A Vaccinology Approach to the Identification and Characterization of Dermanyssus gallinae Candidate Protective Antigens for the Control of Poultry Red Mite Infestations
Abstract
1. Introduction
2. Materials and Methods
2.1. Mite Collection and Proteins Extraction
2.2. Proteomics Data Acquisition and Analysis
2.3. Criteria for Selection of Candidate Protective Antigens
2.4. Cloning of Antigens and Production of Recombinant Proteins
2.5. Sequence Analysis for Deg-CALU
2.6. Vaccine Formulations
2.7. Hen Vaccination and PRM Infestation
2.8. Vaccine Efficacy
2.9. Analysis of Hen IgY Antibody Response by ELISA
2.10. Statistical Analysis
2.11. Ethics Approval
3. Results and Discussion
3.1. Proteomics Analysis of D. gallinae Development Stages and Feeding Status
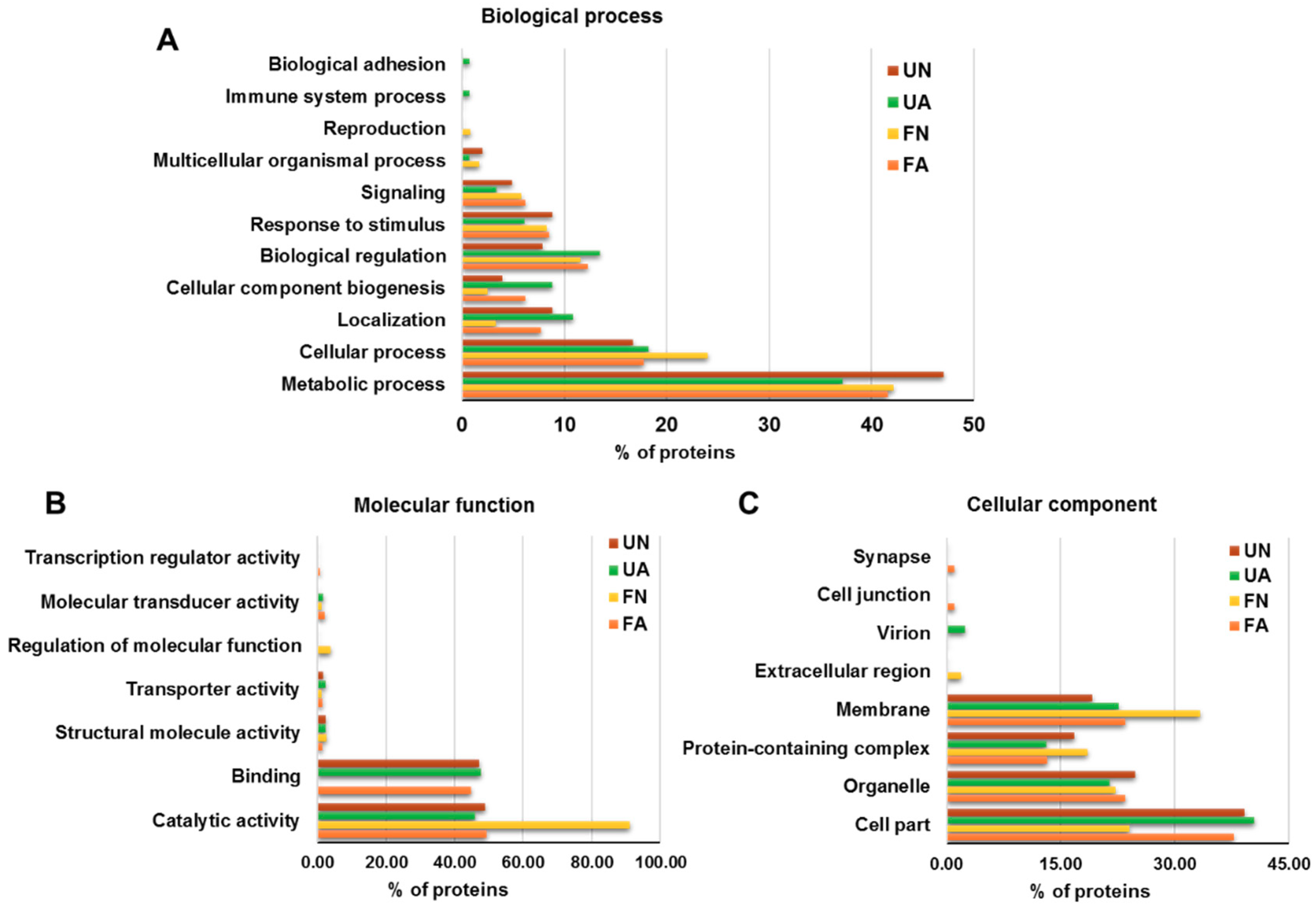
3.2. Functional Analysis of Identified Proteins in D. gallinae Development Stages and Feeding Status
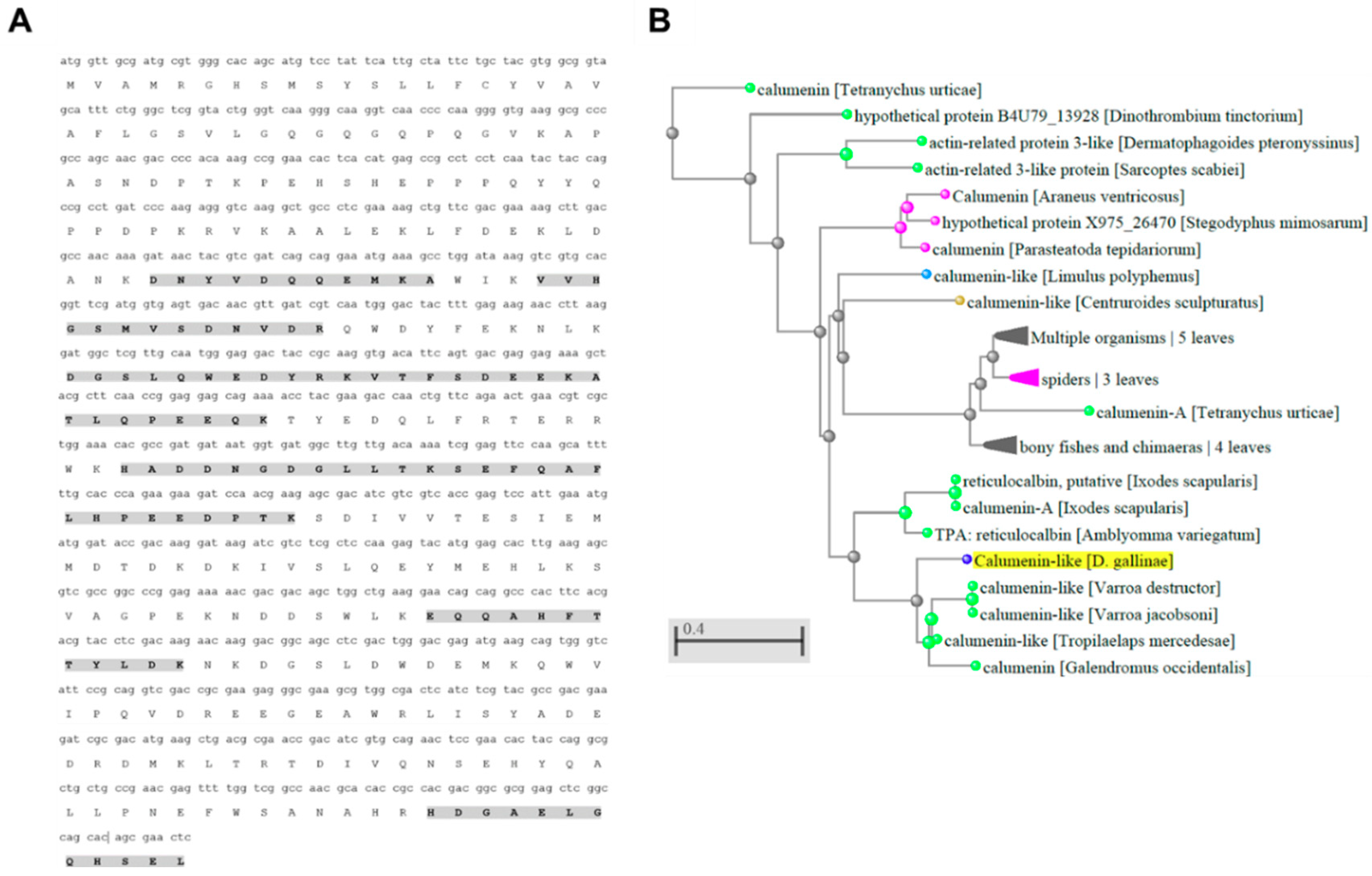
3.3. Characterization of PRM Candidate Protective Antigens
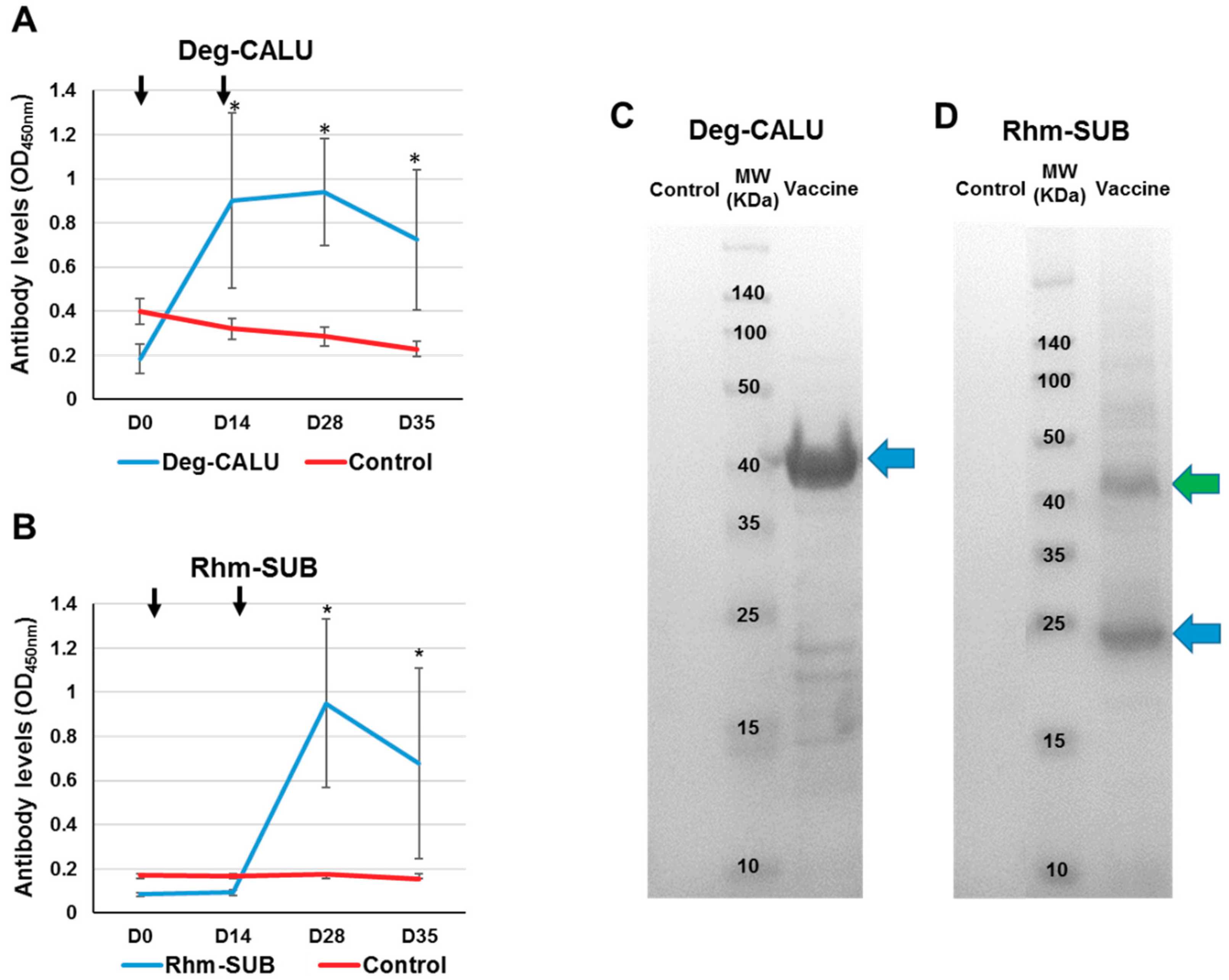
3.4. Immune Response to Vaccination
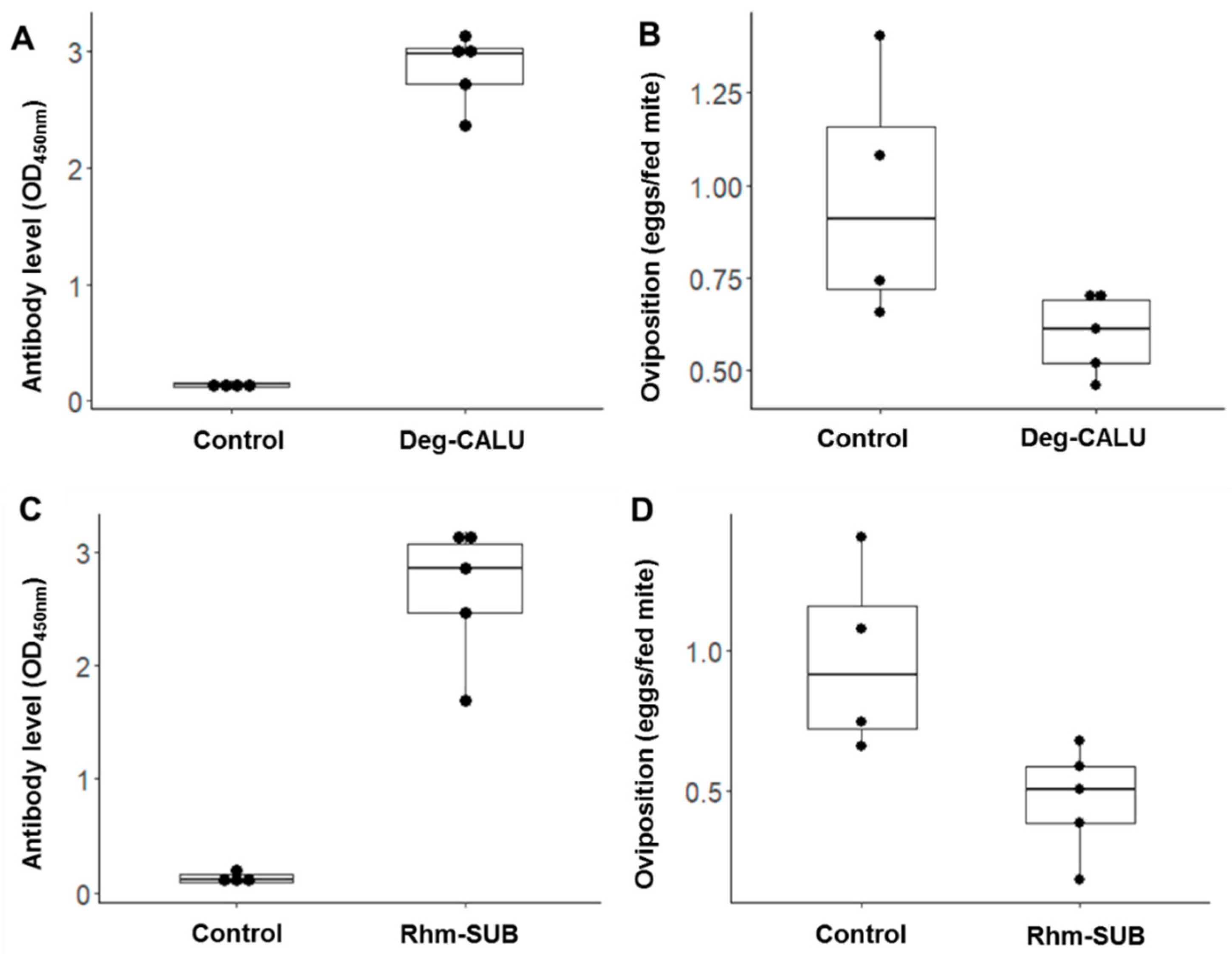
3.5. Vaccines Protective Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chauve, C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): Current situation and future prospects for control. Vet. Parasitol. 1998, 79, 239–245. [Google Scholar] [CrossRef]
- Sparagano, O.A.E.; George, D.R.; Harrington, D.W.J.; Giangaspero, A. Significance and Control of the Poultry Red Mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014, 59, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Maurer, V.; Bieri, M.; Foelsch, D.W. Das Suchverhalten von Dermanyssus gallinae in Huhnerstallen. Host-finding of Dermanyssus gallinae in poultry-houses. Eur. Poult. Sci. 1988, 52, 209–215. [Google Scholar]
- Koenraadt, C.J.M.; Dicke, M. The role of volatiles in aggregation and host-seeking of the haematophagous poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae). Exp. Appl. Acarol. 2010, 50, 191–199. [Google Scholar] [CrossRef]
- Roy, L.; Chauve, C.M.; Buronfosse, T. Contrasted ecological repartition of the Northern Fowl Mite Ornithonyssus sylviarum (Mesostigmata: Macronyssidae) and the Chicken Red Mite Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Acarologia 2010, 50, 207–219. [Google Scholar] [CrossRef][Green Version]
- Kilpinen, O. Activation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae), by increasing temperatures. Exp. Appl. Acarol. 2001, 25, 859–867. [Google Scholar] [CrossRef]
- Cosoroaba, I. Massive Dermanyssus gallinae invasion in battery-husbandry raised fowls. Rev. De Méd. Vét. 2001, 152, 89–96. [Google Scholar]
- Kilpinen, O.; Roepstorff, A.; Permin, A.; Nørgaard-Nielsen, G.; Lawson, L.G.; Simonsen, H.B. Influence of Dermanyssus gallinae and infections on behaviour and health of laying hens (Gallus gallus domesticus). Br. Poult. Sci. 2005, 46, 26–34. [Google Scholar] [CrossRef]
- Sigognault Flochlay, A.; Thomas, E.; Sparagano, O.A.E. Poultry red mite (Dermanyssus gallinae) infestation: A broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasites Vectors 2017, 10, 357. [Google Scholar] [CrossRef]
- Sommer, D.; Heffels-Redmann, U.; Köhler, K.; Lierz, M.; Kaleta, E.F. Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 26–33. [Google Scholar] [CrossRef]
- Valiente Moro, C.; De Luna, C.J.; Tod, A.; Guy, J.H.; Sparagano, O.A.E.; Zenner, L. The poultry red mite (Dermanyssus gallinae): A potential vector of pathogenic agents. Exp. Appl. Acarol. 2009, 48, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mul, M.F.; Van Niekerk, T.; Chirico, J.; Maurer, V.; Kilpinen, O.; Sparagano, O.; Thind, B.; Zoons, J.; Moore, D.; Bell, B.; et al. Control methods for Dermanyssus gallinae in systems for laying hens: Results of an international seminar. Worlds Poult. Sci. J. 2009, 65, 589–600. [Google Scholar] [CrossRef]
- Van Emous, R.A. Verwachtte Schade Bloedluis 21 Miljoen Euro. Available online: https://www.pluimveeweb.nl/artikelen/2017/01/schade-bloedluis-21-miljoen-euro/ (accessed on 5 January 2018).
- Marangi, M.; Cafiero, M.A.; Capelli, G.; Camarda, A.; Sparagano, O.A.; Giangaspero, A. Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp. Appl. Acarol. 2009, 48, 11–18. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Moreno-Cid, J.A.; Canales, M.; Villar, M.; de la Lastra, J.M.; Kocan, K.M.; Galindo, R.C.; Almazán, C.; Blouin, E.F. Targeting arthropod Subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 2011, 181, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bartley, K.; Wright, H.W.; Huntley, J.F.; Manson, E.D.; Inglis, N.F.; McLean, K.; Nath, M.; Bartley, Y.; Nisbet, A.J. Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae). Int. J. Parasitol. 2015, 45, 819–830. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Bartley, K.; Huntley, J.F.; Wright, H.W.; Nath, M.; Nisbet, A.J. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology 2012, 139, 755–765. [Google Scholar] [CrossRef]
- Makert, G.R.; Vorbrüggen, S.; Krautwald-Junghanns, M.E.; Voss, M.; Sohn, K.; Buschmann, T.; Ulbert, S. A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitol. Res. 2016, 115, 2705–2713. [Google Scholar] [CrossRef]
- Schicht, S.; Qi, W.; Poveda, L.; Strube, C. Whole transcriptome analysis of the poultry red mite Dermanyssus gallinae (De Geer, 1778). Parasitology 2014, 141, 336–346. [Google Scholar] [CrossRef]
- Burgess, S.T.G.; Bartley, K.; Nunn, F.; Wright, H.W.; Hughes, M.; Gemmell, M.; Haldenby, S.; Paterson, S.; Rombauts, S.; Tomley, F.M.; et al. Draft Genome Assembly of the Poultry Red Mite, Dermanyssus gallinae. Microbiol. Resour. Announc. 2018, 7, e01221-18. [Google Scholar] [CrossRef]
- Harrington, D.; Canales, M.; de la Fuente, J.; de Luna, C.; Robinson, K.; Guy, J.; Sparagano, O. Immunisation with recombinant proteins Subolesin and Bm86 for the control of Dermanyssus gallinae in poultry. Vaccine 2009, 27, 4056–4063. [Google Scholar] [CrossRef] [PubMed]
- Lima-Barbero, J.F.; Díaz-Sanchez, S.; Sparagano, O.; Finn, R.D.; de la Fuente, J.; Villar, M. Metaproteomics characterization of the alphaproteobacteria microbiome in different developmental and feeding stages of the poultry red mite Dermanyssus gallinae (De Geer, 1778). Avian Pathol. 2019, 1, 179. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.; Popara, M.; Mangold, A.J.; de la Fuente, J. Comparative proteomics for the characterization of the most relevant Amblyomma tick species as vectors of zoonotic pathogens worldwide. J. Proteom. 2014, 105, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Schicht, S.; Qi, W.; Poveda, L.; Strube, C. The predicted secretome and transmembranome of the poultry red mite Dermanyssus gallinae. Parasites Vectors 2013, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org (accessed on 10 September 2018).
- Wright, H.W.; Bartley, K.; Huntley, J.F.; Nisbet, A.J. Characterisation of tropomyosin and paramyosin as vaccine candidate molecules for the poultry red mite, Dermanyssus gallinae. Parasites Vectors 2016, 9, 544. [Google Scholar] [CrossRef]
- Bartley, K.; Nisbet, A.J.; Offer, J.E.; Sparks, N.H.; Wright, H.W.; Huntley, J.F. Histamine Release Factor from Dermanyssus gallinae (De Geer): Characterization and in vitro assessment as a protective antigen. Int. J. Parasitol. 2009, 39, 447–456. [Google Scholar] [CrossRef]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef]
- Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Villar, M.; Jiménez, M.; Pinal, R.; Estrada-Peña, A.; Molina, R.; Lucientes, J.; Gortázar, C.; de la Fuente, J. Control of multiple arthropod vector infestations with Subolesin/akirin vaccines. Vaccine 2013, 31, 1187–1196. [Google Scholar] [CrossRef]
- Merino, O.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- Price, D.R.; Küster, T.; Øines, Ø.; Margaret Oliver, E.; Bartley, K.; Nunn, F.; Lima-Barbero, J.F.; Pritchard, J.; Karp-Tatham, E.; Hauge, H.; et al. Evaluation of vaccine delivery systems for inducing long-lived antibody responses to Dermanyssus gallinae. Avian Pathol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nunn, F.; Bartley, K.; Palarea-Albaladejo, J.; Innocent, G.T.; Turnbull, F.; Wright, H.W.; Nisbet, A.J. A novel, high-welfare methodology for evaluating poultry red mite interventions in vivo. Vet. Parasitol. 2019, 267, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bartley, K.; Turnbull, F.; Wright, H.W.; Huntley, J.F.; Palarea-Albaladejo, J.; Nath, M.; Nisbet, A.J. Field evaluation of poultry red mite (Dermanyssus gallinae) native and recombinant prototype vaccines. Vet. Parasitol. 2017, 244, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; Villar, M.; Cabezas-Cruz, A.; Valdés, J.J.; Estrada-Peña, A.; Alberdi, P.; de la Fuente, J. Functional evolution of Subolesin/Akirin. Front. Physiol. 2018, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, D.H. Characterization of isoforms and genomic organization of mouse calumenin. Gene 2004, 327, 185–194. [Google Scholar] [CrossRef]
- Vorum, H.; Jacobsen, C.; Honoré, B. Calumenin interacts with serum amyloid P component. FEBS Lett. 2000, 465, 129–134. [Google Scholar] [CrossRef]
- Honoré, B. The rapidly expanding CREC protein family: Members, localization, function, and role in disease. BioEssays 2009, 31, 262–277. [Google Scholar] [CrossRef]
- Cho, J.H.; Song, H.; Singaravelu, G.; Sung, H.; Oh, W.; Kwon, S.; Kim, D.H.; Ahnn, J. Pleiotropic roles of calumenin (calu-1), a calcium-binding ER luminal protein, in Caenorhabditis elegans. FEBS Lett. 2009, 583, 3050–3056. [Google Scholar] [CrossRef]
- Figueiredo, B.C.; Ricci, N.D.; de Assis, N.R.G.; de Morais, S.B.; Fonseca, C.T.; Oliveira, S.C. Kicking in the guts: Schistosoma mansoni digestive tract proteins are potential candidates for vaccine development. Front. Immunol. 2015, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Estrada-Peña, A.; Kocan, K.M.; de la Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of Subolesin vaccination and tick autocidal control after Subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
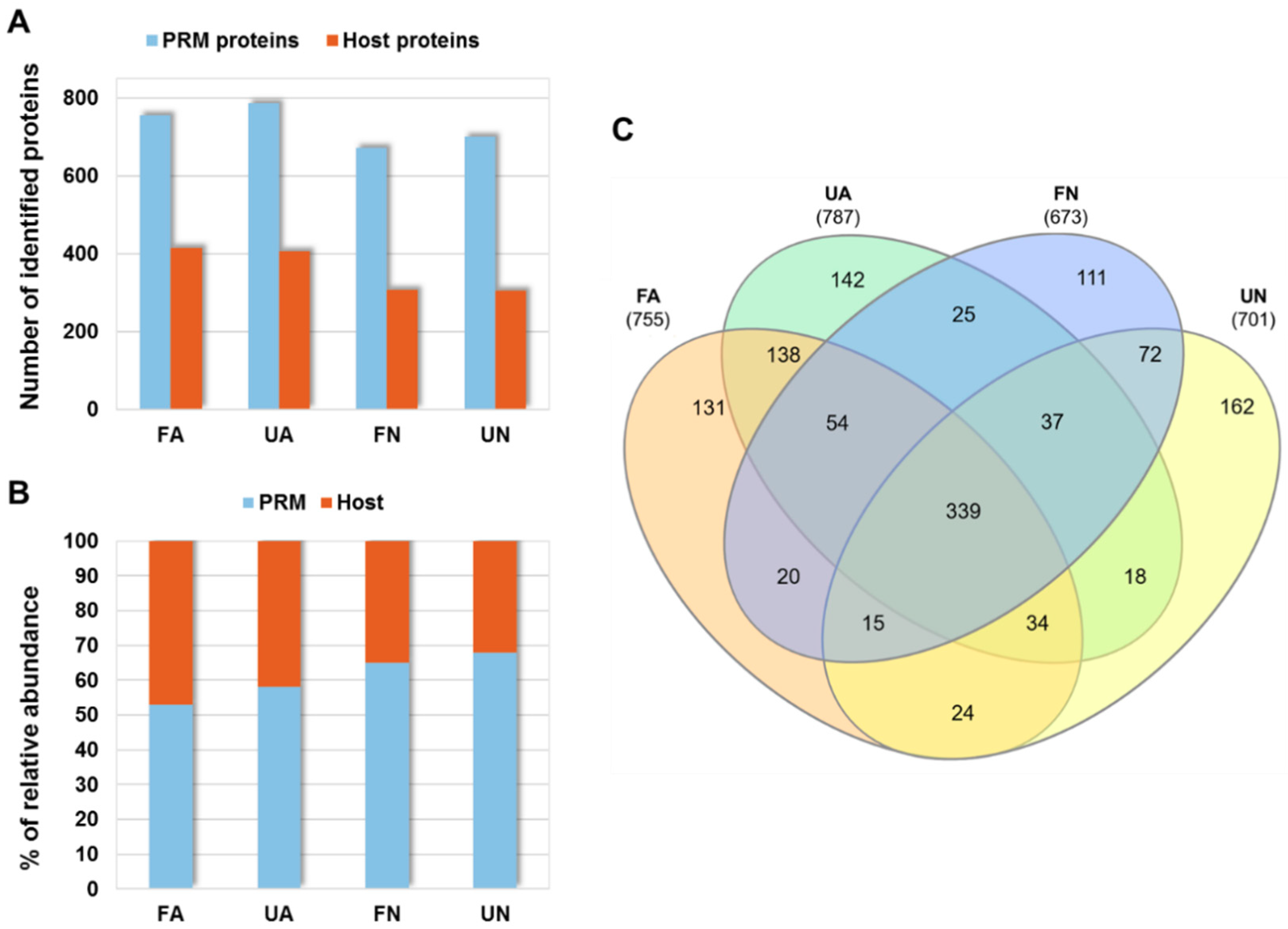
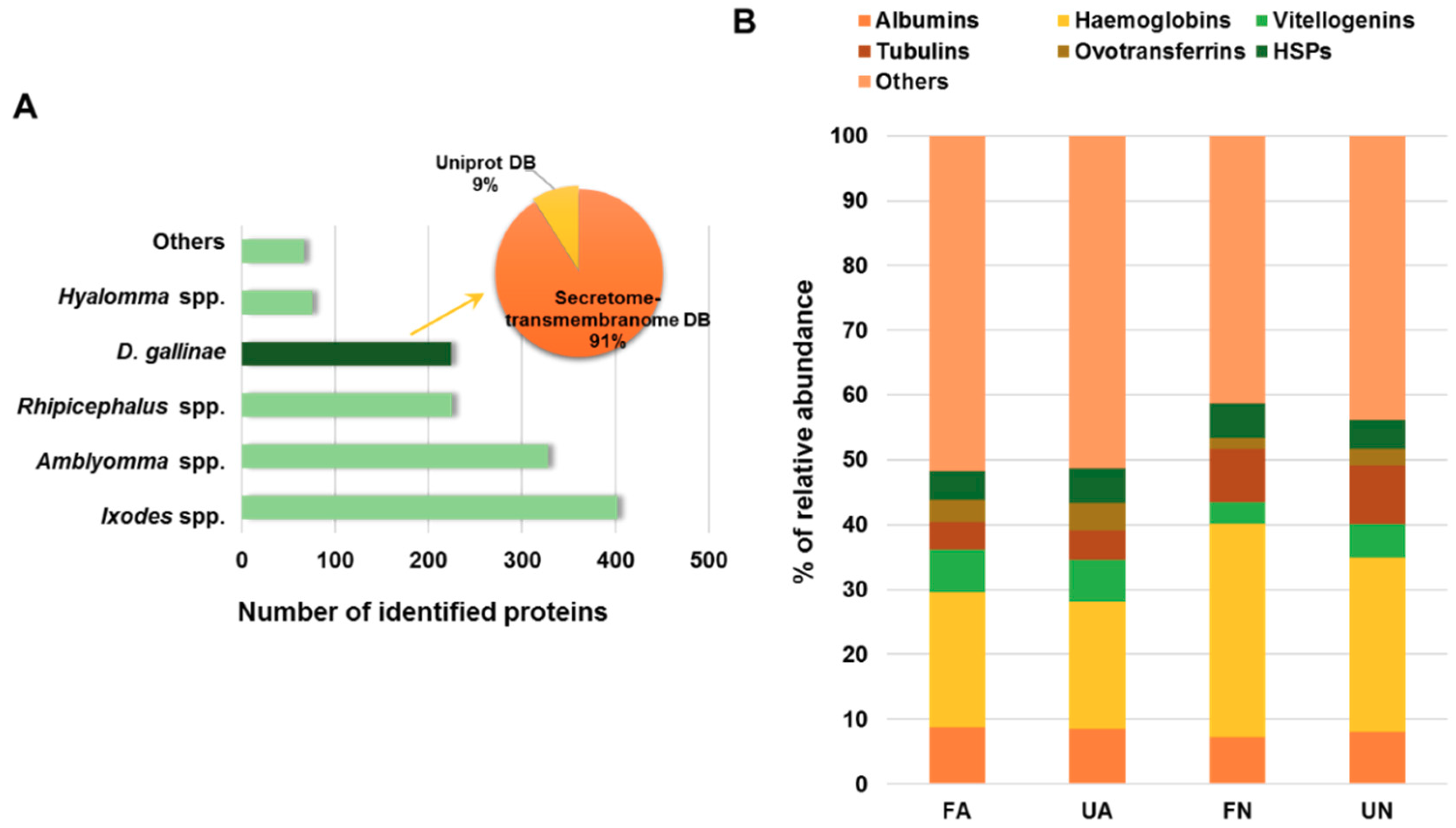
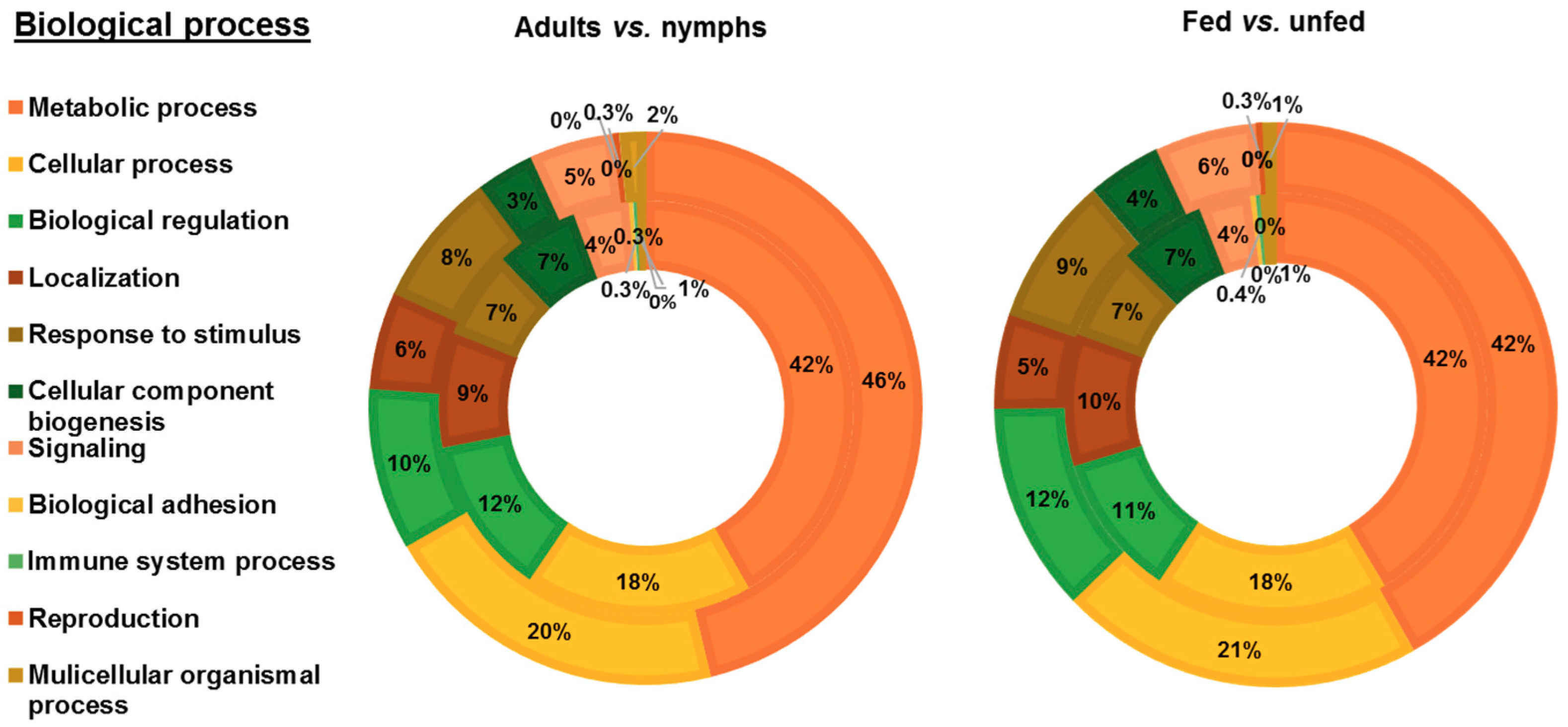
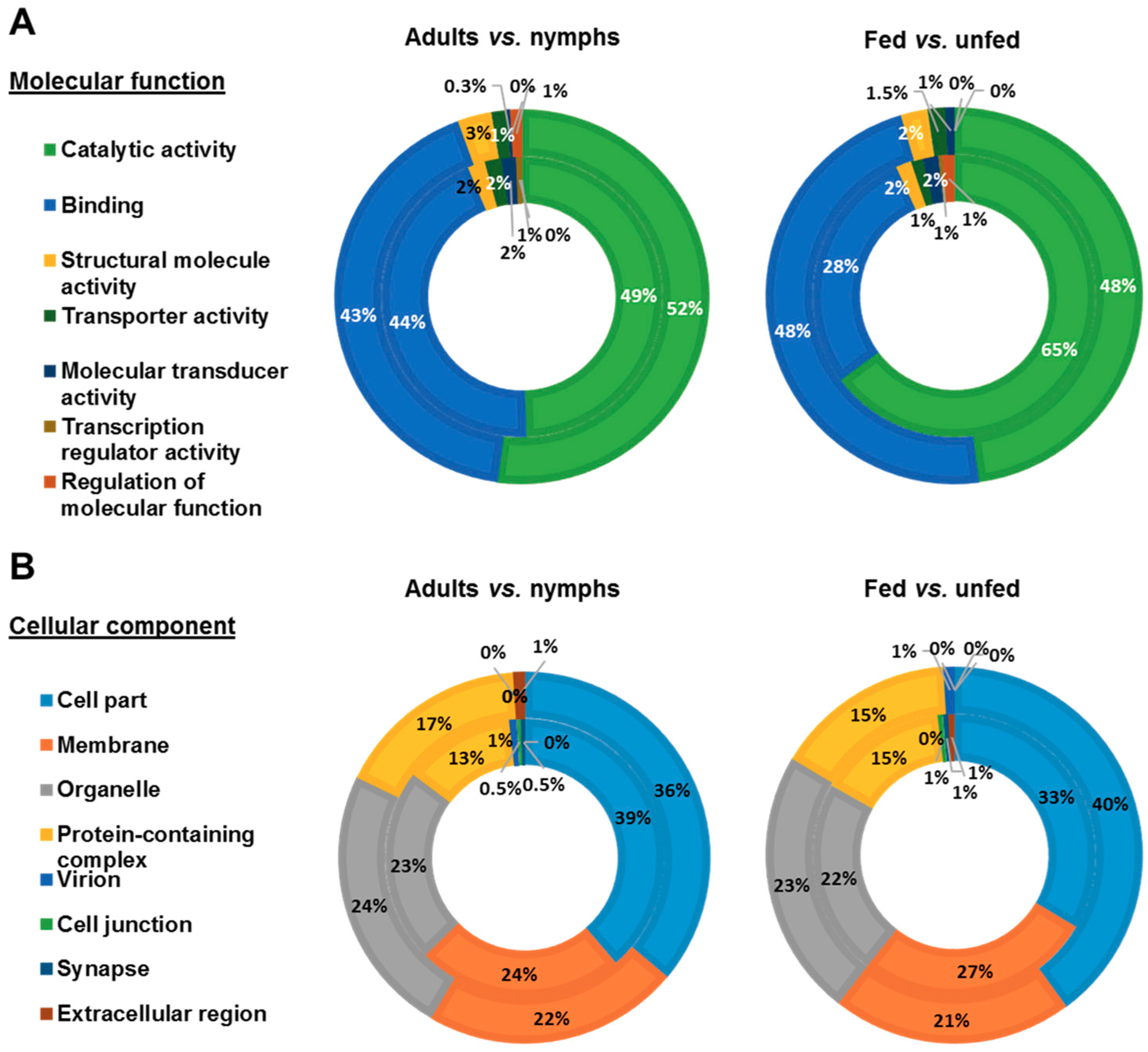
| Accession No. a | Description | Fold Change b | χ2 p-Value | Significance c |
|---|---|---|---|---|
| isotig10396 | Peptidyl-prolyl cis-trans isomerase | ∞ | 0.040 | FA vs. UA |
| 0 | 0.025 | UA vs. UN | ||
| isotig11213 | Vitellogenin 2 | 1.54 | 0.000 | FN vs. UN |
| 1.44 | 0.001 | UA vs. UN | ||
| isotig15430 | Vitellogenin 2 | 1.63 | 0.014 | FA vs. FN |
| 1.36 | 0.037 | UA vs. UN | ||
| A0A0M4FBG8 | Vitellogenin 1 (D. gallinae) | 1.65 | 0.000 | FN vs. UN |
| 0.70 | 0.006 | FA vs. FN | ||
| S5GFP7 | Vitellogenin 2 (Neoseiulus cucumeris) | 0.38 | 0.019 | FA vs. FN |
| 4.20 | 0.002 | FN vs. UN | ||
| 3.00 | 0.027 | UA vs. UN | ||
| B5B8U1 | Histamine release factor (D. gallinae) | 0.29 | 0.020 | FN vs. UN |
| A0A131Y6P3 | Ribosomal protein s7 (Ixodes ricinus) | 0.27 | 0.031 | FN vs. UN |
| 0.18 | 0.012 | UA vs. UN | ||
| A0A023GGI9 | Chromatin remodeling complex rsc subunit rsc1/polybromo (Amblyomma triste) | ∞ | 0.044 | FN vs. UN |
| A0A1R3S3F0 | Paramyosin (D. gallinae) | 0.45 | 0.018 | FA vs. FN |
| Q2WBI0 | Tropomyosin (D. gallinae) | 0.35 | 0.026 | FA vs. FN |
| isotig21684 | Cathepsin l precursor | 0.29 | 0.017 | UA vs. UN |
| isotig16123 | Cathepsin l | 0.22 | 0.033 | UA vs. UN |
| isotig20927 | Cathepsin l | 0 | 0.024 | UA vs. UN |
| isotig21530 | Cathepsin l-like | 0 | 0.014 | UA vs. UN |
| isotig21385 | Cathepsin s-like | 0 | 0.004 | UA vs. UN |
| isotig18930 | Calumenin isoform 2 | 3.33 | 0.047 | FA vs. FN |
| 3.67 | 0.035 | UA vs. UN | ||
| isotig12641 | Protein npc2-like protein | 0.27 | 0.031 | UA vs. UN |
| isotig11090 | Chymotrypsin b-like | 0 | 0.049 | FA vs. FN |
| 0 | 0.014 | UA vs. UN | ||
| isotig21611 | Cytotoxin-like protein | 0 | 0.027 | FA vs. FN |
| V5HC54 | Beta-spectrin (I. ricinus) | 3.33 | 0.047 | FA vs. FN |
| A0A131XHW1 | Beta-spectrin (Hyalomma excavatum) | 3.33 | 0.047 | FA vs. FN |
| isotig18820 | Deoxyribonuclease II | ∞ | 0.007 | FA vs. FN |
| A0A131Y3B8 | Uncharacterized protein (I. ricinus) | 19.00 | 0.000 | FA vs. FN |
| A0A1E1X6V0 | Conserved plasma membrane protein (A. aureolatum) | 3.25 | 0.025 | FA vs. FN |
| 6.50 | 0.005 | UA vs. UN | ||
| isotig08662 | PREDICTED: uncharacterized protein LOC100908559 | 0.22 | 0.033 | UA vs. UN |
| isotig13475 | PREDICTED: uncharacterized protein LOC100900008 | 0 | 0.027 | FA vs. FN |
| Antigen | Hen | Fed Mites | Unfed Mites | Total | % Fed | Average Feeding ± SD | % Reduction | Laying Mites | Total Viable Mites | % Laying | Average Laying ± SD | % Reduction | Egg | Eggs/Fed Mite | Average Oviposition ± SD | % Reduction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhm-SUB | 1 | 154 | 91 | 245 | 62.9 | 60 ± 7 | 15 | 20 | 135 | 15 | 15 ± 5 | 44 * | 59 | 0.44 | 0.48 ± 0.2 | 52 * |
| 2 | 126 | 60 | 186 | 67.7 | 25 | 125 | 20 | 85 | 0.68 | |||||||
| 3 | 82 | 82 | 164 | 50.0 | 5 | 80 | 6 | 15 | 0.19 | |||||||
| 4 | 153 | 115 | 268 | 57.1 | 26 | 148 | 18 | 79 | 0.60 | |||||||
| 5 | 178 | 112 | 290 | 61.4 | 27 | 171 | 16 | 87 | 0.51 | |||||||
| Deg-CALU | 6 | 230 | 84 | 314 | 73.2 | 65 ± 15 | 7 | 29 | 215 | 13 | 17 ± 4 | 35 * | 106 | 0.49 | 0.62 ± 0.1 | 38 * |
| 7 | 253 | 84 | 337 | 75.1 | 40 | 240 | 17 | 155 | 0.65 | |||||||
| 8 | 194 | 78 | 272 | 71.3 | 29 | 186 | 16 | 99 | 0.53 | |||||||
| 9 | 66 | 108 | 174 | 37.9 | 15 | 65 | 23 | 47 | 0.72 | |||||||
| 10 | 135 | 62 | 197 | 68.5 | 23 | 128 | 18 | 93 | 0.73 | |||||||
| Control | 16 | 202 | 45 | 247 | 81.8 | 70 ± 12 | 73 | 190 | 38 | 27 ± 8 | 281 | 1.48 | 1.01 ± 0.4 | |||
| 17 | 158 | 130 | 288 | 54.9 | 29 | 147 | 20 | 103 | 0.70 | |||||||
| 18 | 210 | 59 | 269 | 78.1 | 48 | 204 | 24 | 156 | 0.76 | |||||||
| 20 | 180 | 93 | 273 | 65.9 | 44 | 175 | 25 | 194 | 1.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Barbero, J.F.; Contreras, M.; Mateos-Hernández, L.; Mata-Lorenzo, F.M.; Triguero-Ocaña, R.; Sparagano, O.; Finn, R.D.; Strube, C.; Price, D.R.G.; Nunn, F.; et al. A Vaccinology Approach to the Identification and Characterization of Dermanyssus gallinae Candidate Protective Antigens for the Control of Poultry Red Mite Infestations. Vaccines 2019, 7, 190. https://doi.org/10.3390/vaccines7040190
Lima-Barbero JF, Contreras M, Mateos-Hernández L, Mata-Lorenzo FM, Triguero-Ocaña R, Sparagano O, Finn RD, Strube C, Price DRG, Nunn F, et al. A Vaccinology Approach to the Identification and Characterization of Dermanyssus gallinae Candidate Protective Antigens for the Control of Poultry Red Mite Infestations. Vaccines. 2019; 7(4):190. https://doi.org/10.3390/vaccines7040190
Chicago/Turabian StyleLima-Barbero, José Francisco, Marinela Contreras, Lourdes Mateos-Hernández, Francisco Manuel Mata-Lorenzo, Roxana Triguero-Ocaña, Olivier Sparagano, Robert D. Finn, Christina Strube, Daniel R.G. Price, Francesca Nunn, and et al. 2019. "A Vaccinology Approach to the Identification and Characterization of Dermanyssus gallinae Candidate Protective Antigens for the Control of Poultry Red Mite Infestations" Vaccines 7, no. 4: 190. https://doi.org/10.3390/vaccines7040190
APA StyleLima-Barbero, J. F., Contreras, M., Mateos-Hernández, L., Mata-Lorenzo, F. M., Triguero-Ocaña, R., Sparagano, O., Finn, R. D., Strube, C., Price, D. R. G., Nunn, F., Bartley, K., Höfle, U., Boadella, M., Nisbet, A. J., de la Fuente, J., & Villar, M. (2019). A Vaccinology Approach to the Identification and Characterization of Dermanyssus gallinae Candidate Protective Antigens for the Control of Poultry Red Mite Infestations. Vaccines, 7(4), 190. https://doi.org/10.3390/vaccines7040190








