. Opin. Rheumatol. 2013, 25, 171–178. [Google Scholar] [CrossRef] [PubMed]
- van de Perre, E.; Jones, R.B.; Jayne, D.R.W. IgA vasculitis (Henoch–Schönlein purpura): Refractory and relapsing disease course in the adult population. Clin. Kidney J. 2021, 14, 1953–1960. [Google Scholar] [CrossRef]
- Hines, A.M.; Murphy, N.; Mullin, C.; Barillas, J.; Barrientos, J.C. Henoch-Schönlein purpura presenting post COVID-19 vaccination. Vaccine 2021, 39, 4571–4572. [Google Scholar] [CrossRef]
- Ruperto, N.; Ozen, S.; Pistorio, A.; Dolezalova, P.; Brogan, P.; Cabral, D.A.; Cuttica, R.; Khubchandani, R.; Lovell, D.J.; O’Neil, K.M.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical. characterisation. Ann. Rheum. Dis. 2010, 69, 790–797. [Google Scholar] [CrossRef]
- Hetland, L.E.; Susrud, K.S.; Lindahl, K.H.; Bygum, A. Henoch-Schönlein purpura: A literature review. Acta Derm. Venereol. 2017, 97, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Sugino, H.; Sawada, Y.; Nakamura, M. IgA Vasculitis: Etiology, Treatment, Biomarkers and Epigenetic Changes. Int. J. Mol. Sci. 2021, 22, 7538. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Kantor, R.; Galel, A.; Aviner, S. Henoch-Schönlein Purpura Post-Influenza Vaccination in a Pediatric Patient: A Rare but Possible Adverse Reaction to Vaccine. Isr. Med. Assoc. J. 2020, 22, 654–656. [Google Scholar] [PubMed]
- Jariwala, S.; Vernon, N.; Shliozberg, J. Henoch-Schönlein purpura after hepatitis A vaccination. Ann. Allergy Asthma Immunol. 2011, 107, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.G.; Zheng, Y.; Lu, S.; Hu, Q.; Fang, Y. Rabies post-exposure prophylaxis for a male with severe Henoch Schönlein purpura following rabies vaccination. Hum. Vaccines Immunother. 2018, 14, 2666–2668. [Google Scholar] [CrossRef] [Green Version]
- Borocco, C.; Lafay, C.; Plantard, I.; Gottlieb, J.; Koné-Paut, I.; Galeotti, C. SARS-CoV-2-associated Henoch-Schönlein purpura in a 13-year-old girl. Arch. Pediatr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, N.L.; Papini, A.B.; Shao, T.; Girard, L. Immunoglobulin-A Vasculitis with Renal Involvement in a Patient with COVID-19: A Case Report and Review of Acute Kidney Injury Related to SARS-CoV-2. Can. J. Kidney Health Dis. 2021, 8, 2054358121991684. [Google Scholar] [CrossRef]
- Serafinelli, J.; Mastrangelo, A.; Morello, W.; Cerioni, V.F.; Salim, A.; Nebuloni, M.; Montini, G. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr. Nephrol. 2021, 1–5. [Google Scholar] [CrossRef]
- Hoskins, B.; Keeven, N.; Dang, M.; Keller, E.; Nagpal, R. A Child with COVID-19 and Immunoglobulin A Vasculitis. Pediatr Ann. 2021, 50, e44–e48. [Google Scholar] [CrossRef]
- Allez, M.; Denis, B.; Bouaziz, J.D.; Battistella, M.; Zagdanski, A.M.; Bayart, J. COVID-19-Related IgA Vasculitis. Arthritis Rheumatol. 2020, 72, 1952–1953. [Google Scholar] [CrossRef]
- Kumar, G.; Pillai, S.; Norwick, P.; Bukulmez, H. Leucocytoclastic vasculitis secondary to COVID-19 infection in a young child. BMJ Case Rep. 2021, 14, e242192. [Google Scholar] [CrossRef]
- Suso, A.S.; Mon, C.; Oñate Alonso, I.; Galindo Romo, K.; Juarez, R.C.; Ramírez, C.L. IgA Vasculitis With Nephritis (Henoch-Schönlein Purpura) in a COVID-19 Patient. Kidney Int. Rep. 2020, 5, 2074–2078. [Google Scholar] [CrossRef]
- El Hasbani, G.; Taher, A.T.; Jawad, A.S.M.; Uthman, I. Henoch-Schönlein purpura: Another COVID-19 complication. Pediatr. Dermatol. 2021. [Google Scholar] [CrossRef]
- Sandhu, S.; Chand, S.; Bhatnagar, A.; Dabas, R.; Bhat, S.; Kumar, H. Possible association between IgA vasculitis and COVID-19. Dermatol Ther. 2021, 34, e14551. [Google Scholar] [CrossRef]
- Jacobi, M.; Lancrei, H.M.; Brosh-Nissimov, T.; Yeshayahu, Y. Purpurona: A Novel Report of COVID-19-Related Henoch-Schonlein Purpura in a Child. Pediatr. Infect. Dis. J. 2021, 40, e93–e94. [Google Scholar] [CrossRef]
- Oñate, I.; Ortiz, M.; Suso, A.; Mon, C.; Galindo, K.; Lentisco, C.; Camacho, R.; Sánchez, M.; Oliet, A.; Ortega, O.; et al. Vasculitis ig a con nefritis (púrpura de schönlein-henoch) tras COVID-19: Una serie de casos y revisión de la literatura [Ig a vasculitis with nephritis (henoch-schönlein purpura) after COVID-19: A case series and review of the literature]. Nefrologia 2021. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, L.; Filocamo, G.; Passoni, E.; Boggio, F.; Folli, C.; Monzani, V. Henoch-Schönlein purpura with renal and gastrointestinal involvement in course of COVID-19: A case report. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 129), 191–192. [Google Scholar] [PubMed]
- Huang, Y.; Li, X.J.; Li, Y.Q.; Dai, W.; Shao, T.; Liu, W.Y. Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: A case report. BMC Nephrol. 2020, 21, 504. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Fenwick, C.; Pantaleo, G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021. [Google Scholar] [CrossRef]
- Blumberg, S.; Bienfang, D.; Kantrowitz, F.G. A possible association between influenza vaccination and small vessel vasculitis. Arch. Intern. Med. 1980, 140, 847–848. [Google Scholar] [CrossRef]
- Ledford, D.K. Henoch-Schönlein Purpura and Influenza Vaccine. J. Allergy Clin. Immunol. Pract. 2017, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Onda, H. Henoch-Schönlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr. Nephrol. 2001, 16, 458–459. [Google Scholar] [CrossRef]
- Bellanti, J.A. COVID-19 vaccines and vaccine hesitancy: Role of the allergist/immunologist in promotion of vaccine acceptance. Allergy Asthma Proc. 2021. [Google Scholar] [CrossRef]
- Troiano, G.; Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Hadler, S.C.; Moulia, D.; Shimabukuro, T.T.; Su, J.R.; Tepper, N.K.; Ess, K.C.; Woo, E.J.; Mba-Jonas, A.; Alimchandani, M.; et al. Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices—United States, July 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1094–1099. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).