Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice, Infections, and Treatments
2.2. Cells and Treatments
2.3. Real-Time PCR
2.4. Western Blot Analysis
2.5. GLO1 Enzymatic Activity
2.6. MG-H1 Detection
2.7. Statistical Analysis
3. Results
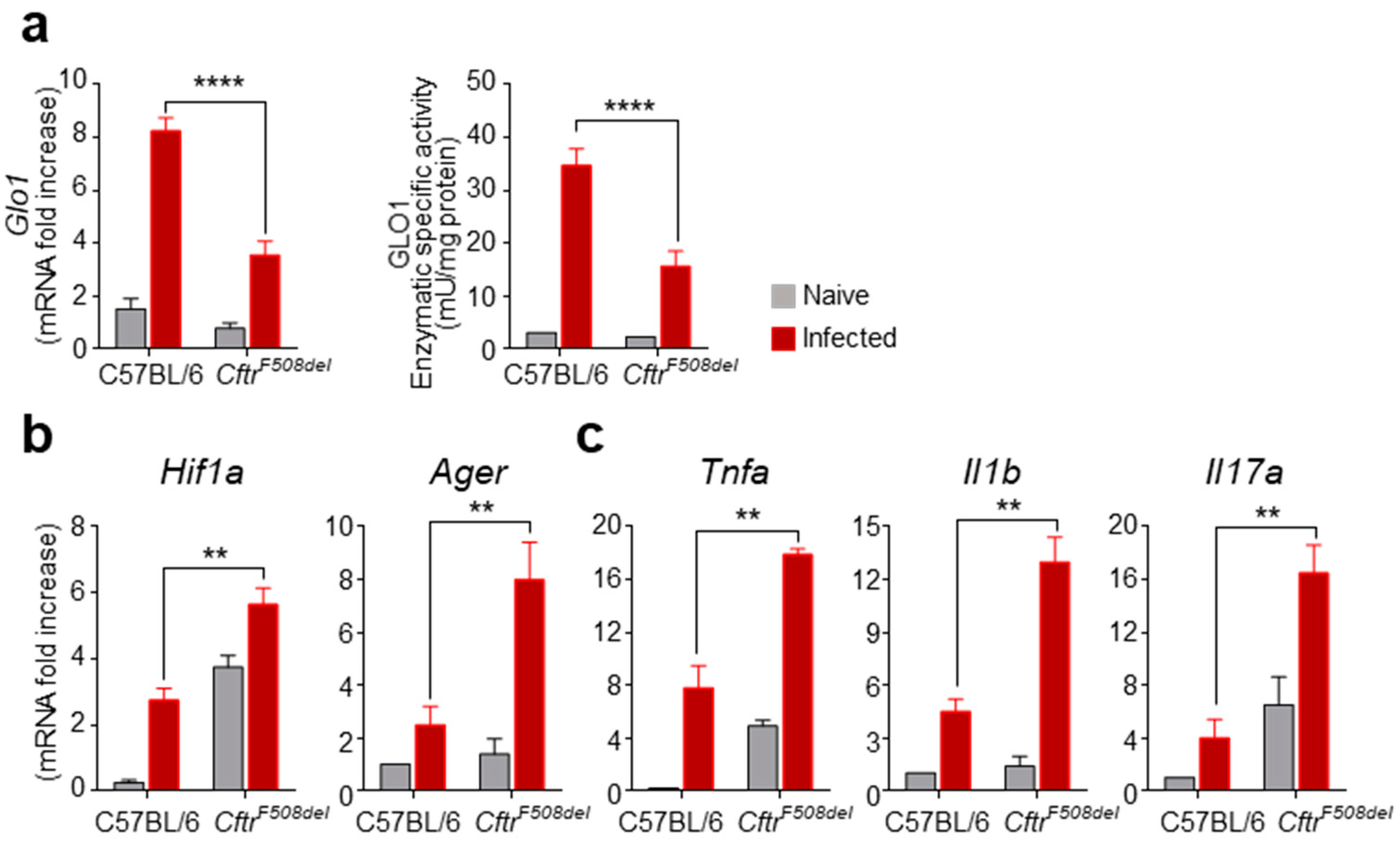
3.1. GLO1 Expression and Activity Are Defective in CF Mice with Aspergillosis
3.2. GLO1 Is Down-Regulated in Human CF
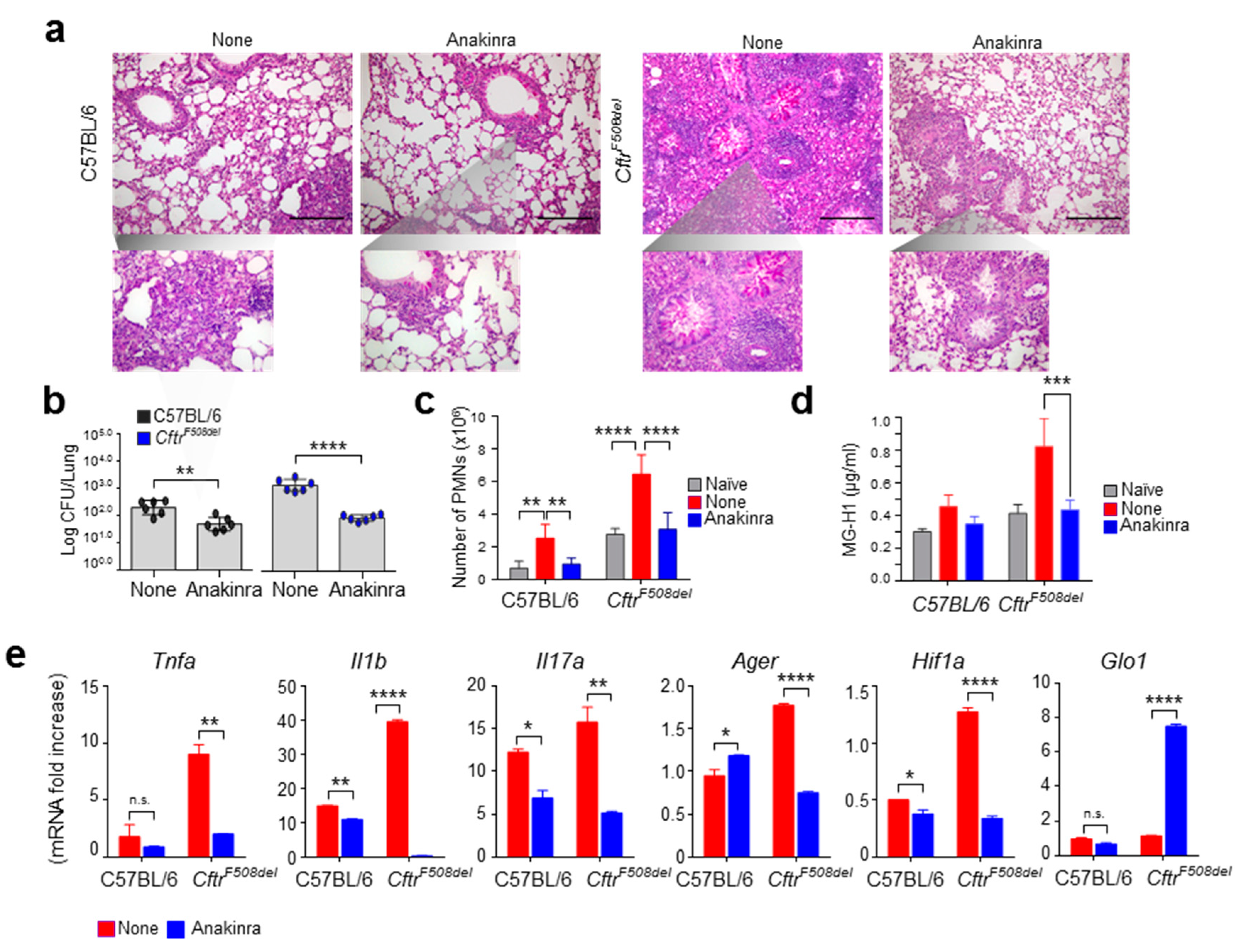
3.3. Anakinra Rescues GLO1 in Murine CF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castanos, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Costantini, C.; Puccetti, M.; Pariano, M.; Renga, G.; Stincardini, C.; D’Onofrio, F.; Bellet, M.M.; Cellini, B.; Giovagnoli, S.; Romani, L. Selectively targeting key inflammatory pathways in cystic fibrosis. Eur. J. Med. Chem. 2020, 206, 112717. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Casagrande, A.; De Luca, A.; Cunha, C.; Sorci, G.; Riuzzi, F.; Borghi, M.; Galosi, C.; Massi-Benedetti, C.; Oury, T.D.; et al. Hypoxia promotes danger-mediated inflammation via receptor for advanced glycation end products in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 1338–1350. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Corvol, H.; Beucher, J.; Boelle, P.Y.; Busson, P.F.; Muselet-Charlier, C.; Clement, A.; Ratjen, F.; Grasemann, H.; Laki, J.; Palmer, C.N.; et al. Ancestral haplotype 8.1 and lung disease severity in European cystic fibrosis patients. J. Cyst. Fibros. 2012, 11, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Beucher, J.; Boelle, P.Y.; Busson, P.F.; Muselet-Charlier, C.; Clement, A.; Corvol, H.; French, C.F. Modifier Gene Study Investigators AGER -429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS ONE 2012, 7, e41913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antognelli, C.; Talesa, V.N. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother 2020, 131, 110663. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin. Sci. 2016, 130, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Fritzsching, B.; Zhou-Suckow, Z.; Trojanek, J.B.; Schubert, S.C.; Schatterny, J.; Hirtz, S.; Agrawal, R.; Muley, T.; Kahn, N.; Sticht, C.; et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 902–913. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Sun, L.; Kato, T.; Okuda, K.; Martino, M.B.; Abzhanova, A.; Lin, J.M.; Gilmore, R.C.; Batson, B.D.; O’Neal, Y.K.; et al. IL-1beta dominates the promucin secretory cytokine profile in cystic fibrosis. J. Clin. Investig. 2019, 129, 4433–4450. [Google Scholar] [CrossRef]
- van Doorninck, J.H.; French, P.J.; Verbeek, E.; Peters, R.H.; Morreau, H.; Bijman, J.; Scholte, B.J. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995, 14, 4403–4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luca, A.; Smeekens, S.P.; Casagrande, A.; Iannitti, R.; Conway, K.L.; Gresnigt, M.S.; Begun, J.; Plantinga, T.S.; Joosten, L.A.; van der Meer, J.W.; et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 3526–3531. [Google Scholar] [CrossRef] [Green Version]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [Green Version]
- Borghi, M.; De Luca, A.; Puccetti, M.; Jaeger, M.; Mencacci, A.; Oikonomou, V.; Pariano, M.; Garlanda, C.; Moretti, S.; Bartoli, A.; et al. Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra. Cell Host Microbe 2015, 18, 198–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariano, M.; Pieroni, S.; De Luca, A.; Iannitti, R.G.; Borghi, M.; Puccetti, M.; Giovagnoli, S.; Renga, G.; D’Onofrio, F.; Bellet, M.M.; et al. Anakinra Activates Superoxide Dismutase 2 to Mitigate Inflammasome Activity. Int. J. Mol. Sci. 2021, 22, 6531. [Google Scholar] [CrossRef]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassoni, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease. Free. Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Gresnigt, M.S.; Rekiki, A.; Rasid, O.; Savers, A.; Jouvion, G.; Dannaoui, E.; Parlato, M.; Fitting, C.; Brock, M.; Cavaillon, J.M.; et al. Reducing hypoxia and inflammation during invasive pulmonary aspergillosis by targeting the Interleukin-1 receptor. Sci. Rep. 2016, 6, 26490. [Google Scholar] [CrossRef]
- Antognelli, C.; Moretti, S.; Frosini, R.; Puxeddu, E.; Sidoni, A.; Talesa, V.N. Methylglyoxal Acts as a Tumor-Promoting Factor in Anaplastic Thyroid Cancer. Cells 2019, 8, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, V.M. Rethinking cystic fibrosis pathology: The critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free. Radic. Biol. Med. 2001, 30, 1440–1461. [Google Scholar] [CrossRef]
- Favia, M.; de Bari, L.; Bobba, A.; Atlante, A. An Intriguing Involvement of Mitochondria in Cystic Fibrosis. J. Clin. Med. 2019, 8, 1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornalley, P.J. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, D.C.; Siefert, M.E.; Lin, S.; Brewington, J.; Sadek, H.; Clancy, J.P.; Plafker, S.M.; Ziady, A.G. Clinically-approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. J. Clin. Investig. 2019, 129, 3448–3463. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pariano, M.; Costantini, C.; Santarelli, I.; Puccetti, M.; Giovagnoli, S.; Talesa, V.N.; Romani, L.; Antognelli, C. Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis. Vaccines 2021, 9, 1311. https://doi.org/10.3390/vaccines9111311
Pariano M, Costantini C, Santarelli I, Puccetti M, Giovagnoli S, Talesa VN, Romani L, Antognelli C. Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis. Vaccines. 2021; 9(11):1311. https://doi.org/10.3390/vaccines9111311
Chicago/Turabian StylePariano, Marilena, Claudio Costantini, Ilaria Santarelli, Matteo Puccetti, Stefano Giovagnoli, Vincenzo N. Talesa, Luigina Romani, and Cinzia Antognelli. 2021. "Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis" Vaccines 9, no. 11: 1311. https://doi.org/10.3390/vaccines9111311
APA StylePariano, M., Costantini, C., Santarelli, I., Puccetti, M., Giovagnoli, S., Talesa, V. N., Romani, L., & Antognelli, C. (2021). Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis. Vaccines, 9(11), 1311. https://doi.org/10.3390/vaccines9111311







