Evaluation of Cross-Protection of African Swine Fever Vaccine ASFV-G-ΔI177L Between ASFV Biotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viruses
2.2. Assessment of Virus Replication
2.3. Animal Experiments
2.4. Real-Time Quantitative PCR for the Detection of ASFV Genome
2.5. Detection of Anti-ASFV Antibodies
2.6. Analysis of Cellular Response to the Vaccine ASFV-G-ΔI177L by IFNg-ELIspot
2.7. Statistical Analysis
3. Results
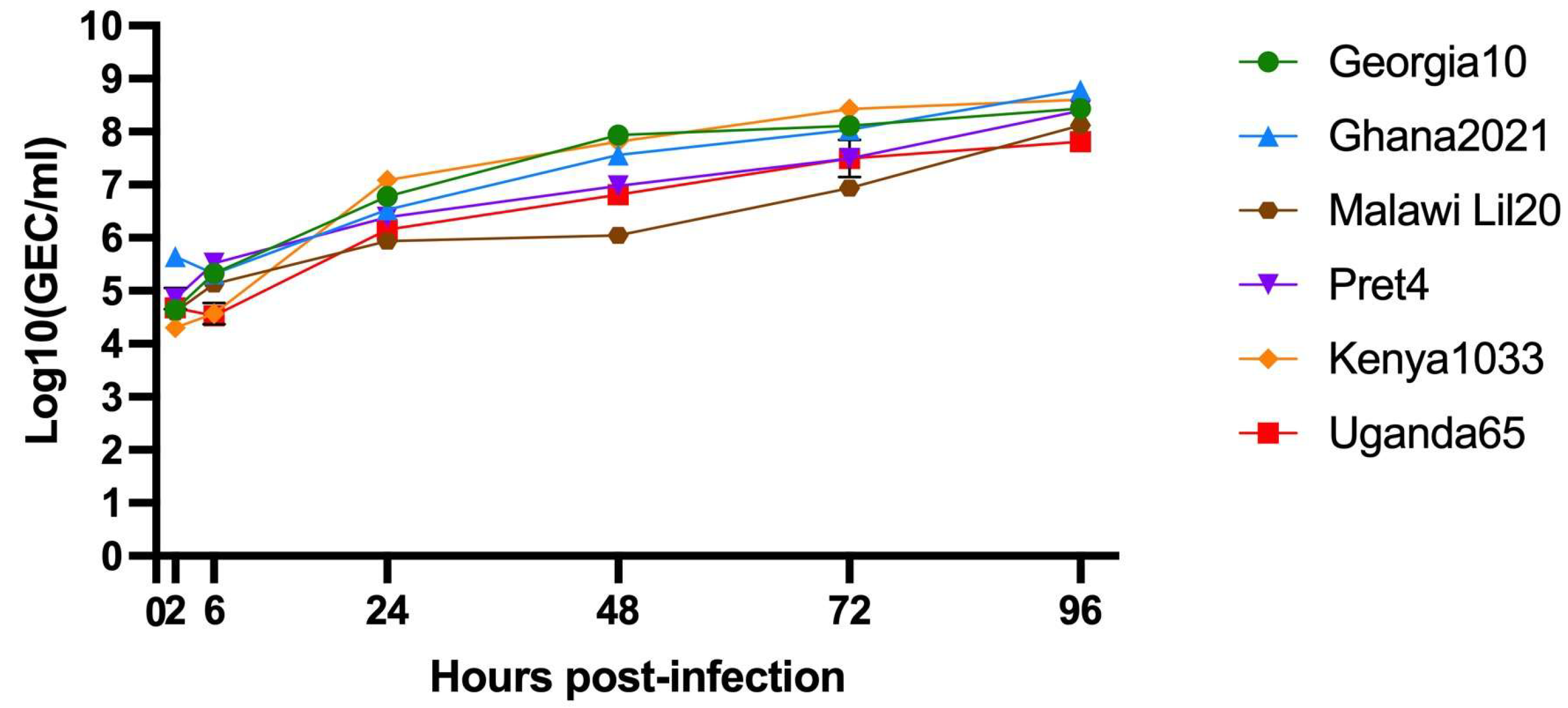
3.1. Comparative Growth Kinetics of the Challenge Viruses
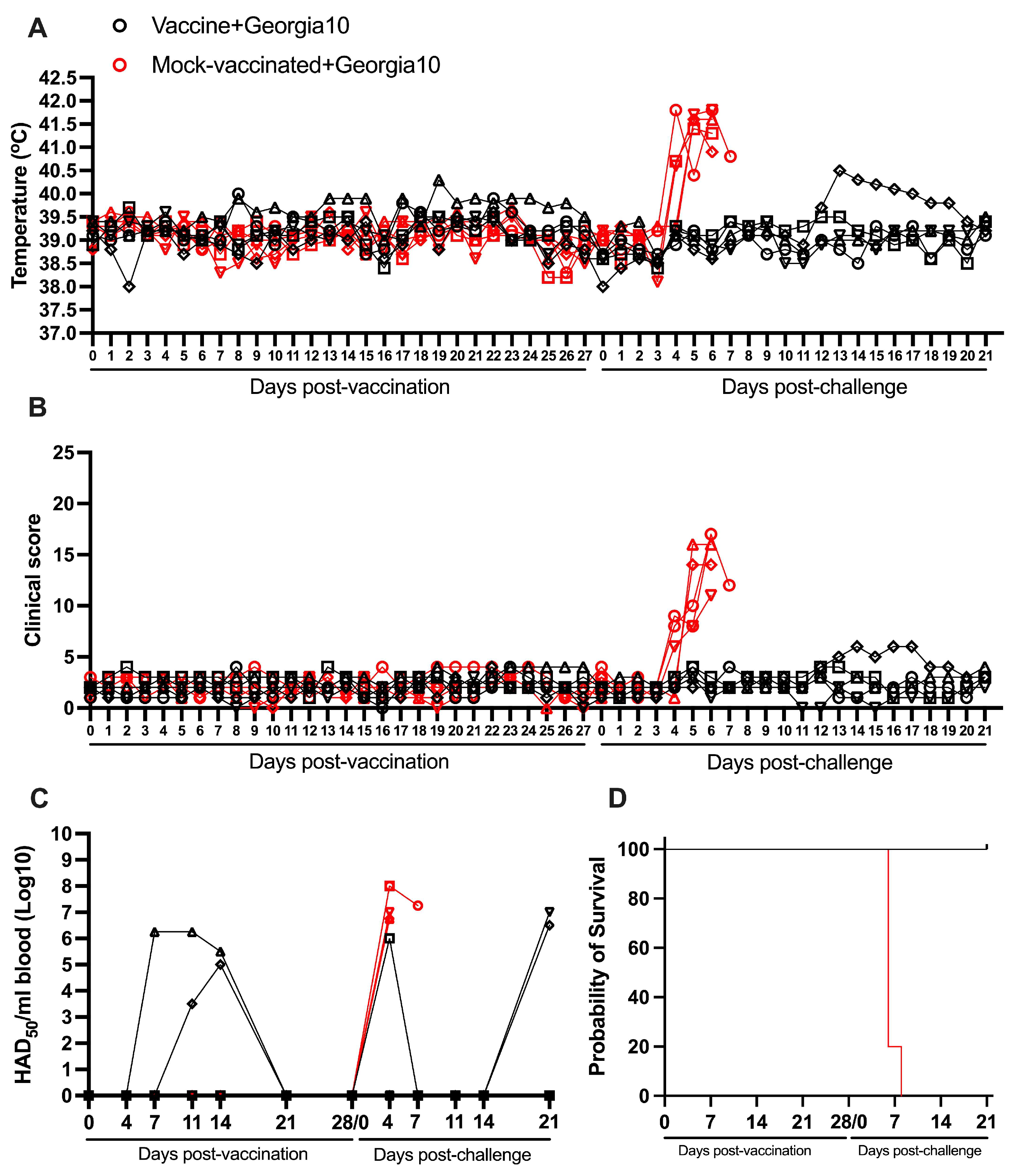
3.2. Protection Induced by ASFV-G-ΔI177L Against the Homologous Challenge
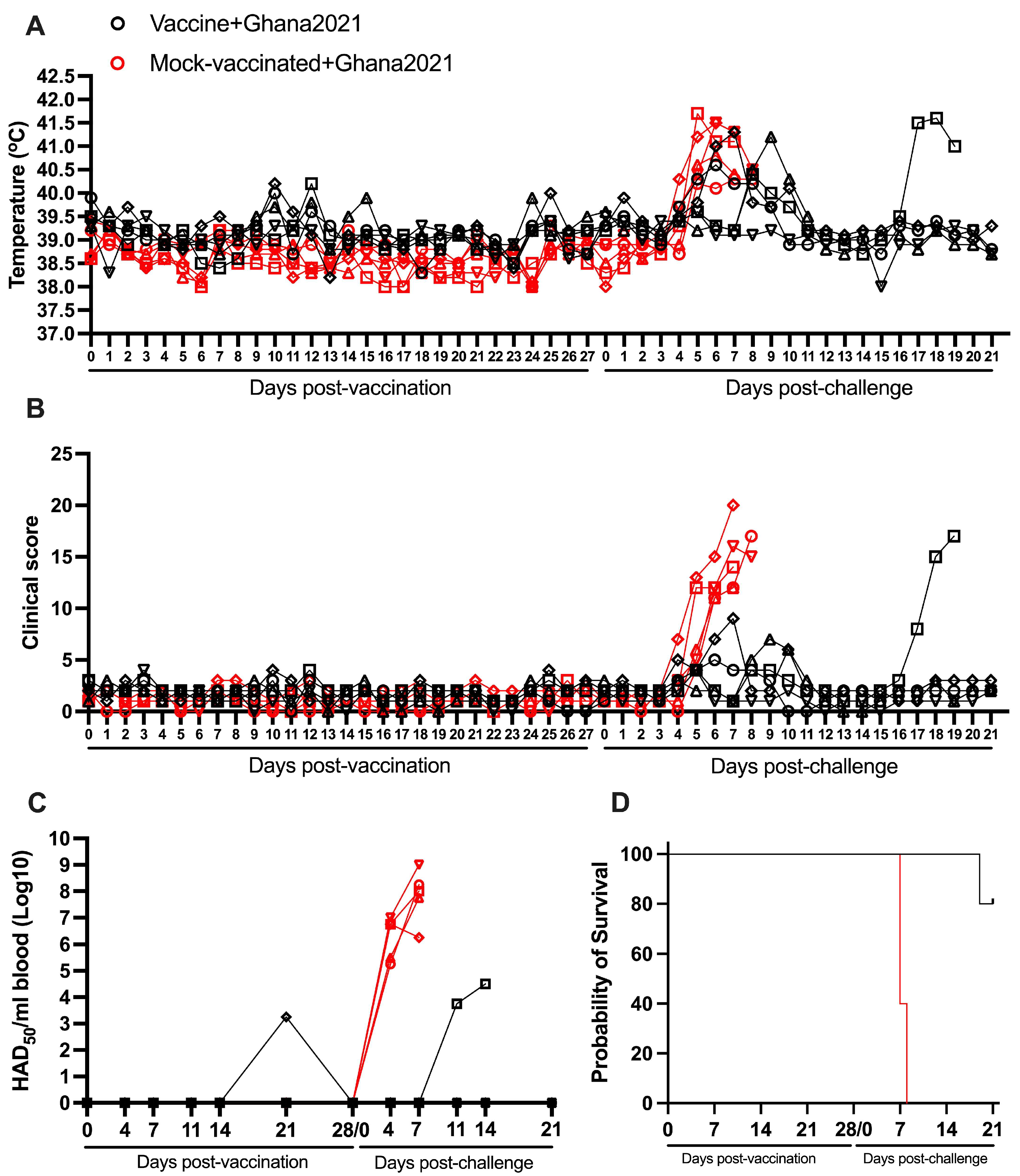
3.3. Heterologous Protection Induced by ASFV-G-ΔI177L Against an ASFV Strain from the Biotype 1 (Ghana2021)
3.4. Protection Induced by ASFV-G-ΔI177L Against a ASFV Strain from the Biotype 6 (Malawi Lil20)
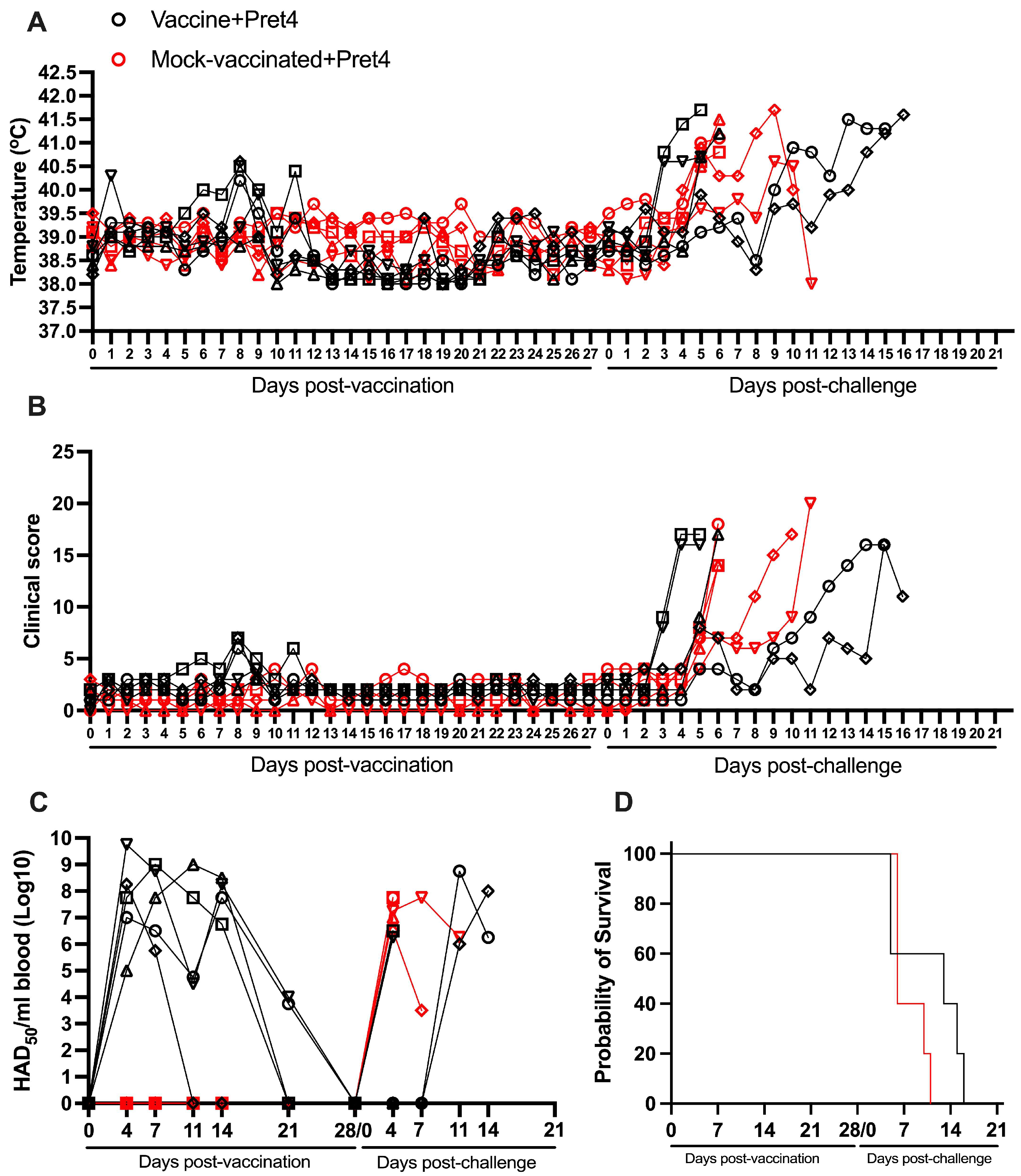
3.5. Protection Induced by ASFV-G-ΔI177L Against a ASFV Strain from the Biotype 3 (Pret4)
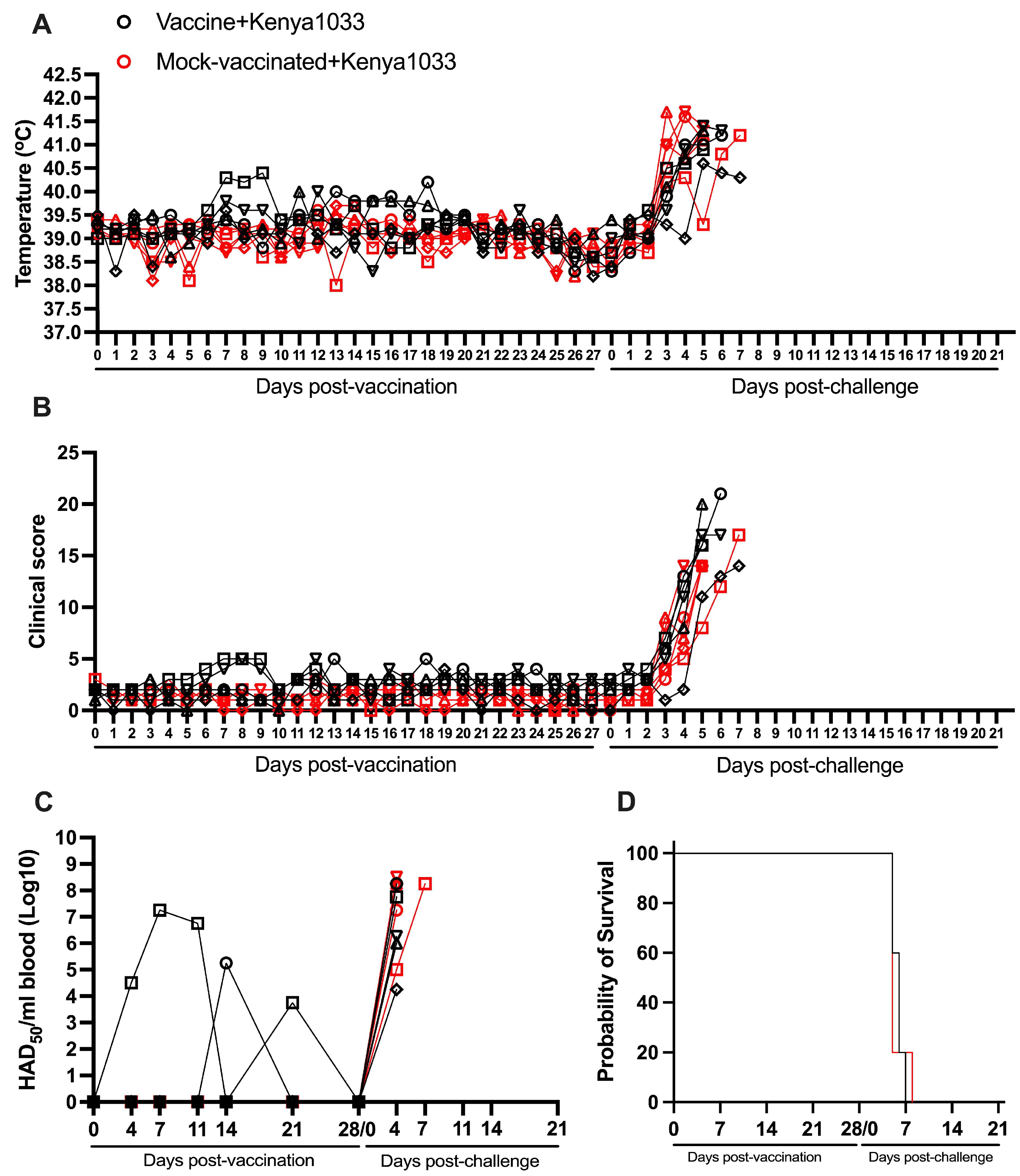
3.6. Protection Induced by ASFV-G-ΔI177L Against an ASFV Strain from the Biotype 5 (Kenya1033)
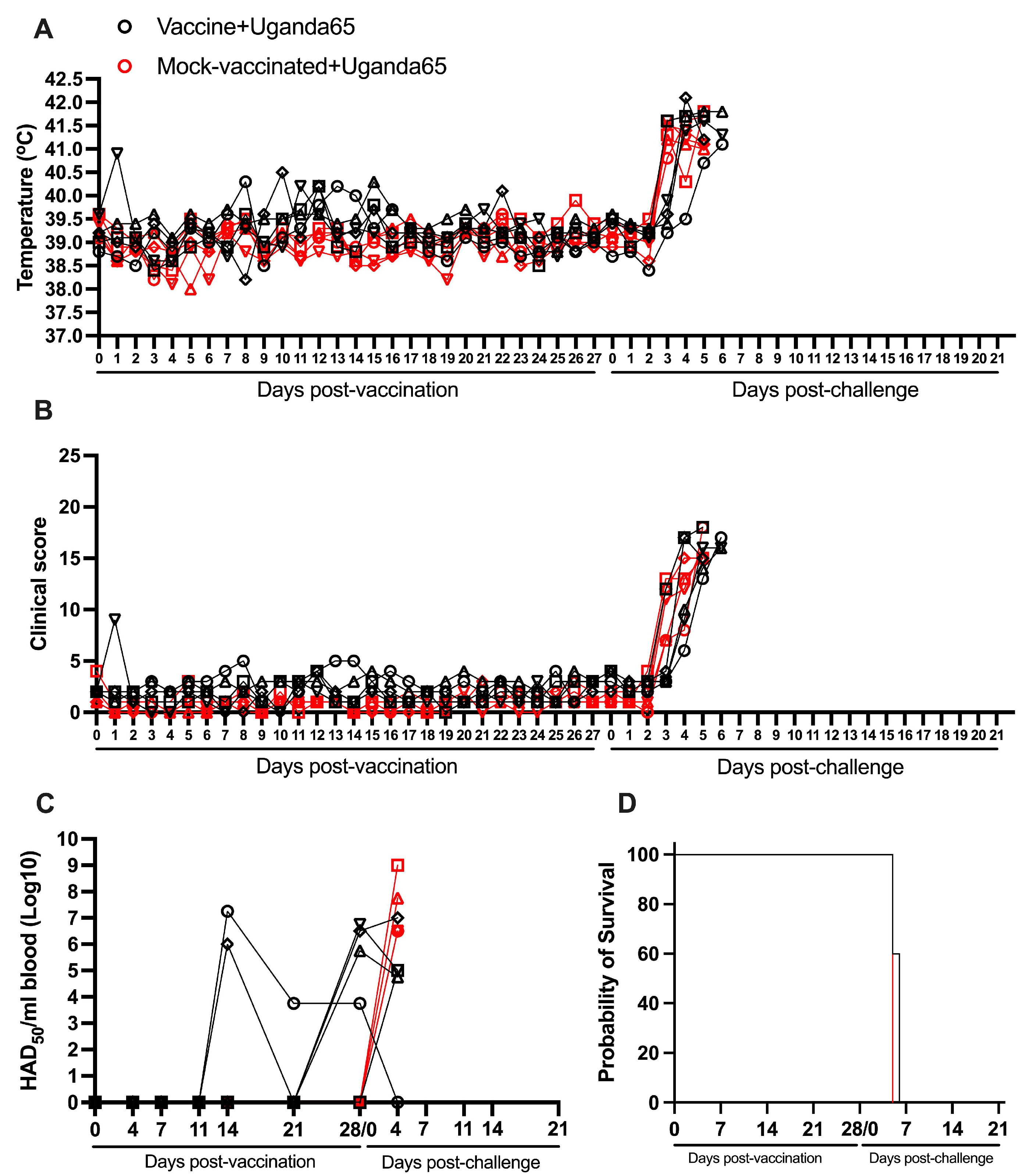
3.7. Protection Induced by ASFV-G-ΔI177L Against an ASFV Strain from the Biotype 4 (Uganda65)
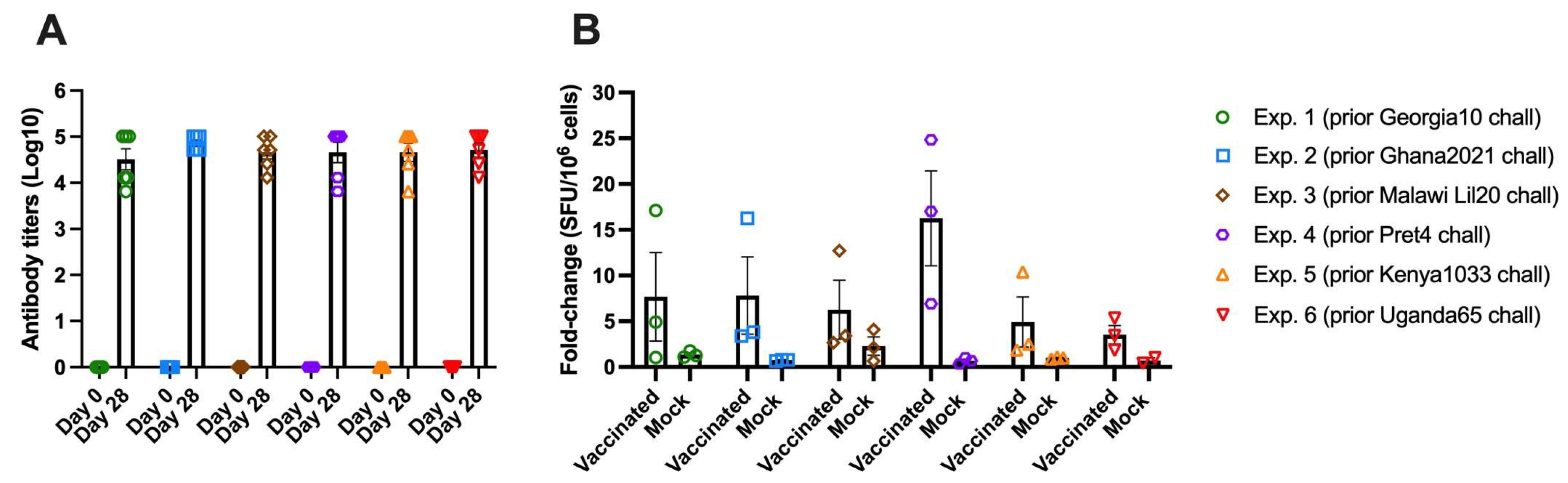
3.8. Detection of ASFV Specific Immune Response in All Groups Vaccinated with ASFV-G-ΔI177L
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Tulman, E.R.; Delhon, G.A.; Ku, B.K.; Rock, D.L. African Swine Fever Virus In Lesser Known Large dsDNA Viruses; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 328, pp. 43–87. [Google Scholar]
- Keßler, C.; Forth, J.H.; Keil, G.M.; Mettenleiter, T.C.; Blome, S.; Karger, A. The intracellular proteome of African swine fever virus. Sci. Rep. 2018, 8, 14714. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, e01293–18. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Gladue, D.P.; Borca, M.V. Recombinant ASF Live Attenuated Virus Strains as Experimental Vaccine Candidates. Viruses 2022, 14, 878. [Google Scholar] [CrossRef]
- Detray, D.E. Persistence of viremia and immunity in African swine fever. Am. J. Vet. Res. 1957, 18, 811–816. [Google Scholar]
- Malmquist, W.A. Serologic and immunologic studies with African swine fever virus. Am. J. Vet. Res. 1963, 24, 450–459. [Google Scholar]
- Manso-Ribeiro, J.; Nunes-Petisca, J.L.; Lopez-Frazao, F.; Sobral, M. Vaccination against ASF. Bull.Off.Int. Epizoot. 1963, 60, 921–937. [Google Scholar]
- Mebus, C.A.; Dardiri, A.H. Western hemisphere isolates of African swine fever virus: Asymptomatic carriers and resistance to challenge inoculation. Am. J. Vet. Res. 1980, 41, 1867–1869. [Google Scholar] [CrossRef]
- Boinas, F.S.; Hutchings, G.H.; Dixon, L.K.; Wilkinson, P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004, 85, 2177–2187. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- King, K.; Chapman, D.; Argilaguet, J.M.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.A.; Netherton, C.L.; Moffat, K.; et al. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine 2011, 29, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- Leitao, A.; Cartaxeiro, C.; Coelho, R.; Cruz, B.; Parkhouse, R.M.; Portugal, F.; Vigario, J.D.; Martins, C.L.V. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol. 2001, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gomez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef]
- Sereda, A.D.; Balyshev, V.M.; Kazakova, A.S.; Imatdinov, A.R.; Kolbasov, D.V. Protective Properties of Attenuated Strains of African Swine Fever Virus Belonging to Seroimmunotypes I-VIII. Pathogens 2020, 9, 274. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017–19. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-DeltaI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound Emerg. Dis. 2021, 69, e497–e504. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes 2022, 58, 77–87. [Google Scholar] [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; Lopez, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71DeltaCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, e01058–17. [Google Scholar] [CrossRef]
- Lopez, E.; van Heerden, J.; Bosch-Camos, L.; Accensi, F.; Navas, M.J.; Lopez-Monteagudo, P.; Argilaguet, J.; Gallardo, C.; Pina-Pedrero, S.; Salas, M.L.; et al. Live Attenuated African Swine Fever Viruses as Ideal Tools to Dissect the Mechanisms Involved in Cross-Protection. Viruses 2020, 12, 1474. [Google Scholar] [CrossRef]
- Souto, R.; Mutowembwa, P.; van Heerden, J.; Fosgate, G.T.; Heath, L.; Vosloo, W. Vaccine Potential of Two Previously Uncharacterized African Swine Fever Virus Isolates from Southern Africa and Heterologous Cross Protection of an Avirulent European Isolate. Transbound Emerg. Dis. 2016, 63, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Cadenas-Fernandez, E.; Barroso-Arevalo, S.; Kosowska, A.; Diaz-Frutos, M.; Gallardo, C.; Rodriguez-Bertos, A.; Bosch, J.; Sanchez-Vizcaino, J.M.; Barasona, J.A. Challenging boundaries: Is cross-protection evaluation necessary for African swine fever vaccine development? A case of oral vaccination in wild boar. Front. Immunol. 2024, 15, 1388812. [Google Scholar] [CrossRef] [PubMed]
- Diep, N.V.D.; Duc, N.V.; Ngoc, N.T.; Dang, V.X.; Tiep, T.N.; Nguyen, V.D.; Than, T.T.; Maydaniuk, D.; Goonewardene, K.; Ambagala, A.; et al. Genotype II Live-Attenuated ASFV Vaccine Strains Unable to Completely Protect Pigs against the Emerging Recombinant ASFV Genotype I/II Strain in Vietnam. Vaccines 2024, 12, 1114. [Google Scholar] [CrossRef]
- Spinard, E.; Dinhobl, M.; Tesler, N.; Birtley, H.; Signore, A.V.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. A Re-Evaluation of African Swine Fever Genotypes Based on p72 Sequences Reveals the Existence of Only Six Distinct p72 Groups. Viruses 2023, 15, 2246. [Google Scholar] [CrossRef]
- Dinhobl, M.; Spinard, E.; Tesler, N.; Birtley, H.; Signore, A.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. Reclassification of ASFV into 7 Biotypes Using Unsupervised Machine Learning. Viruses 2023, 16, 67. [Google Scholar] [CrossRef]
- Borca, M.V.; O’Donnell, V.; Holinka, L.G.; Sanford, B.; Azzinaro, P.A.; Risatti, G.R.; Gladue, D.P. Development of a fluorescent ASFV strain that retains the ability to cause disease in swine. Sci. Rep. 2017, 7, 46747. [Google Scholar] [CrossRef]
- Abworo, E.O.; Onzere, C.; Oluoch Amimo, J.; Riitho, V.; Mwangi, W.; Davies, J.; Blome, S.; Peter Bishop, R. Detection of African swine fever virus in the tissues of asymptomatic pigs in smallholder farming systems along the Kenya-Uganda border: Implications for transmission in endemic areas and ASF surveillance in East Africa. J. Gen. Virol. 2017, 98, 1806–1814. [Google Scholar] [CrossRef]
- Rai, A.; Spinard, E.; Osei-Bonsu, J.; Meyers, A.; Dinhobl, M.; O’Donnell, V.; Ababio, P.T.; Tawiah-Yingar, D.; Arthur, D.; Baah, D.; et al. A Retrospective Analysis Reveals That the 2021 Outbreaks of African Swine Fever Virus in Ghana Were Caused by Two Distinct Genotypes. Viruses 2024, 16, 1265. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Abkallo, H.M.; Hemmink, J.D.; Oduor, B.; Khazalwa, E.M.; Svitek, N.; Assad-Garcia, N.; Khayumbi, J.; Fuchs, W.; Vashee, S.; Steinaa, L. Co-Deletion of A238L and EP402R Genes from a Genotype IX African Swine Fever Virus Results in Partial Attenuation and Protection in Swine. Viruses 2022, 14, 2024. [Google Scholar] [CrossRef]
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Dinhobl, M.; Spinard, E.; Birtley, H.; Tesler, N.; Borca, M.V.; Gladue, D.P. African swine fever virus P72 genotyping tool. Microbiol. Resour. Announc. 2024, 13, e0089123. [Google Scholar] [CrossRef]
- Zhu, J.J. African Swine Fever Vaccinology: The Biological Challenges from Immunological Perspectives. Viruses 2022, 14, 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, X.; Zhang, C.; Wang, H.; Zhang, Z.; Feng, Z. ASFV subunit vaccines: Strategies and prospects for future development. Microb. Pathog. 2024, 197, 107063. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, K.; Liu, Z.; Liu, W.; Gao, T.; Yuan, F.; Guo, R.; Tian, Y.; Zhoud, D. Advancement in the Antigenic Epitopes and Vaccine Adjuvants of African Swine Fever Virus. Pathogens 2024, 13, 706. [Google Scholar] [CrossRef] [PubMed]
| Fever Post-Vaccination | Viremia (HAD50) Post-Vaccination | |||||
|---|---|---|---|---|---|---|
| Group (Biotype) | Days to Onset | Duration (Days) | Animals with Fever | Days to Onset | Duration (Days) | Animals Viremic |
| Georgia10 (2) | 13.50 (±5.50) | 1.00 (±0.00) | 2/6 | 13.50 (±5.50) | 1.00 (±0.00) | 2/6 |
| Ghana2021 (1) | 10.50 (±0.50) | 1.25 (±0.25) | 4/6 | 10.50 (±0.50) | 1.25 (±0.25) | 4/6 |
| Malawi Lil20 (6) | 11.25 (±2.29) | 1.00 (±0.00) | 4/4 | 11.25 (±2.29) | 1.00 (±0.00) | 4/4 |
| Pret 4 (3) | 7.33 (±0.67) | 2.67 (±1.67) | 3/6 | 7.33 (±0.67) | 2.67 (±1.67) | 3/6 |
| Kenya1033 (5) | 12.00 (±1.76) | 2.20 (±0.80) | 5/6 | 12.00 (±1.76) | 2.20 (±0.80) | 5/6 |
| Uganda65 (4) | 11.67 (±1.05) | 1.67 (±0.42) | 6/6 | 11.67 (±1.05) | 1.67 (±0.42) | 6/6 |
| Fever (>40 °C) Post-Challenge | Viremia (HAD50) Post-Challenge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vacc. | Challenge | Num. Survivors | Euthanasia (Day) | Num. Days to Onset | Duration (Days) | Num. Fever | Num. Days to Onset | Duration (Days) | Num. Viremic |
| YES | Georgia10 | 5/5 (100%) | 21.0 (+/−0.0) | 13.0 (±0.0) | 5.0 (±0.0) | 1/5 | 15.33 (+/−5.67) | 1.20 (+/−0.73) | 3/5 |
| NO | Georgia10 | 0/5 (0%) | 6.4 (+/−0.4) | 4.4 (±0.2) | 3.0 (±0.5) | 5/5 | 4.00 (+/−0.00) | 3.40 (+/−0.40) | 5/5 |
| YES | Ghana2021 | 4/5 (80%) | 20.6 (+/−0.4) | 6.8 (±0.8) | 2.8 (±0.5) | 4/5 | 11.00 (+/−0.00) | 1.80 (+/−1.80) | 1/5 |
| NO | Ghana2021 | 0/5 (0%) | 7.4 (+/−0.2) | 4.8 (±0.2) | 3.6 (±0.2) | 5/5 | 4.00 (+/−0.00) | 4.40 (+/−0.24) | 5/5 |
| YES | MalawiLil20 | 0/5 (0%) | 8.4 (+/−1.2) | 5.6 (±0.6) | 2.8 (±0.4) | 5/5 | 6.00 (+/−1.00) | 4.40 (+/−1,63) | 3/5 |
| NO | MalawiLil20 | 0/5 (0%) | 7.0 (+/−0.0) | 4.2 (±0.2) | 2.8 (±0.2) | 5/5 | 4.00 (+/−0.00) | 3.00 (+/−0.00) | 5/5 |
| YES | Pret4 | 0/5 (0%) | 10.8 (+/−2.4) | 6.6 (±1.9) | 5.2 (±1.2) | 5/5 | 7.50 (+/−2.02) | 3.00 (+/−1.10) | 4/5 |
| NO | Pret4 | 0/5 (0%) | 7.8 (+/−1.1) | 5.6 (±0.9) | 3.0 (±1.0) | 5/5 | 4.00 (+/−0.00) | 4.80 (+/−1.11) | 5/5 |
| YES | Ken1033 | 0/5 (0%) | 5.8 (+/−0.4) | 3.8 (±0.4) | 3.0 (±0.0) | 5/5 | 4.00 (+/−0.00) | 2.80 (+/−0.37) | 5/5 |
| NO | Ken1033 | 0/5 (0%) | 5.6 (+/−0.6) | 3.0 (±0.0) | 3.6 (±0.6) | 5/5 | 4.00 (+/−0.00) | 2.60 (+/−0.60) | 5/5 |
| YES | Uganda65 | 0/5 (0%) | 5.6 (+/−0.2) | 4.0 (±0.3) | 2.6 (±0.2) | 5/5 | 4.00 (+/−0.00) | 2.60 (+/−0.24) | 5/5 |
| NO | Uganda65 | 0/5 (0%) | 5.0 (+/−0.0) | 3 (±0.0) | 3.0 (±0.0) | 5/5 | 4.00 (+/−0.00) | 2.00 (+/−0.00) | 5/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borca, M.V.; Ramirez-Medina, E.; Mutisya, C.; Ojuok, R.; Odaba, J.; Dihbol, M.; Lacasta, A.; Gladue, D.P. Evaluation of Cross-Protection of African Swine Fever Vaccine ASFV-G-ΔI177L Between ASFV Biotypes. Vaccines 2025, 13, 858. https://doi.org/10.3390/vaccines13080858
Borca MV, Ramirez-Medina E, Mutisya C, Ojuok R, Odaba J, Dihbol M, Lacasta A, Gladue DP. Evaluation of Cross-Protection of African Swine Fever Vaccine ASFV-G-ΔI177L Between ASFV Biotypes. Vaccines. 2025; 13(8):858. https://doi.org/10.3390/vaccines13080858
Chicago/Turabian StyleBorca, Manuel V., Elizabeth Ramirez-Medina, Christine Mutisya, Rose Ojuok, Josiah Odaba, Mark Dihbol, Anna Lacasta, and Douglas P. Gladue. 2025. "Evaluation of Cross-Protection of African Swine Fever Vaccine ASFV-G-ΔI177L Between ASFV Biotypes" Vaccines 13, no. 8: 858. https://doi.org/10.3390/vaccines13080858
APA StyleBorca, M. V., Ramirez-Medina, E., Mutisya, C., Ojuok, R., Odaba, J., Dihbol, M., Lacasta, A., & Gladue, D. P. (2025). Evaluation of Cross-Protection of African Swine Fever Vaccine ASFV-G-ΔI177L Between ASFV Biotypes. Vaccines, 13(8), 858. https://doi.org/10.3390/vaccines13080858






