Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus Coioides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
2.2. Animals
2.3. Inactivated V. harveyi (Vh MML-1) Bacteria
2.4. Bacterial Lysate
2.5. Grouper Anti-V. harveyi Antibodies (Ab1)
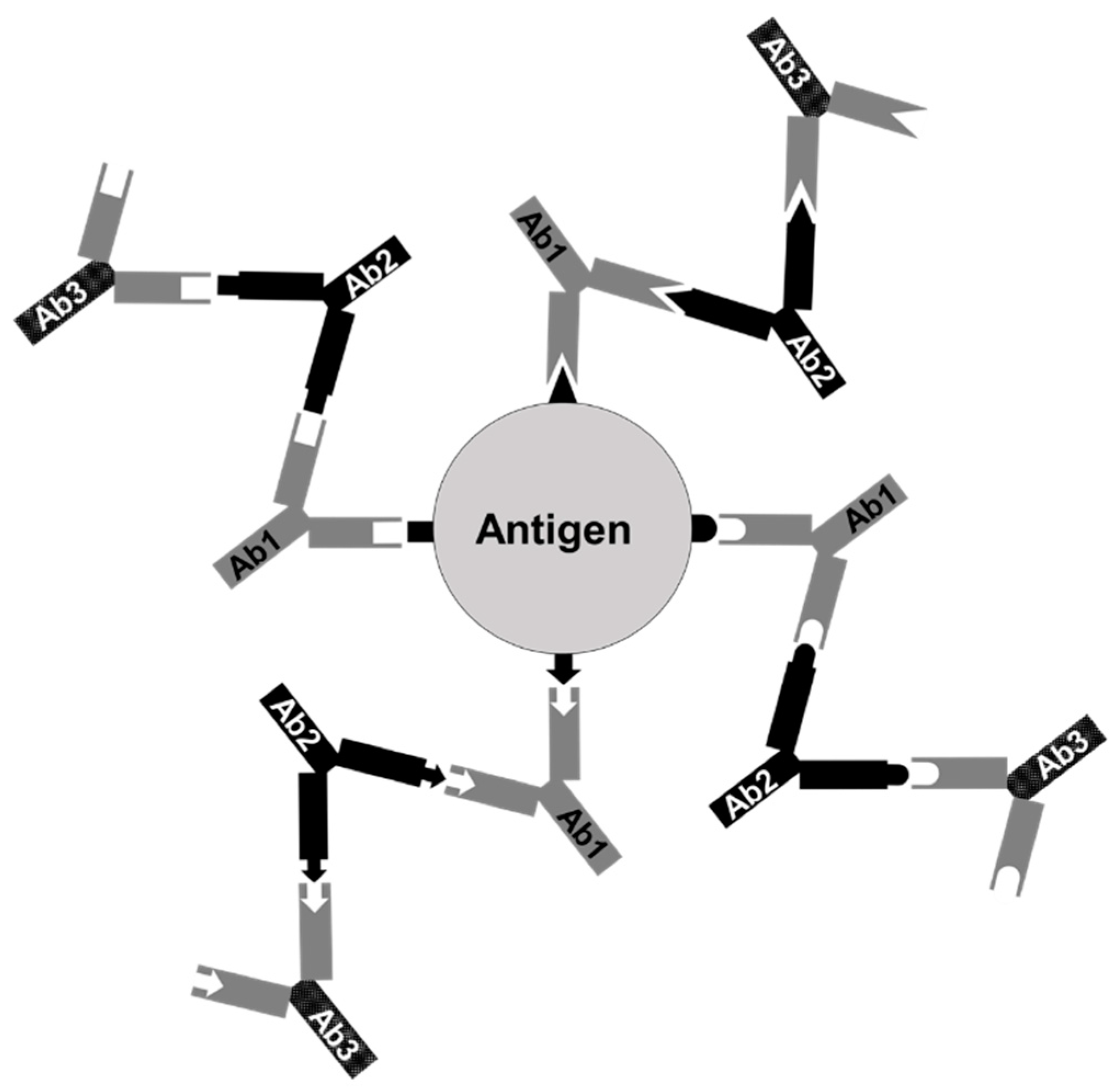
2.6. Production of the Anti-Id Vaccine, Anti-Id IgG (Fab)
2.7. Immunization in Grouper
2.8. Antigenic Specificity of Immunized Grouper Sera
2.9. Grouper Serum Titer Assay
2.10. Lymphocyte Proliferation Assay
2.11. Bacterial Challenge
2.12. Statistical Analysis
3. Results
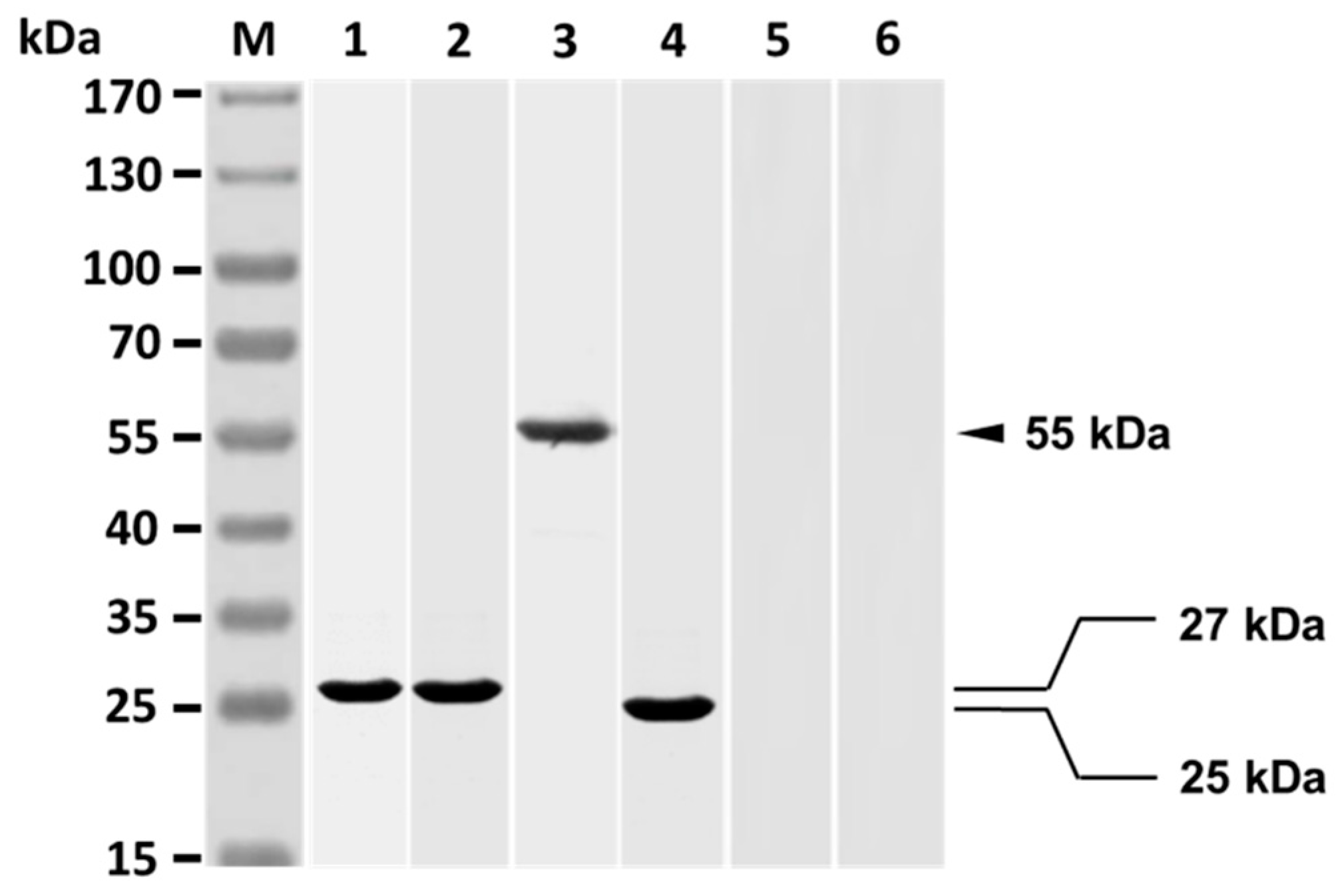
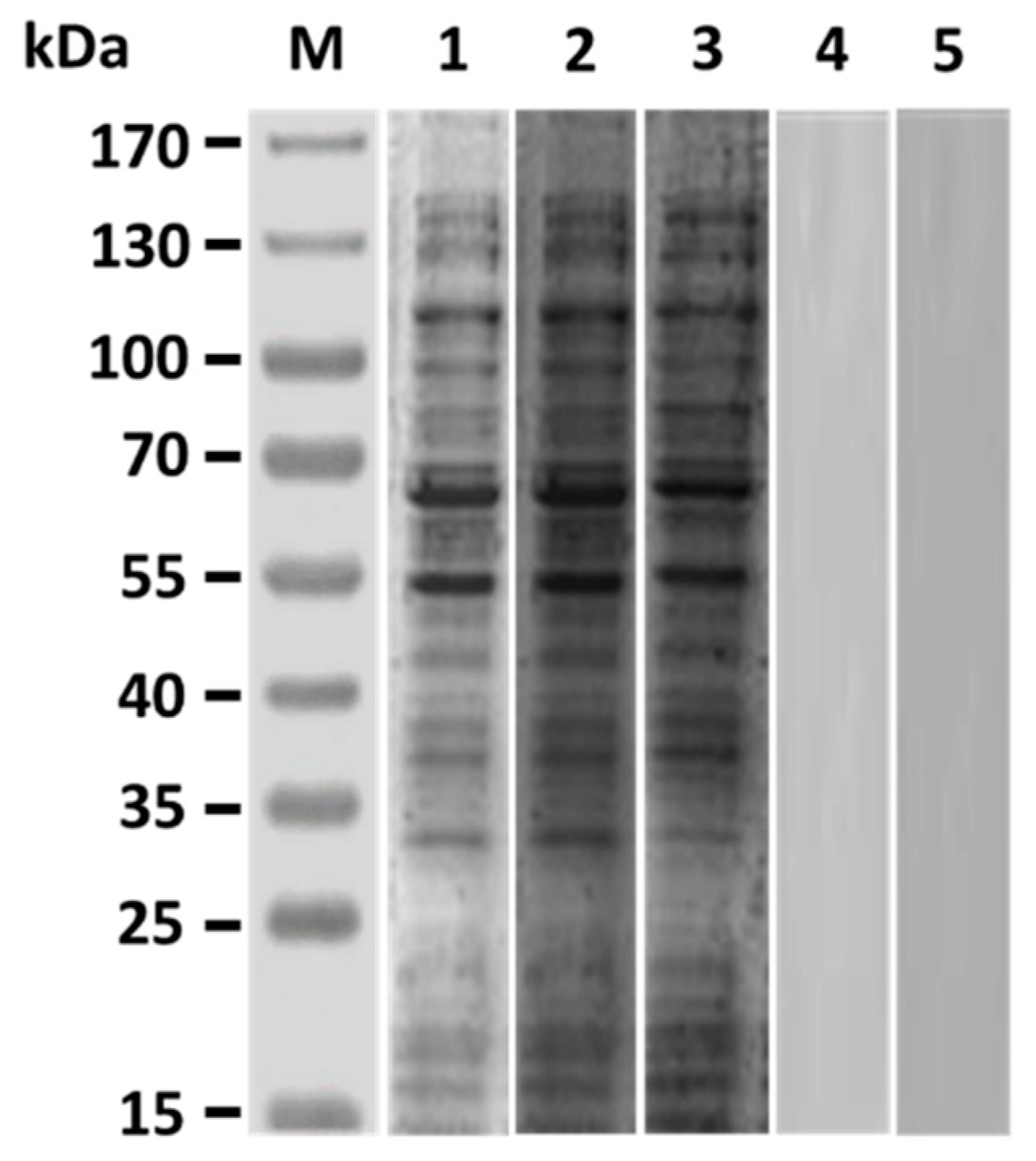
3.1. V. harveyi-Like Antigenicity of Rabbit Anti-Id IgG (Fab)
3.2. Strong Antibody Responses Elicited by Anti-Id IgG (Fab) in Grouper
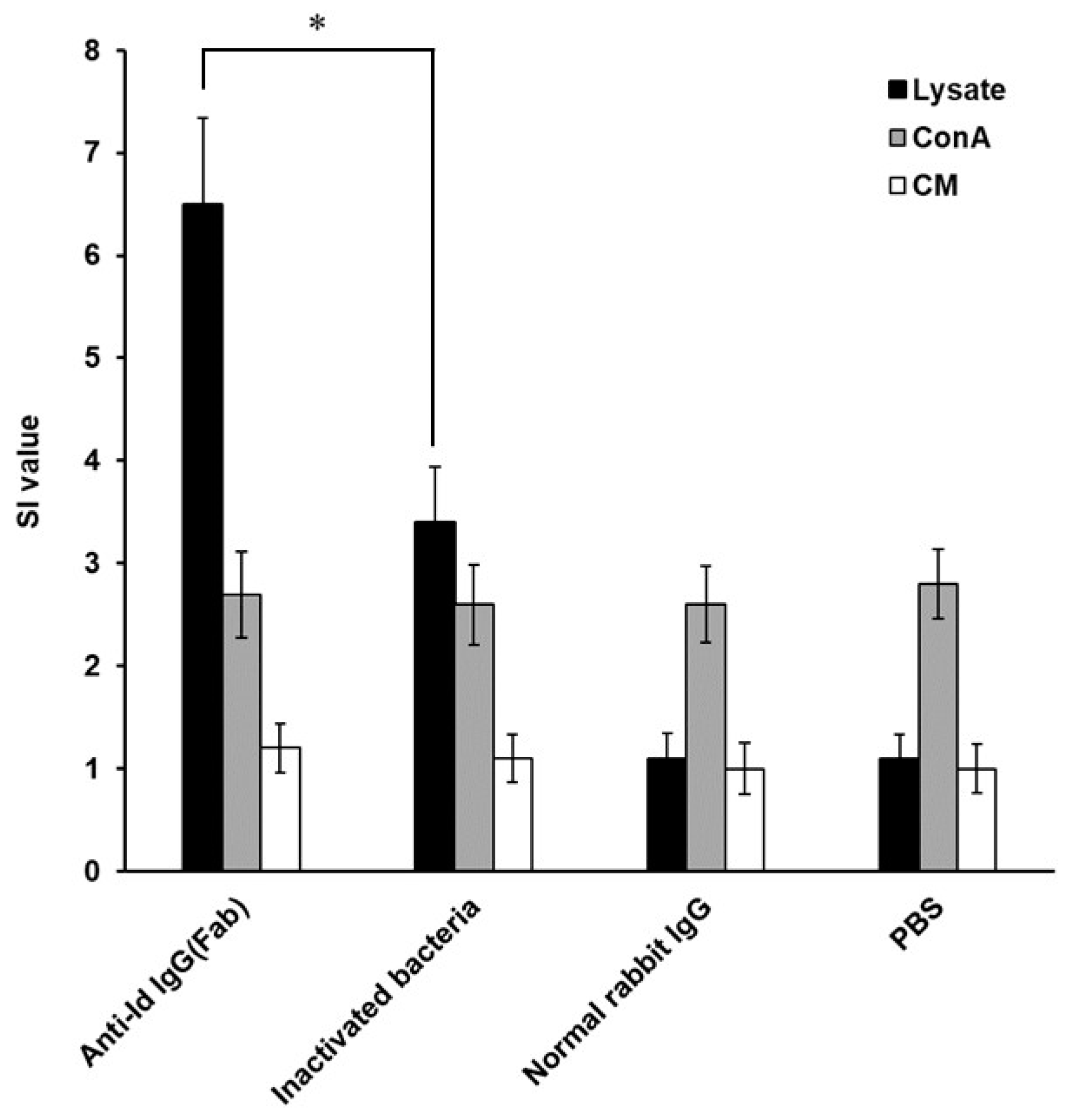
3.3. High Lymphocyte Proliferation Induced by Anti-Id IgG (Fab) in Grouper
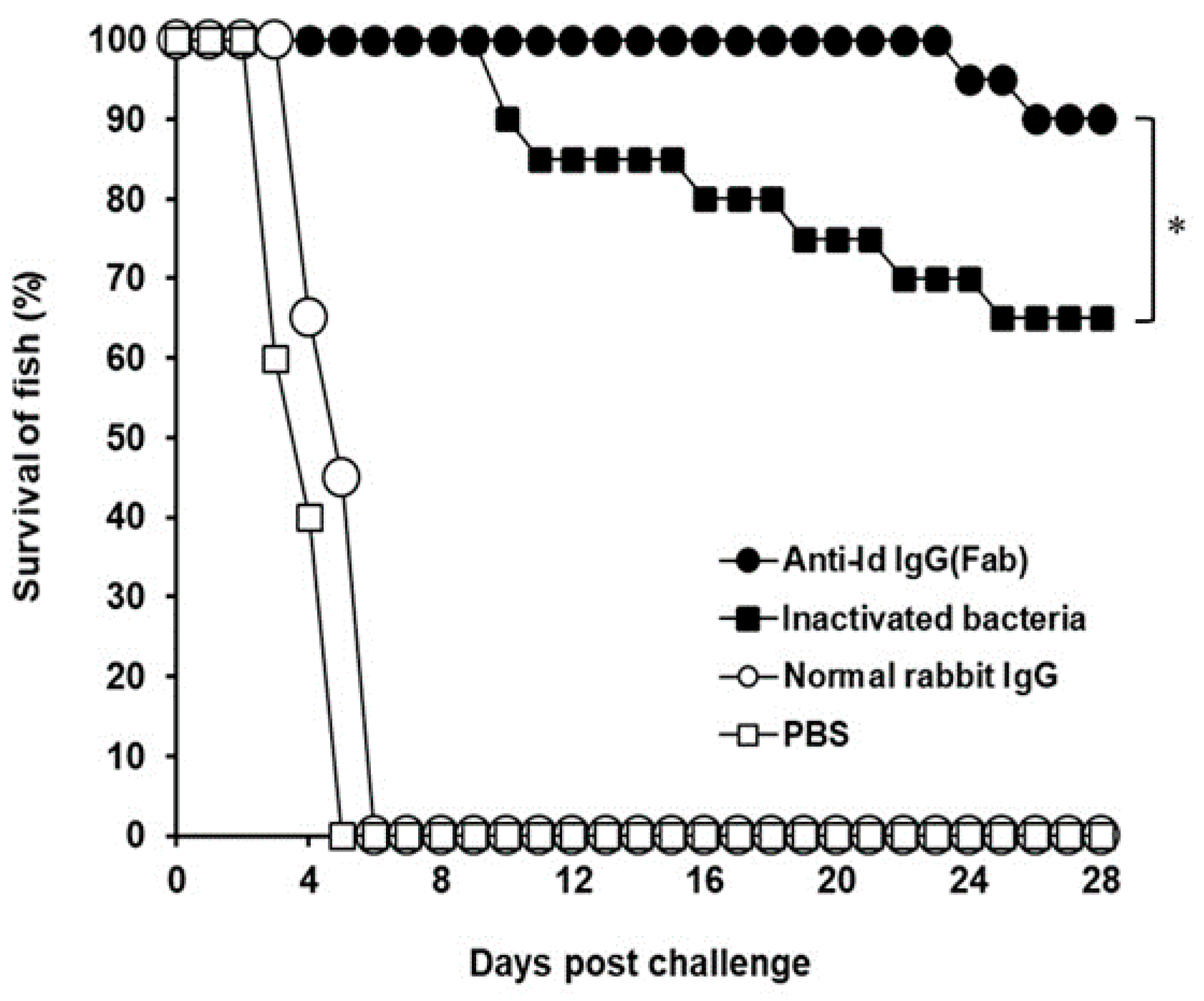
3.4. Protection Against V. harveyi Challenge in Grouper
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Molecular studies, disease status and prophylactic measures in grouper aquaculture: Economic importance, diseases and immunology. Aquaculture 2010, 309, 1–14. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Diet enriched with mushroom Phellinus linteus extract enhances the growth, innate immune response, and disease resistance of kelp grouper, Epinephelus bruneus against vibriosis. Fish Shellfish Immunol. 2011, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Zhang, X.H. Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Lee, K.K.; Chen, S.N. Susceptibility of different isolates of Vibrio harveyi to antibiotics. Microbios 1997, 91, 175–180. [Google Scholar]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 64. [Google Scholar] [CrossRef]
- Yu, L.P.; Hu, Y.H.; Sun, B.G.; Sun, L. Immunological study of the outer membrane proteins of Vibrio harveyi: Insights that link immunoprotectivity to interference with bacterial infection. Fish Shellfish Immunol. 2013, 35, 1293–1300. [Google Scholar] [CrossRef]
- Ningqiu, L.; Junjie, B.; Shuqin, W.; Xiaozhe, F.; Haihua, L.; Xing, Y.; Cunbin, S. An outer membrane protein, OmpK, is an effective vaccine candidate for Vibrio harveyi in Orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2008, 25, 829–833. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, L.; Qian, R. Characterization of OmpK, GAPDH and their fusion OmpK-GAPDH derived from Vibrio harveyi outer membrane proteins: Their immunoprotective ability against vibriosis in large yellow croaker (Pseudosciaena crocea). J. Appl. Microbiol. 2007, 103, 1587–1599. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, L.; Qian, R. Cloning and expression of Vibrio harveyi OmpK* and GAPDH* genes and their potential application as vaccines in large yellow croakers Pseudosciaena crocea. J. Aquat. Anim. Health 2008, 20, 1–11. [Google Scholar] [CrossRef]
- Li, N.; Yang, Z.; Bai, J.; Fu, X.; Liu, L.; Shi, C.; Wu, S. A shared antigen among Vibrio species: Outer membrane protein-OmpK as a versatile Vibriosis vaccine candidate in Orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Pang, H.Y.; Li, Y.; Wu, Z.H.; Jian, J.C.; Lu, Y.S.; Cai, S.H. Immunoproteomic analysis and identification of novel immunogenic proteins from Vibrio harveyi. J. Appl. Microbiol. 2010, 109, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D.; Chang, G.N.; Chao, D. Protective immunity against Toxoplasma gondii in mice induced by a chimeric protein rSAG1/2. Parasitol. Res. 2004, 92, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.A. Polyvalent vaccines in fish: The interactive effects of multiple antigens. Dev. Biol. Stand. 1997, 90, 245–256. [Google Scholar] [PubMed]
- Pace, J.L.; Rossi, H.A.; Esposito, V.M.; Frey, S.M.; Tucker, K.D.; Walker, R.I. Inactivated whole-cell bacterial vaccines: Current status and novel strategies. Vaccine 1998, 16, 1563–1574. [Google Scholar] [CrossRef]
- Hajam, I.A.; Dar, P.A.; Won, G.; Lee, J.H. Bacterial ghosts as adjuvants: Mechanisms and potential. Vet. Res. 2017, 48, 37. [Google Scholar] [CrossRef]
- Naveed, A.; Rahman, S.U.; Arshad, M.I.; Aslam, B. Recapitulation of the anti-Idiotype antibodies as vaccine candidate. Transl. Med. Commun. 2018, 3, 1. [Google Scholar] [CrossRef]
- Kohler, H.; Pashov, A.; Kieber-Emmons, T. The Promise of Anti-idiotype Revisited. Front. Immunol. 2019, 10, 808. [Google Scholar] [CrossRef]
- De Cerio, A.L.; Zabalegui, N.; Rodriguez-Calvillo, M.; Inoges, S.; Bendandi, M. Anti-idiotype antibodies in cancer treatment. Oncogene 2007, 26, 3594–3602. [Google Scholar] [CrossRef]
- Yongjuan, X.; Weiquan, H.; Baocheng, H.; Xiaohang, J.; Rongqing, Z. Production and characterisation of monoclonal anti-idiotype antibody to Vibrio anguillarum. Fish Shellfish Immunol. 2002, 12, 273–281. [Google Scholar] [CrossRef]
- Qin, H.; Jin, X.; Huang, W.; Liu, Y. Production of an anti-idiotypic antibody single chain variable fragment vaccine against Edwardsiella tarda. Acta Biochim. Biophys. Sin. 2010, 42, 129–136. [Google Scholar] [CrossRef]
- Chuang, S.C.; Huang, W.L.; Kau, S.W.; Yang, Y.P.; Yang, C.D. Pleurocidin Peptide Enhances Grouper Anti-Vibrio harveyi Immunity Elicited by Poly(lactide-co-glycolide)-Encapsulated Recombinant Glyceraldehyde-3-phosphate Dehydrogenase. Vaccines 2014, 2, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.C.; Yang, C.D. Sustained release of recombinant surface antigen 2 (rSAG2) from poly(lactide-co-glycolide) microparticles extends protective cell-mediated immunity against Toxoplasma gondii in mice. Parasitology 2014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.A.; John, J.A.; Wu, M.S.; Lee, C.Y.; Lin, C.H.; Lin, C.H.; Chang, C.Y. Characterization of serum immunoglobulin M of grouper and cDNA cloning of its heavy chain. Vet. Immunol. Immunopathol. 2006, 109, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D.; Chang, G.N.; Chao, D. Protective immunity against Toxoplasma gondii in mice induced by the SAG2 internal image of anti-idiotype antibody. Parasitol. Res. 2003, 91, 452–457. [Google Scholar] [CrossRef]
- Peterson, J.W. Bacterial Pathogenesis. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Chuang, S.C.; Ko, J.C.; Chen, C.P.; Du, J.T.; Yang, C.D. Induction of long-lasting protective immunity against Toxoplasma gondii in BALB/c mice by recombinant surface antigen 1 protein encapsulated in poly (lactide-co-glycolide) microparticles. Parasites Vectors 2013, 6, 34. [Google Scholar] [CrossRef]
- Chuang, S.C.; Ko, J.C.; Chen, C.P.; Du, J.T.; Yang, C.D. Encapsulation of chimeric protein rSAG1/2 into poly(lactide-co-glycolide) microparticles induces long-term protective immunity against Toxoplasma gondii in mice. Exp. Parasitol. 2013, 134, 430–437. [Google Scholar] [CrossRef]
- Chuang, S.C.; Chung, Y.C.; Yang, C.D. Protective immunity against toxoplasmosis in mice induced by single-dose immunization with rSAG1/2 protein released from poly(lactide-co-glycolide) microparticles. Parasite 2017, 24, 5. [Google Scholar] [CrossRef]
- Munford, R.S. Sensing gram-negative bacterial lipopolysaccharides: A human disease determinant? Infect. Immun. 2008, 76, 454–465. [Google Scholar] [CrossRef]
- Irene, C.; Fantappie, L.; Caproni, E.; Zerbini, F.; Anesi, A.; Tomasi, M.; Zanella, I.; Stupia, S.; Prete, S.; Valensin, S.; et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc. Natl. Acad. Sci. USA 2019, 116, 21780–21788. [Google Scholar] [CrossRef]
- Abrahams, K.A.; Besra, G.S. Mycobacterial cell wall biosynthesis: A multifaceted antibiotic target. Parasitology 2018, 145, 116–133. [Google Scholar] [CrossRef]
- Barbier, T.; Zuniga-Ripa, A.; Moussa, S.; Plovier, H.; Sternon, J.F.; Lazaro-Anton, L.; Conde-Alvarez, R.; De Bolle, X.; Iriarte, M.; Moriyon, I.; et al. Brucella central carbon metabolism: An update. Crit. Rev. Microbiol. 2018, 44, 182–211. [Google Scholar] [CrossRef] [PubMed]
- Matthysse, A.G.; Deora, R.; Mishra, M.; Torres, A.G. Polysaccharides cellulose, poly-beta-1,6-n-acetyl-D-glucosamine, and colanic acid are required for optimal binding of Escherichia coli O157:H7 strains to alfalfa sprouts and K-12 strains to plastic but not for binding to epithelial cells. Appl. Environ. Microbiol. 2008, 74, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Arjunaraja, S.; Massari, P.; Wetzler, L.M.; Lees, A.; Colino, J.; Snapper, C.M. The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain. J. Immunol. 2012, 188, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, W.; Ahrendt, T.; Bozhuyuk, K.A.; Bode, H.B. A multifunctional enzyme is involved in bacterial ether lipid biosynthesis. Nat. Chem. Biol. 2014, 10, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Bajestani, M.I.; Mousavi, S.M.; Jafari, A.; Shojaosadati, S.A. Biosynthesis and physicochemical characterization of a bacterial polysaccharide/polyamide blend, applied for microfluidics study in porous media. Int. J. Biol. Macromol. 2017, 96, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Munang‘andu, H.M.; Evensen, O. Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef]
- Dalmo, R.A. DNA vaccines for fish: Review and perspectives on correlates of protection. J. Fish Dis. 2018, 41, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.H.; Deng, T.; Sun, B.G.; Sun, L. Development and efficacy of an attenuated Vibrio harveyi vaccine candidate with cross protectivity against Vibrio alginolyticus. Fish Shellfish Immunol. 2012, 32, 1155–1161. [Google Scholar] [CrossRef]
 ) with anti-Id IgG (Fab) (●), inactivated bacteria (■), normal rabbit IgG (○) or PBS (□). Sera were collected from three fish per group on days 0, 21, and 42 and their anti-V. harveyi serum titers were determined by ELISA. Results were presented as the mean of log10 titers ± SD. * A significant difference (p < 0.05) exists when comparing the anti-Id IgG (Fab) group to the inactivated bacteria group.
) with anti-Id IgG (Fab) (●), inactivated bacteria (■), normal rabbit IgG (○) or PBS (□). Sera were collected from three fish per group on days 0, 21, and 42 and their anti-V. harveyi serum titers were determined by ELISA. Results were presented as the mean of log10 titers ± SD. * A significant difference (p < 0.05) exists when comparing the anti-Id IgG (Fab) group to the inactivated bacteria group.
 ) with anti-Id IgG (Fab) (●), inactivated bacteria (■), normal rabbit IgG (○) or PBS (□). Sera were collected from three fish per group on days 0, 21, and 42 and their anti-V. harveyi serum titers were determined by ELISA. Results were presented as the mean of log10 titers ± SD. * A significant difference (p < 0.05) exists when comparing the anti-Id IgG (Fab) group to the inactivated bacteria group.
) with anti-Id IgG (Fab) (●), inactivated bacteria (■), normal rabbit IgG (○) or PBS (□). Sera were collected from three fish per group on days 0, 21, and 42 and their anti-V. harveyi serum titers were determined by ELISA. Results were presented as the mean of log10 titers ± SD. * A significant difference (p < 0.05) exists when comparing the anti-Id IgG (Fab) group to the inactivated bacteria group.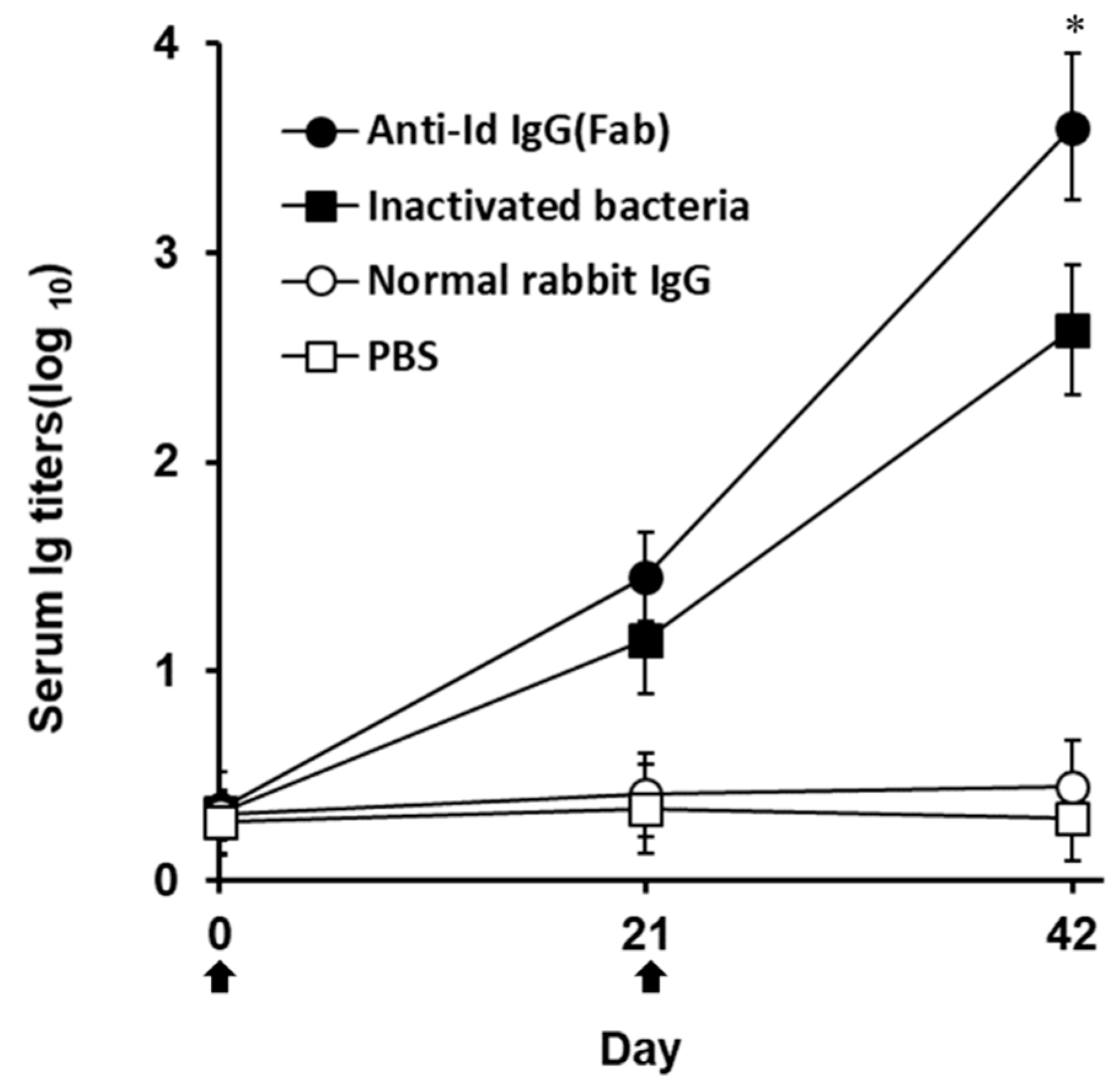
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-L.; Chuang, S.-C.; Yang, C.-D. Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus Coioides). Vaccines 2019, 7, 210. https://doi.org/10.3390/vaccines7040210
Huang W-L, Chuang S-C, Yang C-D. Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus Coioides). Vaccines. 2019; 7(4):210. https://doi.org/10.3390/vaccines7040210
Chicago/Turabian StyleHuang, Wan-Ling, Shu-Chun Chuang, and Chung-Da Yang. 2019. "Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus Coioides)" Vaccines 7, no. 4: 210. https://doi.org/10.3390/vaccines7040210
APA StyleHuang, W.-L., Chuang, S.-C., & Yang, C.-D. (2019). Anti-Idiotype Vaccine Provides Protective Immunity Against Vibrio Harveyi in Grouper (Epinephelus Coioides). Vaccines, 7(4), 210. https://doi.org/10.3390/vaccines7040210




