Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Lines
2.3. DENV1-4 Viral Stocks
2.4. Quantification of Mouse Viral Load by DENV Plaque Assay
2.5. PRNT Protocol
2.6. Nanopatch Fabrication and Application
2.7. Vaccine Delivery Efficiency
2.8. Immunization Study
2.9. DENV Challenge Study
2.10. Anti-sE IgG Enzyme Linked Immunosorbent Assay
2.11. NS1 Quantification
2.12. Statistical Analysis
3. Results
3.1. Nanopatch Coating and Delivery
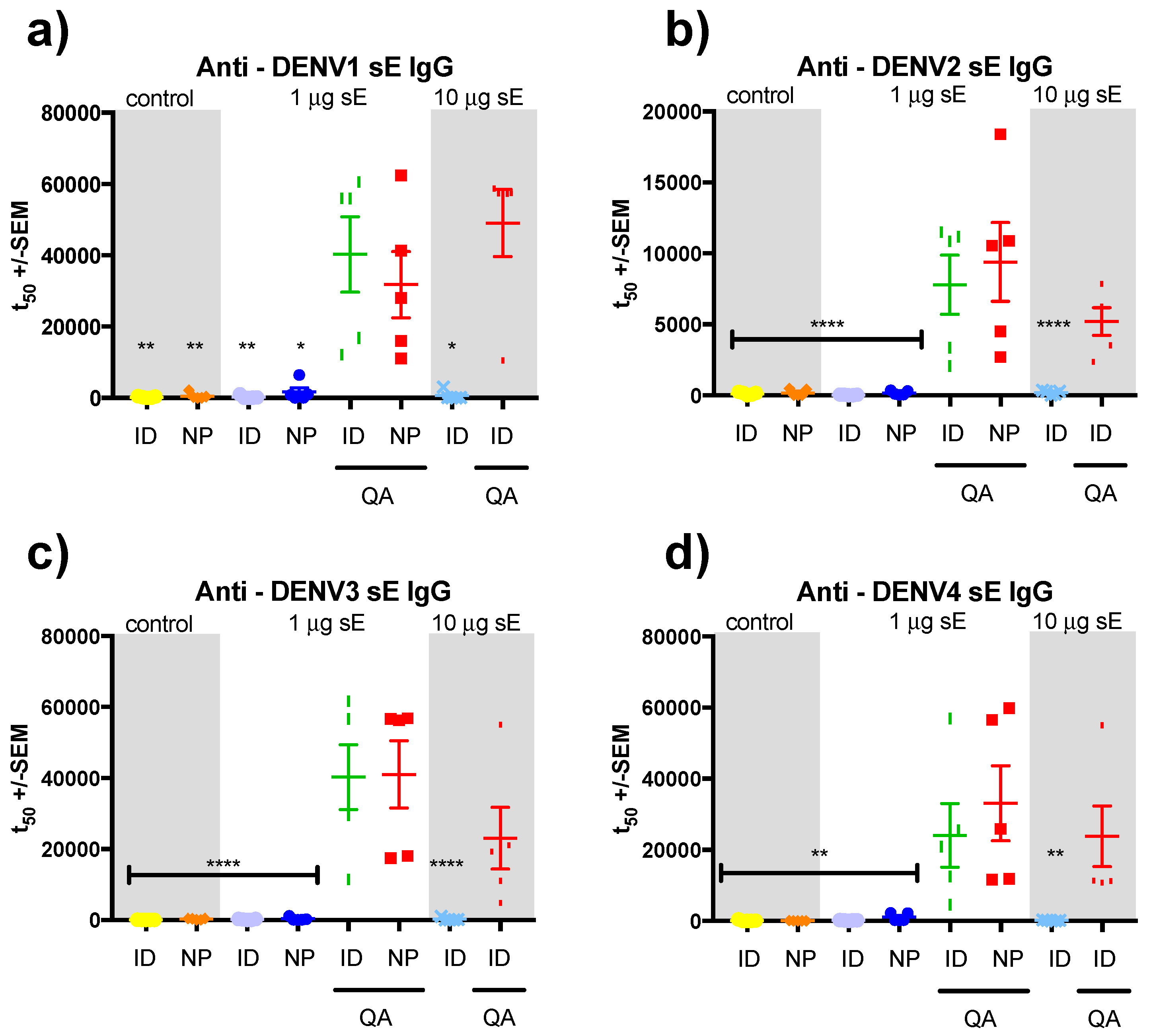
3.2. sE Immunization of SV129 Mice
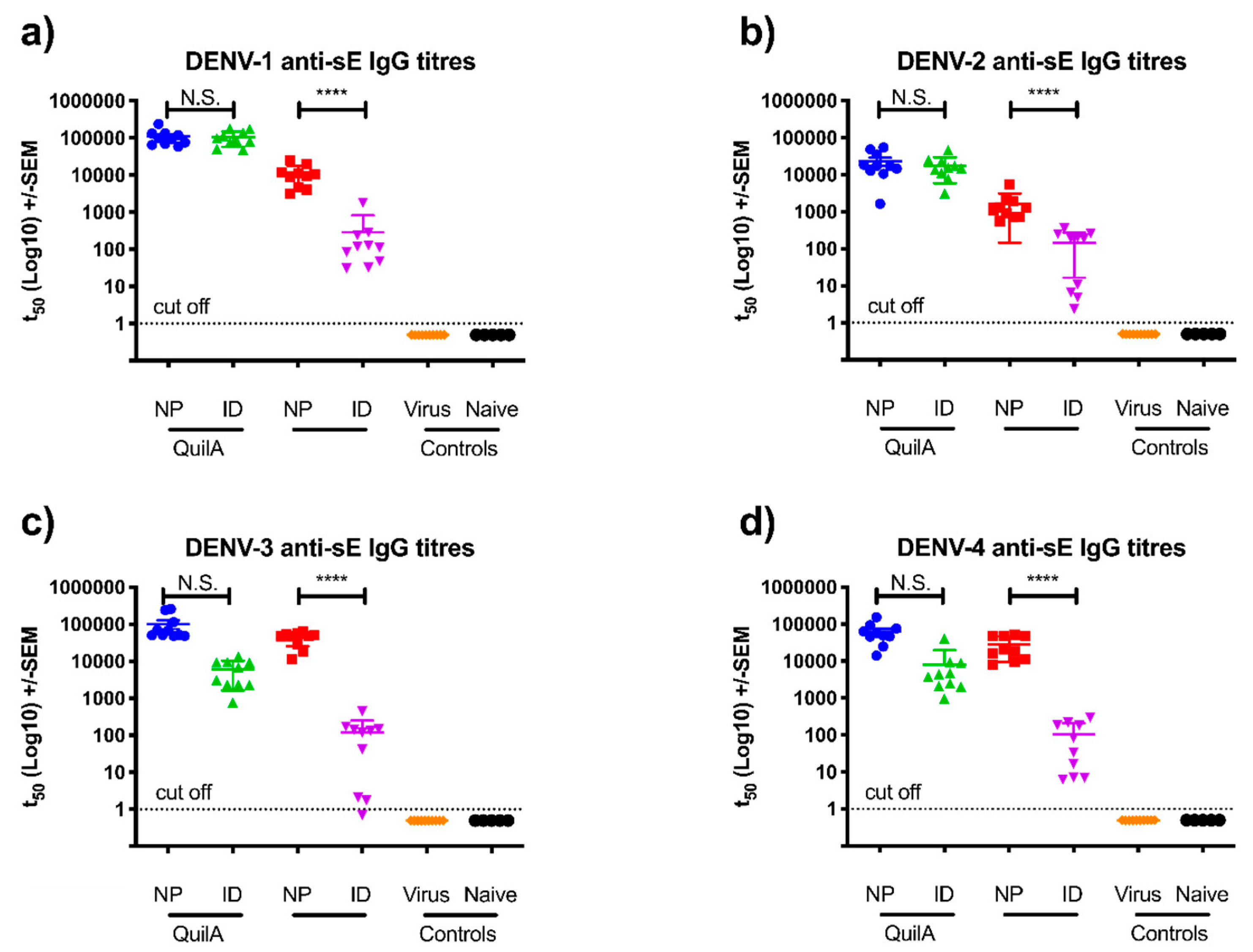
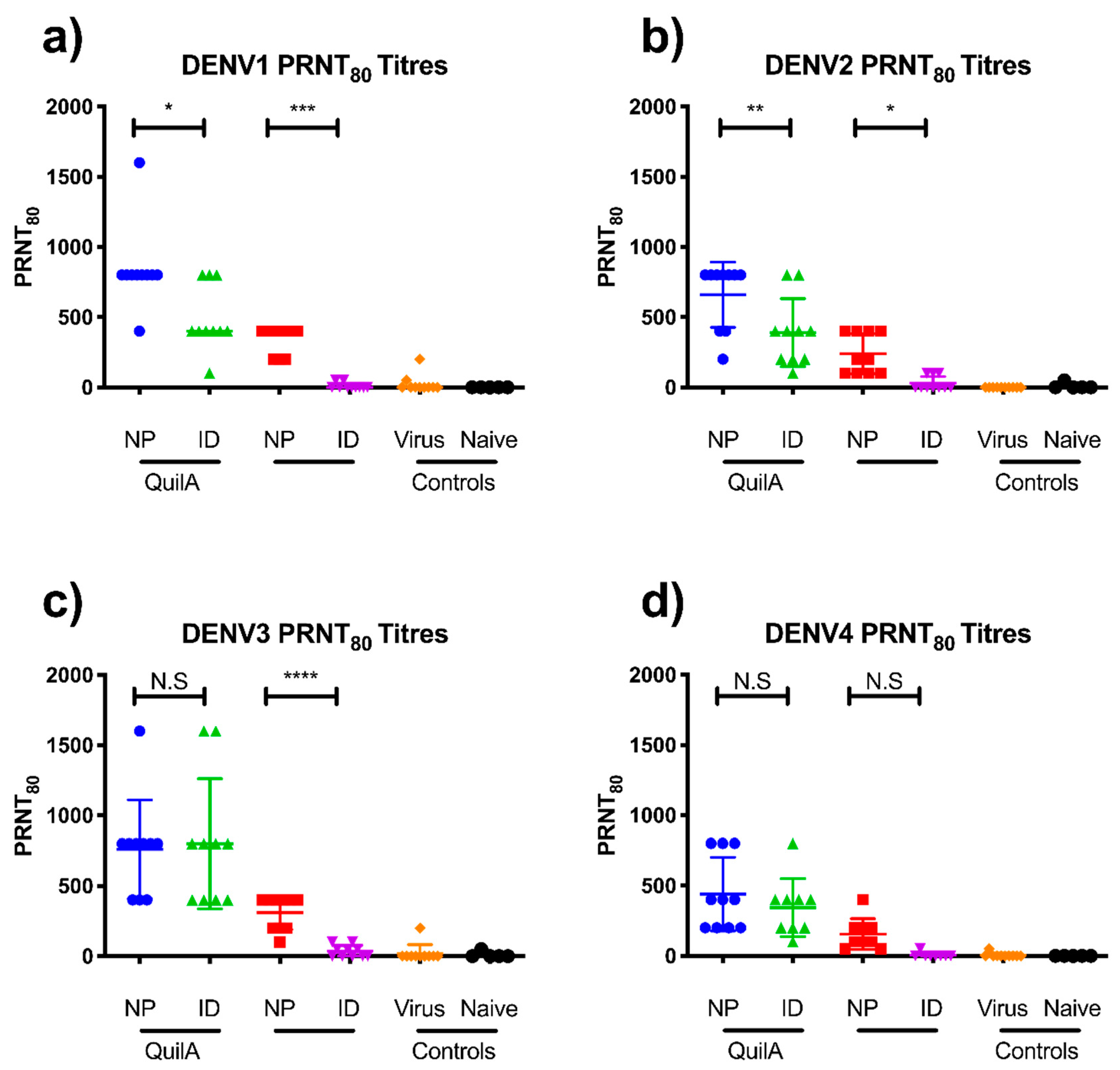
3.3. sE Nanopatch Immunization of AG129 Mice
3.4. DENV Challenge of Vaccinated AG129 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Halstead, S.B. Etiologies of the experimental dengues of Siler and Simmons. Am. J. Trop. Med. Hyg. 1974, 23, 974–982. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Lee, Y.S.; Namkung, S.; Lim, J.K.; Yoon, I.K. Prospects for dengue vaccines for travelers. Clin. Exp. Vaccine Res. 2016, 5, 89–100. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Rodriguez-Barraquer, I.; Dorigatti, I.; Mier, Y.T.-R.L.; Laydon, D.J.; Cummings, D.A. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 2016, 353, 1033–1036. [Google Scholar] [CrossRef]
- WHO. WHO Advises Dengvaxia be used only in People Infected with Dengue. Available online: http://www.who.int/medicines/news/2017/WHO-advises-dengvaxia-used-only-in-people-previously-infected/en/ (accessed on 19 January 2018).
- Clements, D.E.; Coller, B.A.; Lieberman, M.M.; Ogata, S.; Wang, G.; Harada, K.E.; Putnak, J.R.; Ivy, J.M.; McDonell, M.; Bignami, G.S.; et al. Development of a recombinant tetravalent dengue virus vaccine: Immunogenicity and efficacy studies in mice and monkeys. Vaccine 2010, 28, 2705–2715. [Google Scholar] [CrossRef]
- da Costa, V.G.; Marques-Silva, A.C.; Floriano, V.G.; Moreli, M.L. Safety, immunogenicity and efficacy of a recombinant tetravalent dengue vaccine: A meta-analysis of randomized trials. Vaccine 2014, 32, 4885–4892. [Google Scholar] [CrossRef]
- Gouvea, I.E.; Izidoro, M.A.; Judice, W.A.; Cezari, M.H.; Caliendo, G.; Santagada, V.; dos Santos, C.N.; Queiroz, M.H.; Juliano, M.A.; Young, P.R.; et al. Substrate specificity of recombinant dengue 2 virus NS2B-NS3 protease: Influence of natural and unnatural basic amino acids on hydrolysis of synthetic fluorescent substrates. Arch. Biochem. Biophys. 2007, 457, 187–196. [Google Scholar] [CrossRef]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Smith, J.S.; Wolf, J.J.; Gindy, M.E.; Casimiro, D.R.; Bett, A.J. A tetravalent sub-unit dengue vaccine formulated with ionizable cationic lipid nanoparticle induces significant immune responses in rodents and non-human primates. Sci. Rep. 2016, 6, 34215. [Google Scholar] [CrossRef]
- Crichton, M.L.; Ansaldo, A.; Chen, X.; Prow, T.W.; Fernando, G.J.; Kendall, M.A. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials 2010, 31, 4562–4572. [Google Scholar] [CrossRef]
- Fernando, G.J.; Chen, X.; Primiero, C.A.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control. Release 2012, 159, 215–221. [Google Scholar] [CrossRef]
- Pearson, F.E.; McNeilly, C.L.; Crichton, M.L.; Primiero, C.A.; Yukiko, S.R.; Fernando, G.J.; Chen, X.; Gilbert, S.C.; Hill, A.V.; Kendall, M.A. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS ONE 2013, 8, e67888. [Google Scholar] [CrossRef]
- Prow, T.W.; Chen, X.; Prow, N.A.; Fernando, G.J.; Tan, C.S.; Raphael, A.P.; Chang, D.; Ruutu, M.P.; Jenkins, D.W.; Pyke, A.; et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small 2010, 6, 1776–1784. [Google Scholar] [CrossRef]
- Muller, D.A.; Pearson, F.E.; Fernando, G.J.; Agyei-Yeboah, C.; Owens, N.S.; Corrie, S.R.; Crichton, M.L.; Wei, J.C.; Weldon, W.C.; Oberste, M.S.; et al. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci. Rep. 2016, 6, 22094. [Google Scholar] [CrossRef]
- Muller, D.A.; Fernando, G.J.P.; Owens, N.S.; Agyei-Yeboah, C.; Wei, J.C.J.; Depelsenaire, A.C.I.; Forster, A.; Fahey, P.; Weldon, W.C.; Oberste, M.S.; et al. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci. Rep. 2017, 7, 12644. [Google Scholar] [CrossRef]
- Corbett, H.J.; Fernando, G.J.; Chen, X.; Frazer, I.H.; Kendall, M.A. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS ONE 2010, 5, e13460. [Google Scholar] [CrossRef]
- Pearson, F.E.; Muller, D.A.; Roalfe, L.; Zancolli, M.; Goldblatt, D.; Kendall, M.A. Functional anti-polysaccharide IgG titres induced by unadjuvanted pneumococcal-conjugate vaccine when delivered by microprojection-based skin patch. Vaccine 2015, 33, 6675–6683. [Google Scholar] [CrossRef]
- Crichton, M.L.; Muller, D.A.; Depelsenaire, A.C.; Pearson, F.E.; Wei, J.; Coffey, J.; Zhang, J.; Fernando, G.J.; Kendall, M.A. The changing shape of vaccination: Improving immune responses through geometrical variations of a microdevice for immunization. Sci. Rep. 2016, 6, 27217. [Google Scholar] [CrossRef]
- Fernando, G.J.; Chen, X.; Prow, T.W.; Crichton, M.L.; Fairmaid, E.J.; Roberts, M.S.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE 2010, 5, e10266. [Google Scholar] [CrossRef]
- Watterson, D.; Robinson, J.; Chappell, K.J.; Butler, M.S.; Edwards, D.J.; Fry, S.R.; Bermingham, I.M.; Cooper, M.A.; Young, P.R. A generic screening platform for inhibitors of virus induced cell fusion using cellular electrical impedance. Sci. Rep. 2016, 6, 22791. [Google Scholar] [CrossRef]
- Li, J.; Watterson, D.; Chang, C.W.; Che, X.Y.; Li, X.Q.; Ericsson, D.J.; Qiu, L.W.; Cai, J.P.; Chen, J.; Fry, S.R.; et al. Structural and functional characterization of a cross-reactive dengue virus neutralizing antibody that recognizes a cryptic epitope. Structure 2018, 26, 51e54–59e54. [Google Scholar] [CrossRef]
- Jenkins, D.; Corrie, S.R.; Flaim, C.; Kendall, M.A. High density and high aspect ratio solid micro-nanoprojection arrays for targeted skin vaccine delivery and specific antibody extraction. RSC Adv. 2012, 2, 3490–3495. [Google Scholar] [CrossRef]
- Chen, X.; Kask, A.S.; Crichton, M.L.; McNeilly, C.; Yukiko, S.; Dong, L.; Marshak, J.O.; Jarrahian, C.; Fernando, G.J.; Chen, D.; et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Control. Release 2010, 148, 327–333. [Google Scholar] [CrossRef]
- Young, P.R. Antigenic analysis of dengue virus using monoclonal antibodies. Southeast Asian J. Trop. Med. Public Health 1990, 21, 646–651. [Google Scholar]
- Muller, D.A.; Corrie, S.R.; Coffey, J.; Young, P.R.; Kendall, M.A. Surface modified microprojection arrays for the selective extraction of the dengue virus NS1 protein as a marker for disease. Anal. Chem. 2012, 84, 3262–3268. [Google Scholar] [CrossRef]
- Sarathy, V.V.; Milligan, G.N.; Bourne, N.; Barrett, A.D. Mouse models of dengue virus infection for vaccine testing. Vaccine 2015, 33, 7051–7060. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Shresta, S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Depelsenaire, A.C.; Meliga, S.C.; McNeilly, C.L.; Pearson, F.E.; Coffey, J.W.; Haigh, O.L.; Flaim, C.J.; Frazer, I.H.; Kendall, M.A. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J. Investig. Dermatol. 2014, 134, 2361–2370. [Google Scholar] [CrossRef]
- Moyle, P.M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017, 35, 375–389. [Google Scholar] [CrossRef]
- Ng, H.I.; Fernando, G.J.; Depelsenaire, A.C.; Kendall, M.A. Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing. Sci. Rep. 2016, 6, 29368. [Google Scholar] [CrossRef]
- Ng, H.I.; Tuong, Z.K.; Fernando, G.J.P.; Depelsenaire, A.C.I.; Meliga, S.C.; Frazer, I.H.; Kendall, M.A.F. Microprojection arrays applied to skin generate mechanical stress, induce an inflammatory transcriptome and cell death, and improve vaccine-induced immune responses. NPJ Vaccines 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fernando, G.J.; Crichton, M.L.; Flaim, C.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Primiero, C.A.; Ansaldo, A.B.; Frazer, I.H.; et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J. Control. Release 2011, 152, 349–355. [Google Scholar] [CrossRef]
- Govindarajan, D.; Meschino, S.; Guan, L.M.; Clements, D.E.; ter Meulenc, J.H.; Casimiro, D.R.; Collera, B.A.G.; Bett, A.J. Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates. Vaccine 2015, 33, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.A.; Clements, D.E.; Bett, A.J.; Sagar, S.L.; Ter Meulen, J.H. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 2011, 29, 7267–7275. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garcon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Brewoo, J.N.; Kinney, R.M.; Powell, T.D.; Arguello, J.J.; Silengo, S.J.; Partidos, C.D.; Huang, C.Y.; Stinchcomb, D.T.; Osorio, J.E. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine 2012, 30, 1513–1520. [Google Scholar] [CrossRef]
- Hertz, T.; Beatty, P.R.; MacMillen, Z.; Killingbeck, S.S.; Wang, C.; Harris, E. Antibody epitopes identified in critical regions of dengue virus nonstructural 1 protein in mouse vaccination and natural human infections. J. Immunol. 2017, 198, 4025–4035. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, D.A.; Depelsenaire, A.C.I.; Shannon, A.E.; Watterson, D.; Corrie, S.R.; Owens, N.S.; Agyei-Yeboah, C.; Cheung, S.T.M.; Zhang, J.; Fernando, G.J.P.; et al. Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. Vaccines 2019, 7, 189. https://doi.org/10.3390/vaccines7040189
Muller DA, Depelsenaire ACI, Shannon AE, Watterson D, Corrie SR, Owens NS, Agyei-Yeboah C, Cheung STM, Zhang J, Fernando GJP, et al. Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. Vaccines. 2019; 7(4):189. https://doi.org/10.3390/vaccines7040189
Chicago/Turabian StyleMuller, David A., Alexandra C. I. Depelsenaire, Ashleigh E. Shannon, Daniel Watterson, Simon R. Corrie, Nick S. Owens, Christiana Agyei-Yeboah, Stacey T. M. Cheung, Jin Zhang, Germain J. P. Fernando, and et al. 2019. "Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays" Vaccines 7, no. 4: 189. https://doi.org/10.3390/vaccines7040189
APA StyleMuller, D. A., Depelsenaire, A. C. I., Shannon, A. E., Watterson, D., Corrie, S. R., Owens, N. S., Agyei-Yeboah, C., Cheung, S. T. M., Zhang, J., Fernando, G. J. P., Kendall, M. A. F., & Young, P. R. (2019). Efficient Delivery of Dengue Virus Subunit Vaccines to the Skin by Microprojection Arrays. Vaccines, 7(4), 189. https://doi.org/10.3390/vaccines7040189




