Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strain
2.2. Drugs
2.3. Influenza Vaccines
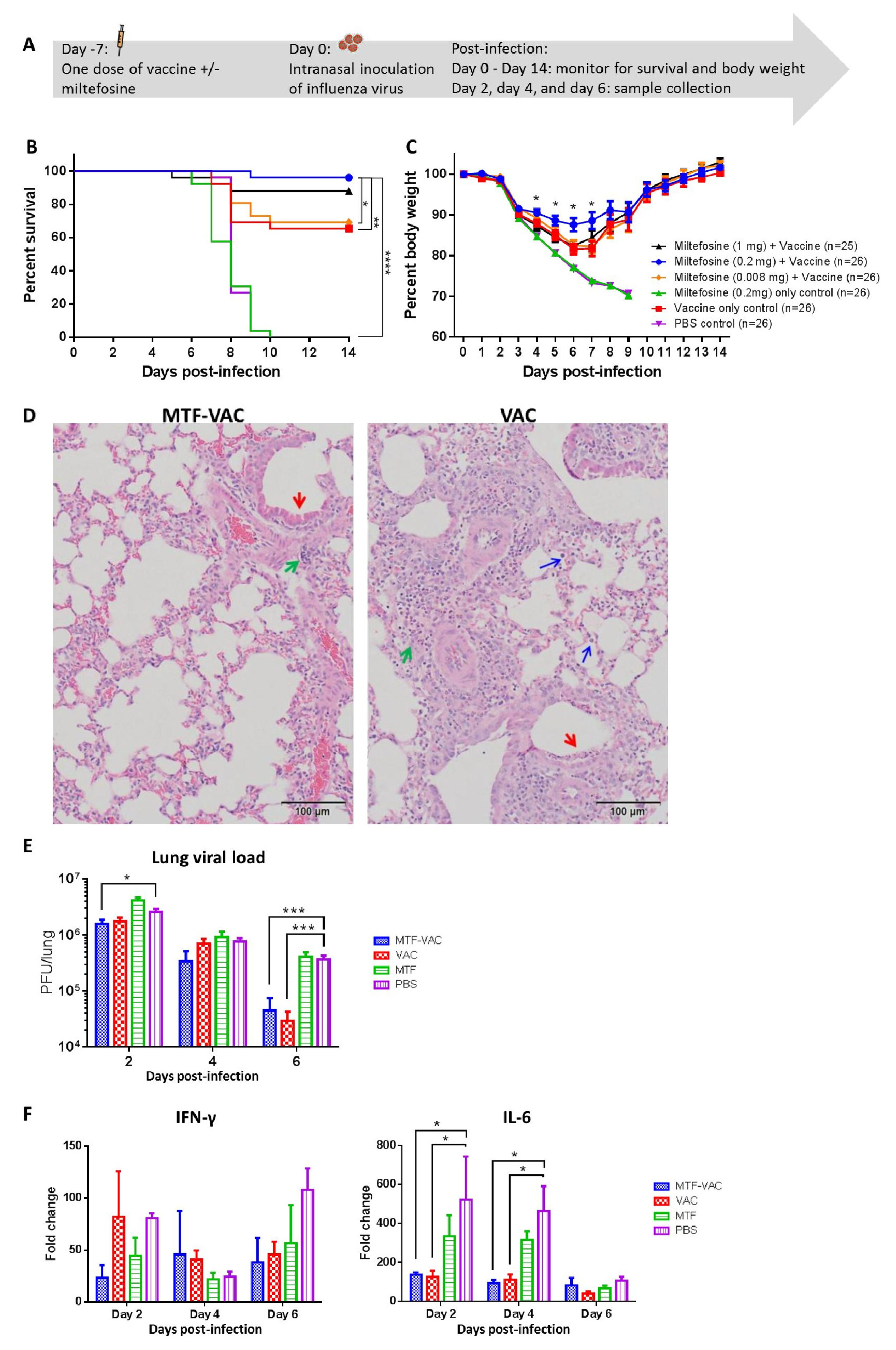
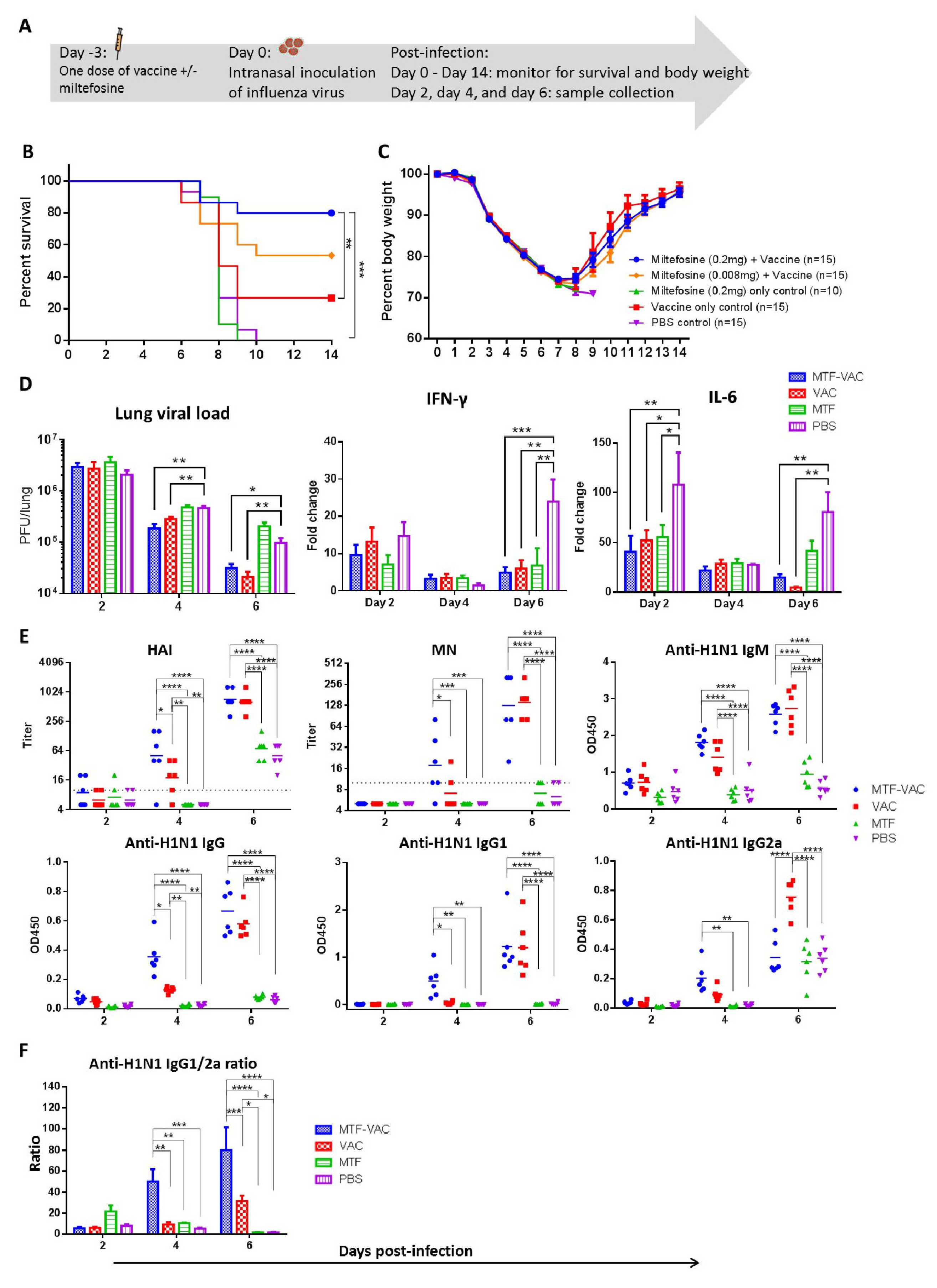
2.4. Animal Infection Experiments
2.5. Pulmonary Examinations
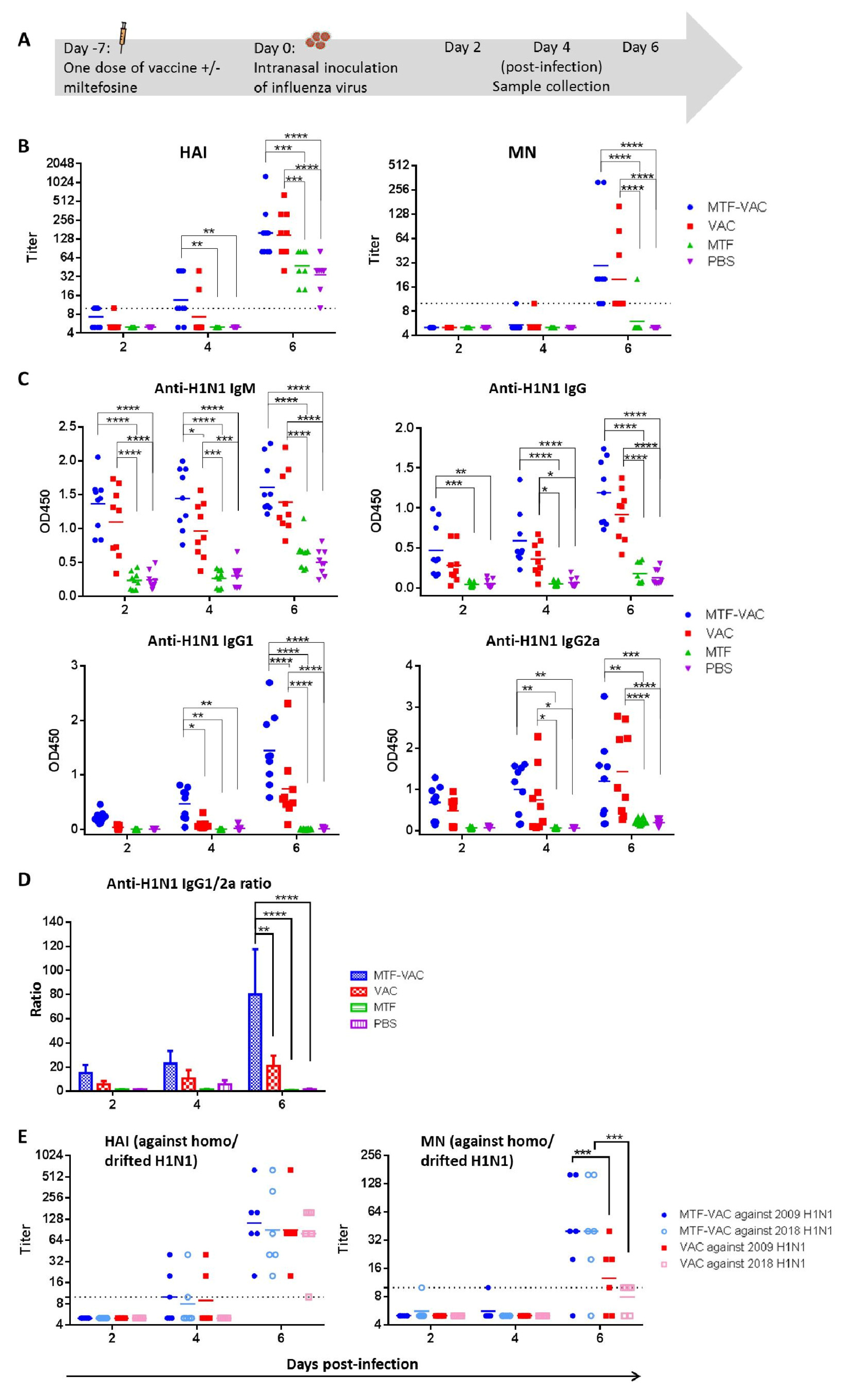
2.6. Antibody Titers
2.7. Splenocyte Single Cell Suspension Preparation
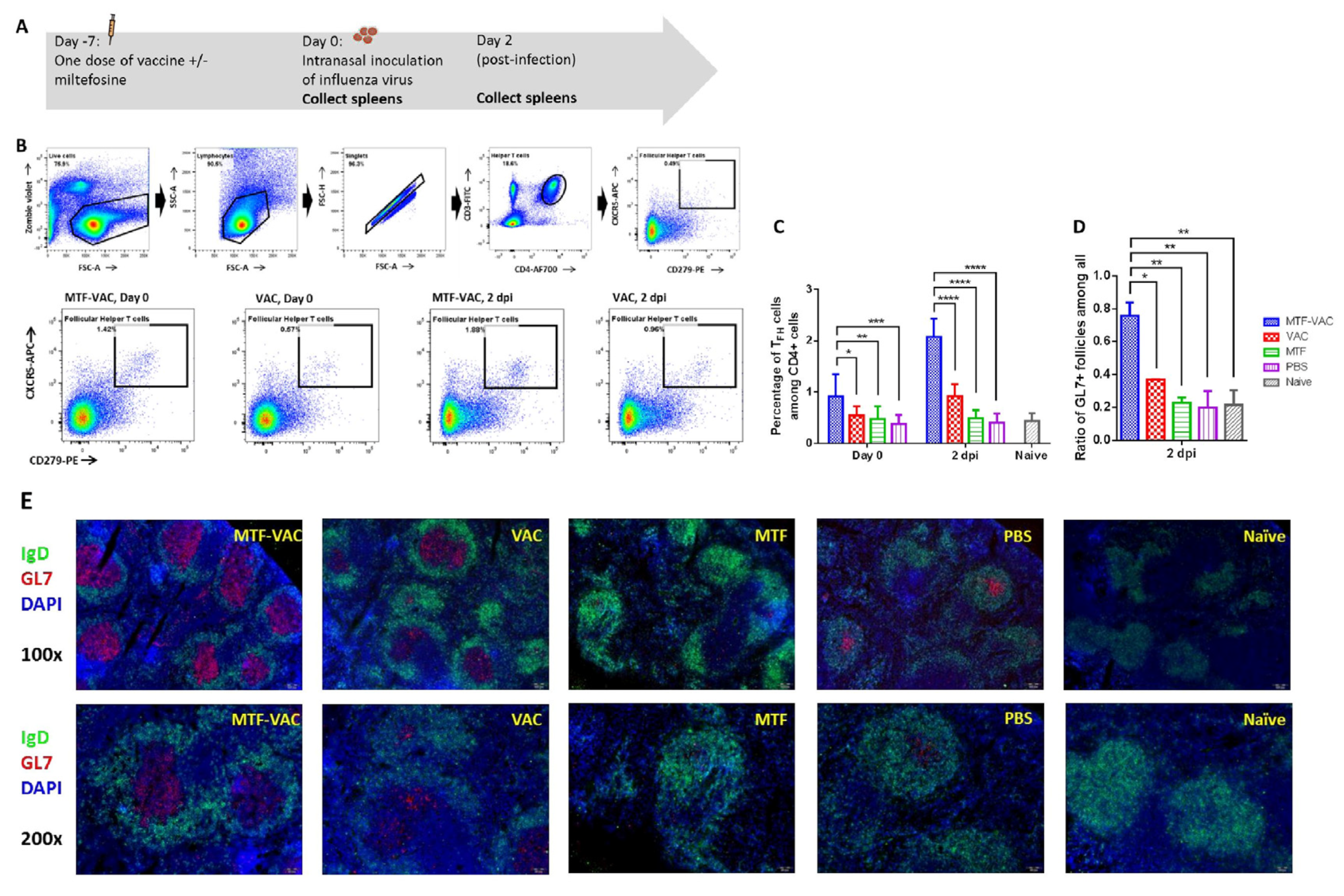
2.8. TFH Cells Flow Cytometry
2.9. GC Examination
2.10. Antigen-Specific Cytokine Responses
2.11. Statistical Analysis
3. Results
3.1. MTF Adjuvant Improved Vaccine Efficacy in 7 Days
3.2. MTF Adjuvant Augmented and Broadened the Antibody Response of Influenza Vaccine When Given 7 Days before Viral Challenge
3.3. MTF Adjuvant Improved Vaccine Efficacy in 3 Days
3.4. MTF Adjuvant Significantly Increased TFH Cell Frequency and Activity in the Spleen
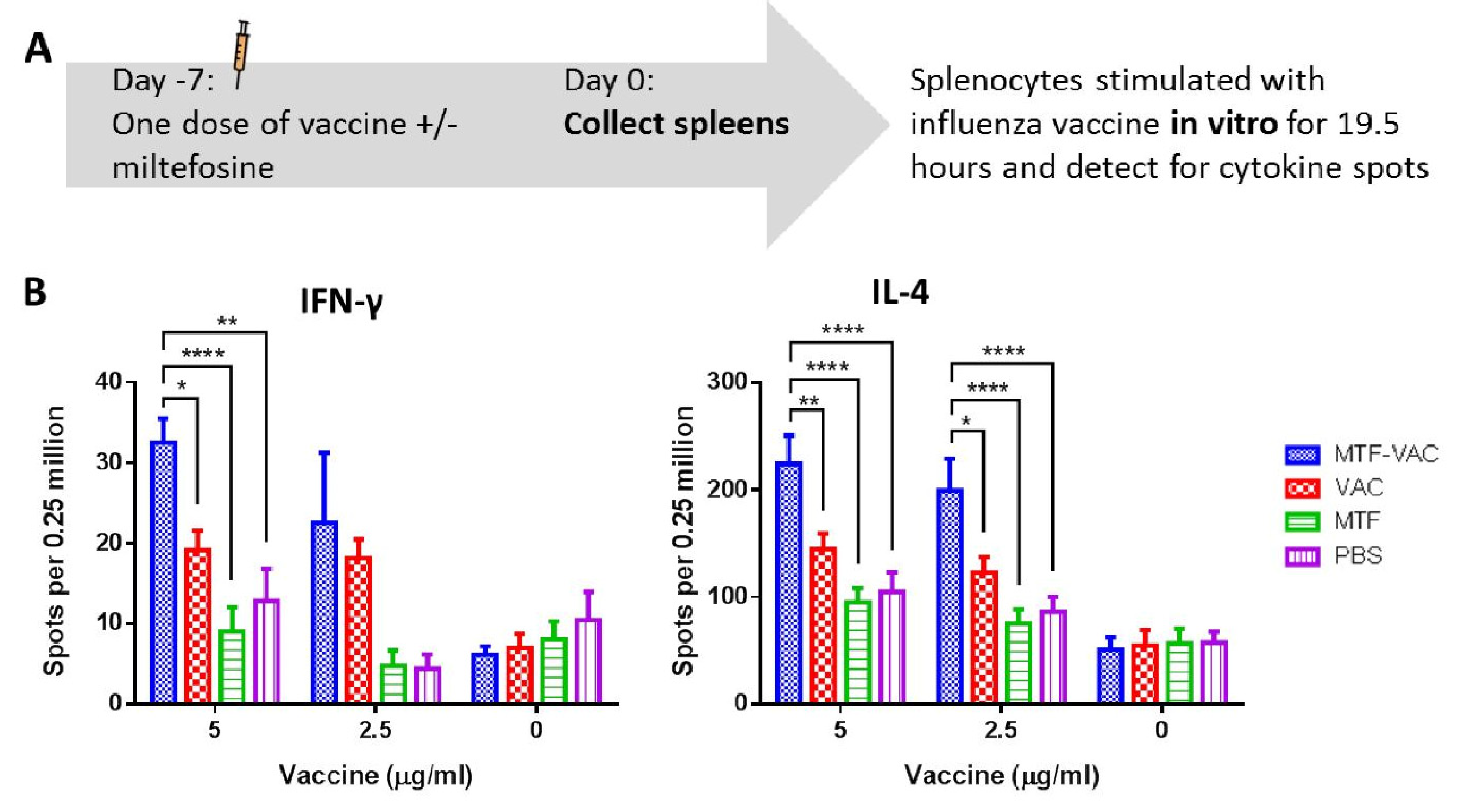
3.5. MTF Adjuvanted Influenza Vaccine Enhanced the Antigen-Specific TH1 and TH2 Cytokine Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- To, K.K.W.; Chan, J.F.W.; Chen, H.; Li, L.; Yuen, K.-Y. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: A tale of two cities. Lancet Infect. Dis. 2013, 13, 809–821. [Google Scholar] [CrossRef]
- Muthuri, S.G.; Venkatesan, S.; Myles, P.R.; Leonardi-Bee, J.; Al Khuwaitir, T.S.A.; Al Mamun, A.; Anovadiya, A.P.; Azziz-Baumgartner, E.; Báez, C.; Bassetti, M.; et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: A meta-analysis of individual participant data. Lancet Respir. Med. 2014, 2, 395–404. [Google Scholar] [CrossRef]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Leung, A.Y.; Chu, D.W.S.; Leung, R.; Cheung, T.; Chan, C.; Lam, C.L.K.; Liu, S.; Chu, C.; Ho, P.-L.; et al. Prevention of Acute Myocardial Infarction and Stroke among Elderly Persons by Dual Pneumococcal and Influenza Vaccination: A Prospective Cohort Study. Clin. Infect. Dis. 2010, 51, 1007–1016. [Google Scholar] [CrossRef]
- Weinberger, B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 2018, 41, 34–41. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Marra, F.; Young, F.; Richardson, K.; Marra, C.A. A Meta-analysis of intradermal versus intramuscular influenza vaccines: Immunogenicity and Adverse Events. Influenza Other Respir. Viruses 2012, 7, 584–603. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 Influenza Season. MMWR Recomm. Rep. 2019, 68, 1–21. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Han, T.; Liu, C.; Li, X.; Yan, L.; Yang, B.; Yang, X. Effectiveness, immunogenicity, and safety of influenza vaccines with MF59 adjuvant in healthy people of different age groups: A systematic review and meta-analysis. Medicine 2020, 99, e19095. [Google Scholar] [CrossRef]
- Vesikari, T.; Kirstein, J.; Go, G.D.; Leav, B.; Ruzycky, M.E.; Isakov, L.; De Bruijn, M.; Oberye, J.; Heijnen, E. Efficacy, immunogenicity, and safety evaluation of an MF59-adjuvanted quadrivalent influenza virus vaccine compared with non-adjuvanted influenza vaccine in children: A multicentre, randomised controlled, observer-blinded, phase 3 trial. Lancet Respir. Med. 2018, 6, 345–356. [Google Scholar] [CrossRef]
- To, K.K.W.; Zhang, A.J.X.; Chan, A.S.F.; Li, C.; Cai, J.-P.; Lau, C.C.Y.; Li, C.-G.; Jahan, A.S.; Wu, W.-L.; Li, L.-J.; et al. Recombinant influenza A virus hemagglutinin HA2 subunit protects mice against influenza A(H7N9) virus infection. Arch. Virol. 2015, 160, 777–786. [Google Scholar] [CrossRef]
- Zhang, A.J.X.; Li, C.; To, K.K.W.; Zhu, H.-S.; Lee, A.C.Y.; Li, C.-G.; Chan, J.F.W.; Hung, I.F.N.; Yuen, K.-Y. Toll-Like Receptor 7 Agonist Imiquimod in Combination with Influenza Vaccine Expedites and Augments Humoral Immune Responses against Influenza A(H1N1)pdm09 Virus Infection in BALB/c Mice. Clin. Vaccine Immunol. 2014, 21, 570–579. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Zhang, A.J.; To, K.K.-W.; Chan, J.F.-W.; Li, P.; Wong, T.-L.; Zhang, R.; Chan, T.-C.; Chan, B.C.-Y.; Wai, H.H.; et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: A single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect. Dis. 2016, 16, 209–218. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Zhang, A.J.; To, K.K.W.; Chan, J.F.W.; Li, C.; Zhu, H.-S.; Li, P.; Li, C.; Chan, T.-C.; Cheng, V.C.C.; et al. Immunogenicity of Intradermal Trivalent Influenza Vaccine With Topical Imiquimod: A Double Blind Randomized Controlled Trial. Clin. Infect. Dis. 2014, 59, 1246–1255. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Gupta, G.; Adhikari, A.; Majumder, S.; Mahapatra, S.K.; Majumdar, S.B.; Majumdar, S. Miltefosine triggers a strong proinflammatory cytokine response during visceral leishmaniasis: Role of TLR4 and TLR9. Int. Immunopharmacol. 2012, 12, 565–572. [Google Scholar] [CrossRef]
- Iacano, A.J.; Lewis, H.; Hazen, J.E.; Andro, H.; Smith, J.D.; Gulshan, K. Miltefosine increases macrophage cholesterol release and inhibits NLRP3-inflammasome assembly and IL-1β release. Sci. Rep. 2019, 9, 11128. [Google Scholar] [CrossRef]
- Hochhuth, C.H.; Vehmeyer, K.; Eibl, H.; Unger, C. Hexadecylphosphocholine induces interferon-gamma secretion and expression of GM-CSF mRNA in human mononuclear cells. Cell. Immunol. 1992, 141, 161–168. [Google Scholar] [CrossRef]
- Eue, I. Hexadecylphosphocholine selectively upregulates expression of intracellular adhesion molecule-1 and class I major histocompatibility complex antigen in human monocytes. J. Exp. Ther. Oncol. 2002, 2, 333–336. [Google Scholar] [CrossRef]
- Debache, K.; Hemphill, A. Effects of miltefosine treatment in fibroblast cell cultures and in mice experimentally infected with Neospora caninum tachyzoites. Parasitology 2012, 139, 934–944. [Google Scholar] [CrossRef]
- Gupta, S.; Sane, S.A.; Shakya, N.; Vishwakarma, P.; Haq, W. CpG Oligodeoxynucleotide 2006 and Miltefosine, a Potential Combination for Treatment of Experimental Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 3461–3464. [Google Scholar] [CrossRef] [PubMed]
- Wargo, M.J.; Gross, M.J.; Rajamani, S.; Allard, J.L.; Lundblad, L.K.A.; Allen, G.B.; Vasil, M.L.; LeClair, L.W.; Hogan, D.A. Hemolytic Phospholipase C Inhibition Protects Lung Function duringPseudomonas aeruginosaInfection. Am. J. Respir. Crit. Care Med. 2011, 184, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Najvar, L.K.; Bocanegra, R.; Kirkpatrick, W.R.; Sorrell, T.C.; Patterson, T.F. Limited Activity of Miltefosine in Murine Models of Cryptococcal Meningoencephalitis and Disseminated Cryptococcosis. Antimicrob. Agents Chemother. 2012, 57, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Rehg, J.E. A Comparison of Anticryptosporidial Activity of Paromomycin with that of Other Aminoglycosides and Azithromycin in Immunosuppressed Rats. J. Infect. Dis. 1994, 170, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Kanyok, T.P.; Reddy, M.V.; Chinnaswamy, J.; Danziger, L.H.; Gangadharam, P.R. In vivo activity of paromomycin against susceptible and multidrug-resistant Mycobacterium tuberculosis and M. avium complex strains. Antimicrob. Agents Chemother. 1994, 38, 170–173. [Google Scholar] [CrossRef][Green Version]
- Ndao, M.; Nath-Chowdhury, M.; Sajid, M.; Marcus, V.; Mashiyama, S.T.; Sakanari, J.; Chow, E.; Mackey, Z.; Land, K.M.; Jacobson, M.P.; et al. A Cysteine Protease Inhibitor Rescues Mice from a Lethal Cryptosporidium parvum Infection. Antimicrob. Agents Chemother. 2013, 57, 6063–6073. [Google Scholar] [CrossRef]
- Li, C.; To, K.K.W.; Zhang, A.J.X.; Lee, A.C.Y.; Zhu, H.; Mak, W.W.N.; Hung, I.F.N.; Yuen, K.-Y. Co-stimulation With TLR7 Agonist Imiquimod and Inactivated Influenza Virus Particles Promotes Mouse B Cell Activation, Differentiation, and Accelerated Antigen Specific Antibody Production. Front. Immunol. 2018, 9, 2370. [Google Scholar] [CrossRef]
- WHO. WHO Manual on Animal Influenza Diagnosis and Surveillance; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Voordouw, B.C.G.; Beyer, W.; Smith, D.; Sturkenboom, M.; Stricker, B. Evaluation of serological trials submitted for annual re-licensure of influenza vaccines to regulatory authorities between 1992 and 2002. Vaccine 2009, 28, 392–397. [Google Scholar] [CrossRef]
- Belshe, R.B.; Gruber, W.C.; Mendelman, P.M.; Mehta, H.B.; Mahmood, K.; Reisinger, K.; Treanor, J.; Zangwill, K.; Hayden, F.G.; Bernstein, D.I.; et al. Correlates of Immune Protection Induced by Live, Attenuated, Cold-Adapted, Trivalent, Intranasal Influenza Virus Vaccine. J. Infect. Dis. 2000, 181, 1133–1137. [Google Scholar] [CrossRef]
- Ohmit, S.E.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Monto, A.S. Influenza Hemagglutination-Inhibition Antibody Titer as a Correlate of Vaccine-Induced Protection. J. Infect. Dis. 2011, 204, 1879–1885. [Google Scholar] [CrossRef]
- WHO. Serological Diagnosis of Influenza by Microneutralization Assay; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Verschoor, C.P.; Singh, P.; Russell, M.L.; Bowdish, D.M.E.; Brewer, A.; Cyr, L.; Ward, B.J.; Loeb, M. Microneutralization Assay Titres Correlate with Protection against Seasonal Influenza H1N1 and H3N2 in Children. PLoS ONE 2015, 10, e0131531. [Google Scholar] [CrossRef] [PubMed]
- Tsubata, T. Faculty Opinions recommendation of Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc. Natl. Acad. Sci. USA 2014, 111, E3224–E3233. [Google Scholar] [CrossRef]
- Hjertner, B.; Bengtsson, T.; Morein, B.; Paulie, S.; Fossum, C. A novel adjuvant G3 induces both Th1 and Th2 related immune responses in mice after immunization with a trivalent inactivated split-virion influenza vaccine. Vaccine 2018, 36, 3340–3344. [Google Scholar] [CrossRef] [PubMed]
- Vernacchio, L.; Bernstein, H.; Pelton, S.I.; Allen, C.; Macdonald, K.; Dunn, J.; Duncan, D.D.; Tsao, G.; LaPosta, V.; Eldridge, J.; et al. Effect of monophosphoryl lipid A (MPL®) on T-helper cells when administered as an adjuvant with pneumocococcal–CRM197 conjugate vaccine in healthy toddlers. Vaccine 2002, 20, 3658–3667. [Google Scholar] [CrossRef]
- Hill, D.L.; Pierson, W.; Bolland, D.J.; Mkindi, C.; Carr, E.J.; Wang, J.; Houard, S.; Wingett, S.W.; Audran, R.; Wallin, E.F.; et al. The adjuvant GLA-SE promotes human Tfh cell expansion and emergence of public TCRbeta clonotypes. J. Exp. Med. 2019, 216, 1857–1873. [Google Scholar] [CrossRef]
- Lartey, S.; Zhou, F.; Brokstad, K.A.; Mohn, K.G.-I.; Slettevoll, S.A.; Pathirana, R.D.; Cox, R.J. Live-Attenuated Influenza Vaccine Induces Tonsillar Follicular T Helper Cell Responses That Correlate With Antibody Induction. J. Infect. Dis. 2020, 221, 21–32. [Google Scholar] [CrossRef]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef]
- Pallikkuth, S.; De Armas, L.R.; Rinaldi, S.; George, V.K.; Pan, L.; Arheart, K.L.; Pahwa, R.; Pahwa, S. Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: Results from the FLORAH study. PLoS Biol. 2019, 17, e3000257. [Google Scholar] [CrossRef]
- Sage, P.T.; Tan, C.L.; Freeman, G.J.; Haigis, M.; Sharpe, A.H. Defective TFH Cell Function and Increased TFR Cells Contribute to Defective Antibody Production in Aging. Cell Rep. 2015, 12, 163–171. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Pastore, G.; Andersen, P.; Pozzi, G.; Medaglini, D. Modulation of Primary Immune Response by Different Vaccine Adjuvants. Front. Immunol. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.; Fouchier, R.; Rimmelzwaan, G.F. Immune responses to influenza virus infection. Virus Res. 2011, 162, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–259. [Google Scholar] [CrossRef]
- Lilienthal, G.-M.; Rahmöller, J.; Petry, J.; Bartsch, Y.C.; Leliavski, A.; Ehlers, M. Potential of Murine IgG1 and Human IgG4 to Inhibit the Classical Complement and Fcγ Receptor Activation Pathways. Front. Immunol. 2018, 9, 958. [Google Scholar] [CrossRef]
- Tamayo, E.; Fernández, A.; Almansa, R.; Carrasco, E.; Goncalves, L.; Heredia, M.; Andaluz-Ojeda, D.; March, G.; Rico, L.; Gómez-Herreras, J.I.; et al. Beneficial role of endogenous immunoglobulin subclasses and isotypes in septic shock. J. Crit. Care 2012, 27, 616–622. [Google Scholar] [CrossRef]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct Contributions of Vaccine-Induced Immunoglobulin G1 (IgG1) and IgG2a Antibodies to Protective Immunity against Influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef]
- Sun, J.; Madan, R.; Karp, C.L.; Braciale, T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009, 15, 277–284. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Holzer, B.; Morgan, S.B.; Matsuoka, Y.; Edmans, M.; Bodes, F.J.S.; Everett, H.; Brookes, S.M.; Porter, E.; MacLoughlin, R.; Charleston, B.; et al. Comparison of Heterosubtypic Protection in Ferrets and Pigs Induced by a Single-Cycle Influenza Vaccine. J. Immunol. 2018, 200, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Tang, S.Y.; Devine, J.C.; Anderson, S.T.; Nayak, S.; Zhang, S.L.; Valenzuela, A.; Fisher, D.G.; Grant, G.R.; López, C.B.; et al. Circadian control of lung inflammation in influenza infection. Nat. Commun. 2019, 10, 4107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, R.; Li, C.; Jiang, L.; Xiang, M.; Ye, Z.; Kita, H.; Melnick, A.M.; Dent, A.L.; Sun, J. BCL6 modulates tissue neutrophil survival and exacerbates pulmonary inflammation following influenza virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 11888–11893. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Guptill, J.; Naficy, A.; Nachbagauer, R.; Berlanda-Scorza, F.; Feser, J.; Wilson, P.C.; Solórzano, A.; Van Der Wielen, M.; Walter, E.B.; et al. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: Interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020, 20, 80–91. [Google Scholar] [CrossRef]
- Pleguezuelos, O.; Dille, J.; De Groen, S.; Oftung, F.; Niesters, H.G.M.; Islam, A.; Næss, L.M.; Hungnes, O.; Aldarij, N.; Idema, D.L.; et al. Immunogenicity, Safety, and Efficacy of a Standalone Universal Influenza Vaccine, FLU-v, in Healthy Adults: A Randomized Clinical Trial. Ann. Intern. Med 2020, 7, 453–462. [Google Scholar] [CrossRef]
- Kavian, Z.; Alavizadeh, S.H.; Golmohamadzadeh, S.; Badiee, A.; Khamesipour, A.; Jaafari, M.R.; Alavizade, S.H. Development of topical liposomes containing miltefosine for the treatment of Leishmania major infection in susceptible BALB/c mice. Acta Trop. 2019, 196, 142–149. [Google Scholar] [CrossRef]
- Terwogt, J.M.M.; Mandjes, I.A.M.; Sindermann, H.; Beijnen, J.H.; Huinink, W.W.T.B. Phase II trial of topically applied miltefosine solution in patients with skin-metastasized breast cancer. Br. J. Cancer 1999, 79, 1158–1161. [Google Scholar] [CrossRef]
- Bastiani, F.W.M.D.S.D.; Spadari, C.D.C.; De Matos, J.K.R.; Salata, G.C.; Lopes, L.B.; Ishida, K. Nanocarriers Provide Sustained Antifungal Activity for Amphotericin B and Miltefosine in the Topical Treatment of Murine Vaginal Candidiasis. Front. Microbiol. 2020, 10, 2976. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nat. Cell Biol. 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Fong, C.H.-Y.; Zhang, A.J.; Wu, W.-L.; Li, I.C.; Lee, A.C.-Y.; Dissanayake, T.K.; Chen, L.; Hung, I.F.-N.; Chan, K.-H.; et al. Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine. Vaccines 2020, 8, 754. https://doi.org/10.3390/vaccines8040754
Lu L, Fong CH-Y, Zhang AJ, Wu W-L, Li IC, Lee AC-Y, Dissanayake TK, Chen L, Hung IF-N, Chan K-H, et al. Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine. Vaccines. 2020; 8(4):754. https://doi.org/10.3390/vaccines8040754
Chicago/Turabian StyleLu, Lu, Carol Ho-Yan Fong, Anna Jinxia Zhang, Wai-Lan Wu, Iris Can Li, Andrew Chak-Yiu Lee, Thrimendra Kaushika Dissanayake, Linlei Chen, Ivan Fan-Ngai Hung, Kwok-Hung Chan, and et al. 2020. "Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine" Vaccines 8, no. 4: 754. https://doi.org/10.3390/vaccines8040754
APA StyleLu, L., Fong, C. H.-Y., Zhang, A. J., Wu, W.-L., Li, I. C., Lee, A. C.-Y., Dissanayake, T. K., Chen, L., Hung, I. F.-N., Chan, K.-H., Chu, H., Kok, K.-H., Yuen, K.-Y., & To, K. K.-W. (2020). Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine. Vaccines, 8(4), 754. https://doi.org/10.3390/vaccines8040754







