Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Vaccine Components
2.3. Nanoparticle Formulation
2.4. Evaluation of the Loading Efficiency
2.5. Mouse Immunization
2.6. Splenocyte Isolation and Ex Vivo Restimulation
2.7. Lymphocyte Proliferative Response Assay
2.8. Cytokine Detection by ELISA in Mice
2.9. Statistical Analysis
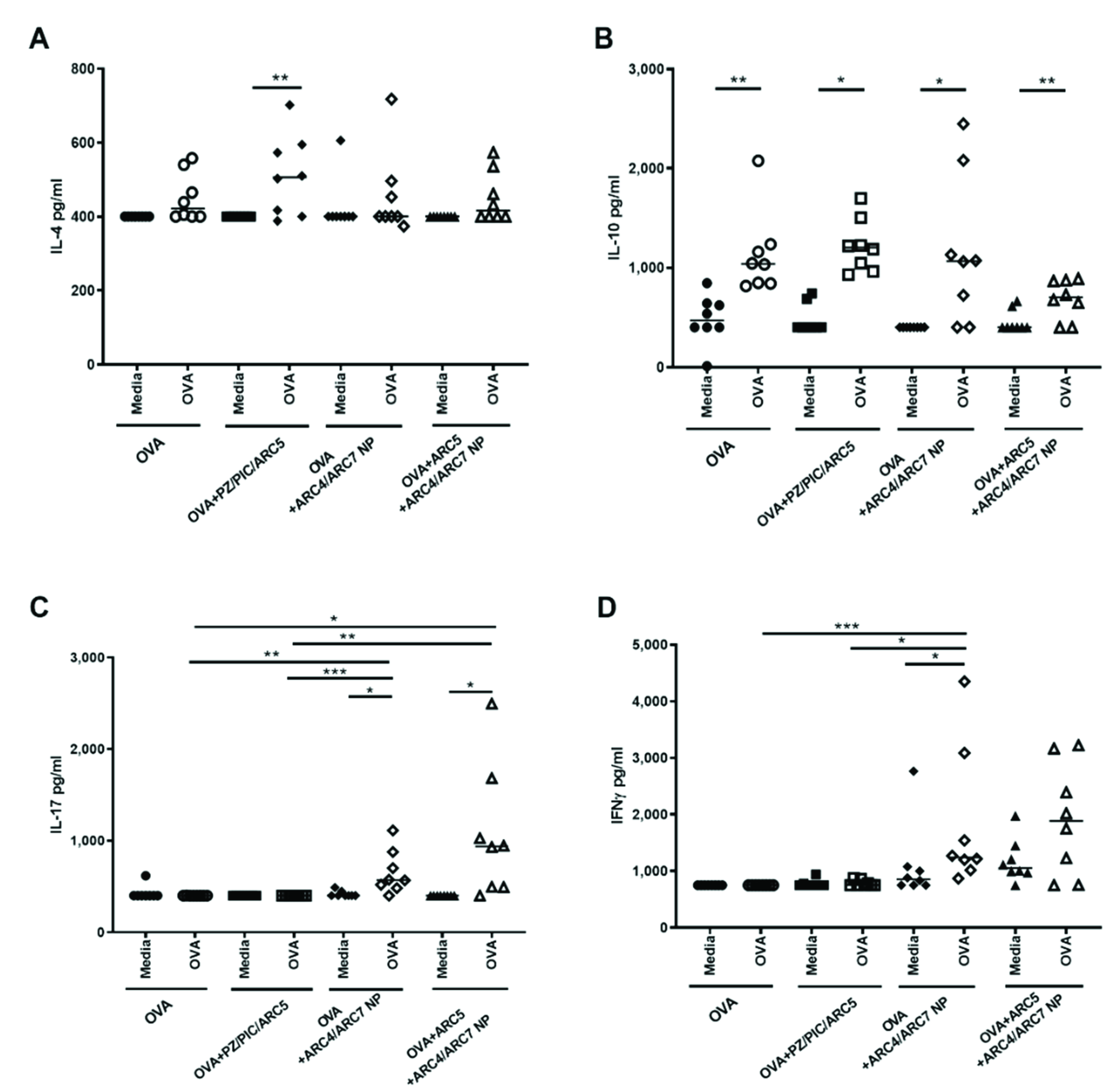
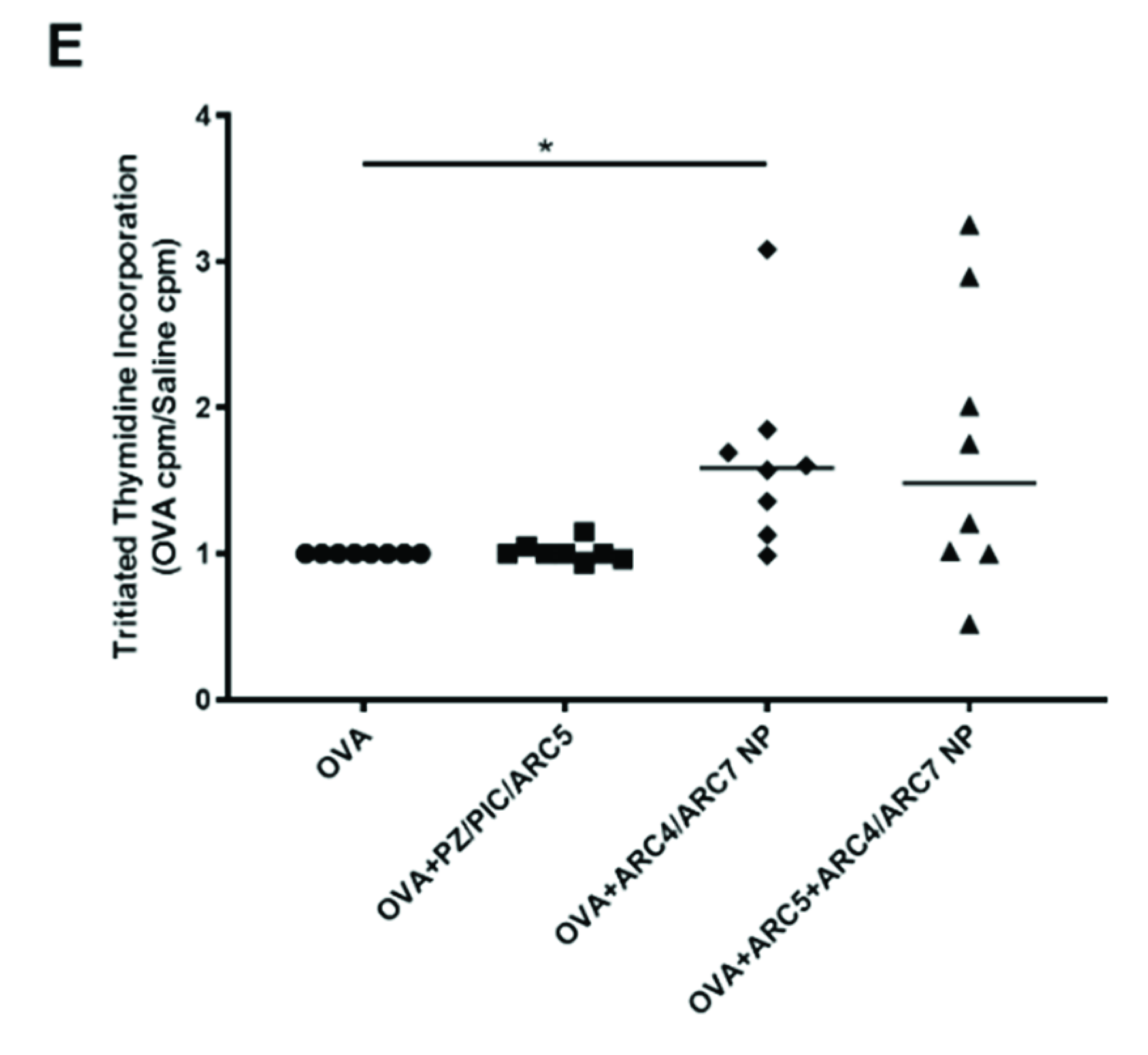
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Herndler-Brandstetter, D.; Weinberger, B.; Grubeck-Loebenstein, B. Persistent viral infections and immune aging. Ageing Res. Rev. 2011, 10, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.L.; Kovacs-Nolan, J.; Latimer, L.; Buchanan, R.; Gomis, S.; Babiuk, L.; van Drunen Littel-van den Hurk, S. A novel triple adjuvant formulation promotes strong, Th1-biased immune responses and significant antigen retention at the site of injection. Vaccine 2010, 28, 8288–8299. [Google Scholar] [CrossRef]
- Myhr, A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017, 22, 79–88. [Google Scholar] [CrossRef]
- Awate, S.; Wilson, H.L.; Lai, K.; Babiuk, L.A.; Mutwiri, G. Activation of adjuvant core response genes by the novel adjuvant PCEP. Mol. Immunol. 2012, 51, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Eng, N.F.; Gerdts, V.; Babiuk, L.A.; Mutwiri, G. Caspase-1 Dependent IL-1β Secretion and Antigen-Specific T-Cell Activation by the Novel Adjuvant, PCEP. Vaccines 2014, 2, 500–514. [Google Scholar] [CrossRef]
- Palmer, C.D.; Ninković, J.; Prokopowicz, Z.M.; Mancuso, C.J.; Marin, A.; Andrianov, A.K.; Dowling, D.J.; Levy, O. The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells. Biomaterials 2014, 35, 8876–8886. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Chen, J. Synthesis, properties; biological activity of poly (di (sodium carboxylatoethylphenoxy) phosphazene). Biomacromolecules 2006, 7, 394–399. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Molecular-Level Interactions of Polyphosphazene Immunoadjuvants and Their Potential Role in Antigen Presentation and Cell Stimulation. Biomacromolecules 2016, 17, 3732–3742. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- Saiki, I.; Fidler, I.J. Synergistic activation by recombinant mouse interferon-gamma and muramyl dipeptide of tumoricidal properties in mouse macrophages. J. Immunol. 1985, 135, 684–688. [Google Scholar] [PubMed]
- Souvannavong, V.; Brown, S.; Adam, A. Muramyl dipeptide (MDP) synergizes with interleukin 2 and interleukin 4 to stimulate, respectively, the differentiation and proliferation of B cells. Cell Immunol. 1990, 126, 106–116. [Google Scholar] [CrossRef]
- Poecheim, J.; Heuking, S.; Brunner, L.; Barnier-Quer, C.; Collin, N.; Borchard, G. Nanocarriers for DNA Vaccines: Co-Delivery of TLR-9 and NLR-2 Ligands Leads to Synergistic Enhancement of Proinflammatory Cytokine Release. Nanomaterials 2015, 5, 2317–2334. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Jadlowsky, J.K.; Lederman, M.M.; Feng, Z.; Weinberg, A.; Sieg, S.F. The Toll-like receptor 1/2 agonists Pam(3) CSK(4) and human beta-defensin-3 differentially induce interleukin-10 and nuclear factor-kappaB signalling patterns in human monocytes. Immunology 2011, 134, 151–160. [Google Scholar] [CrossRef]
- Weir, G.M.; Karkada, M.; Hoskin, D.; Stanford, M.M.; MacDonald, L.; Mansour, M.; Liwski, R.S. Combination of poly I:C and Pam3CSK4 enhances activation of B cells in vitro and boosts antibody responses to protein vaccines in vivo. PLoS ONE 2017, 12, e0180073. [Google Scholar] [CrossRef]
- Jahan, S.T.; Sadat, S.M.; Haddadi, A. Design and immunological evaluation of anti-CD205-tailored PLGA-based nanoparticulate cancer vaccine. Int. J. Nanomed. 2018, 13, 367–386. [Google Scholar] [CrossRef]
- Hamdy, S.; Molavi, O.; Ma, Z.; Haddadi, A.; Alshamsan, A.; Gobti, Z.; Elhasi, S.; Samuel, J.; Lavasanifar, A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008, 26, 5046–5057. [Google Scholar] [CrossRef]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization; controlled delivery of curcumin. J. Biomed. Mater. Res. A 2008, 86, 300–310. [Google Scholar] [CrossRef]
- Haddadi, A.; Hamdy, S.; Ghotbi, Z.; Samuel, J.; Lavasanifar, A. Immunoadjuvant activity of the nanoparticles’ surface modified with mannan. Nanotechnology 2014, 25, 355101. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Samuel, J.; Lavasanifar, A. Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharm. Res. 2011, 28, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Yarahmadi, M.; Haddadi, A. Potentiating Antigen Specific Immune Response by Targeted Delivery of the PLGA-Based Model Cancer Vaccine. Mol. Pharm. 2019, 16, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Haddadi, A.; Somayaji, V.; Ruan, D.; Samuel, J. Pharmaceutical analysis of synthetic lipid A-based vaccine adjuvants in poly (D,L-lactic-co-glycolic acid) nanoparticle formulations. J. Pharm. Biomed. Anal. 2007, 44, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Madiyalakan, R.; Woo, T.; Haddadi, A. Antitumor efficacy of photodynamic therapy using novel nanoformulations of hypocrellin photosensitizer SL052. Photochem. Photobiol. 2012, 88, 188–193. [Google Scholar] [CrossRef]
- Sadat, S.M.A.; Saeidnia, S.; Nazarali, A.J.; Haddadi, A. Nano-pharmaceutical formulations for targeted drug delivery against HER2 in breast cancer. Curr. Cancer Drug Targets 2015, 15, 71–86. [Google Scholar] [CrossRef]
- Haddadi, A.; Farboud, E.S.; Erfan, M.; Aboofazeli, R. Preparation and characterization of biodegradable urea-loaded microparticles as an approach for transdermal delivery. J. Microencapsul. 2006, 23, 698–712. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Ghotbi, Z.; Hung, R.W.; Lavasanifar, A. Part I: Targeted particles for cancer immunotherapy. Curr. Drug Deliv. 2011, 8, 261–273. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Latimer, L.; Landi, A.; Jenssen, H.; Hancock, R.E.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. The novel adjuvant combination of CpG ODN, indolicidin and polyphosphazene induces potent antibody- and cell-mediated immune responses in mice. Vaccine 2009, 27, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddadi, A.; Chaffey, A.; Ng, S.H.; Yalamati, D.; Wilson, H.L. Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice. Vaccines 2020, 8, 569. https://doi.org/10.3390/vaccines8040569
Haddadi A, Chaffey A, Ng SH, Yalamati D, Wilson HL. Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice. Vaccines. 2020; 8(4):569. https://doi.org/10.3390/vaccines8040569
Chicago/Turabian StyleHaddadi, Azita, Alyssa Chaffey, Siew Hon Ng, Damayanthi Yalamati, and Heather L. Wilson. 2020. "Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice" Vaccines 8, no. 4: 569. https://doi.org/10.3390/vaccines8040569
APA StyleHaddadi, A., Chaffey, A., Ng, S. H., Yalamati, D., & Wilson, H. L. (2020). Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice. Vaccines, 8(4), 569. https://doi.org/10.3390/vaccines8040569






