Antibody-Dependent Cellular Cytotoxicity Elicited by the Antibodies Against the E120R Protein of African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Anti-pE120R Antibodies and Virus Strains
2.2. pE120R Sequence Conservation Analysis Across ASFV Genotypes
2.3. Bioinformatics Analysis of the Antigenic Features of the pE120R
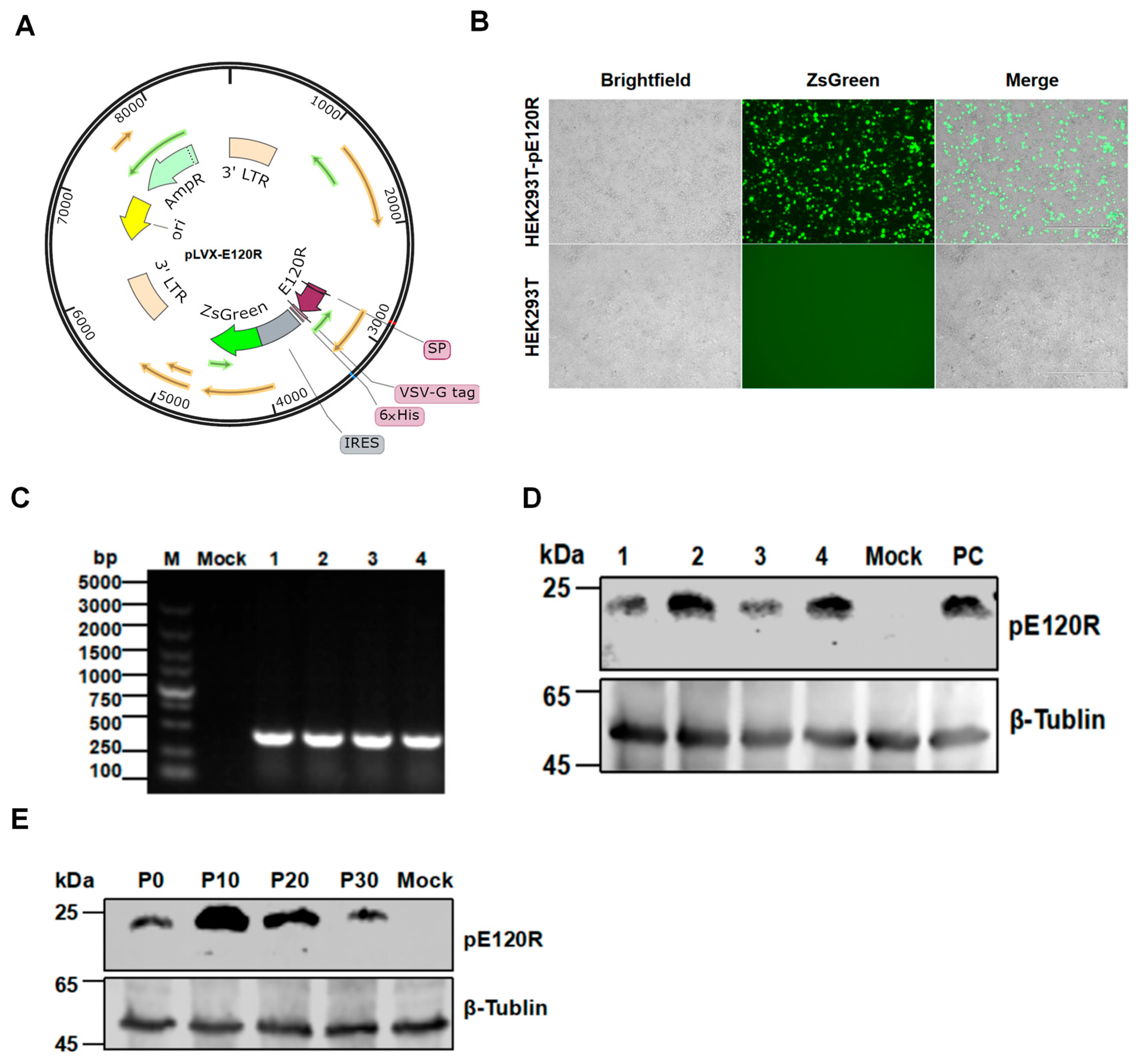
2.4. Design of a Lentiviral Expression Vector
2.5. Lentivirus Packaging and Infection
2.6. Western Blotting
2.7. Screening and Characterization of an HEK293T Cell Line Stably Expressing pE120R
2.8. Laser Confocal Scanning Microscopy
2.9. Membrane Fractionation
2.10. ADCC Measurement
2.11. Effects of pE120R on ASFV Replication
2.12. Statistical Analysis
3. Results
3.1. pE120R Is Conserved Among Various ASFV Genotypes
3.2. Immunogenicity and Epitope Prediction Analysis
3.3. Generation of a Stable HEK293T Cell Line Expressing pE120R
3.4. Anti-pE120R Antibodies Mediate ADCC Activities
3.5. ASFV Replication Is Not Significantly Enhanced in the HEK293T-pE120R Cell Line
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASF | African swine fever |
| ASFV | African swine fever virus |
| LAVs | Live-attenuated vaccines |
| CTLs | Cytotoxic T lymphocytes |
| PAMs | Porcine alveolar macrophages |
| PBMCs | Peripheral blood mononuclear cells |
| LDH | Lactate dehydrogenase |
| hpi | Hours post-infection |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| FACS | Fluorescence-activated cell sorting |
| ADCC | Antibody-dependent cellular cytotoxicity |
| CDC | Complement-dependent cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
References
- Blasco, R.; de la Vega, I.; Almazán, F.; Agüero, M.; Viñuela, E. Genetic variation of African swine fever virus, variable regions near the ends of the viral DNA. Virology 1989, 173, 251–257. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Bui, N.T.T.; Nguyen, L.T.; Ngo, T.N.T.; Van Nguyen, C.; Nguyen, L.M.; Nouhin, J.; Karlsson, E.; Padungtod, P.; Pamornchainavakul, N.; et al. An African swine fever vaccine-like variant with multiple gene deletions caused reproductive failure in a Vietnamese breeding herd. Sci. Rep. 2025, 15, 14919. [Google Scholar] [CrossRef]
- Diep, N.V.; Duc, N.V.; Ngoc, N.T.; Dang, V.X.; Tiep, T.N.; Nguyen, V.D.; Than, T.T.; Maydaniuk, D.; Goonewardene, K.; Ambagala, A.; et al. Genotype II Live-attenuated ASFV vaccine strains unable to completely protect pigs against the emerging recombinant ASFV genotype I/II strain in Vietnam. Vaccines 2024, 12, 1114. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Richt, J.A. Subunit vaccine approaches for African swine fever virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gan, M.; Zhang, Z.; Lin, X.; Ouyang, Q.; Fu, H.; Xu, X.; Wang, Z.; Li, X.; Wang, Y.; et al. A hidden guardian, The stability and spectrum of antibody-dependent cell-mediated cytotoxicity in COVID-19 response in Chinese adults. Vaccines 2025, 13, 262. [Google Scholar] [CrossRef]
- Adeniji, O.S.; Giron, L.B.; Purwar, M.; Zilberstein, N.F.; Kulkarni, A.J.; Shaikh, M.W.; Balk, R.A.; Moy, J.N.; Forsyth, C.B.; Liu, Q.; et al. COVID-19 severity is associated with differential antibody Fc-mediated innate immune functions. mBio 2021, 12, e00281. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, H.; Liu, H.; Chen, Y.; Zhou, J.; Zhu, X.; Liang, C.; Ding, P.; Liu, E.; Zhang, G. Detection of African swine fever virus antibodies using p11.5 and p14.5 protein-based indirect ELISA. Virology 2025, 603, 110335. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Feng, T.; Ma, Z.; Xue, Q.; Wu, P.; Li, P.; Li, S.; Yang, F.; Cao, W.; et al. African swine fever virus E120R protein inhibits interferon beta production by interacting with IRF3 to block its activation. J. Virol. 2021, 95, e0082421. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Han, Y.; Pan, L.; Yang, J.; Sun, M.; Zhou, P.; Sun, Y.; Bi, Y.; Qiu, H.J. Adaptation of African swine fever virus to HEK293T cells. Transbound. Emerg. Dis. 2021, 68, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, D.; Prakash, A.; Nguyen, Q.A.; Suntisukwattana, R.; Atthaapa, W.; Tantituvanont, A.; Nilubol, D. Designing a multi-epitope vaccine against African swine fever virus using immunoinformatics approach. Sci. Rep. 2025, 15, 16044. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, H.; Fan, W.; He, L.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Hu, R.; Luo, T.; et al. Evaluation of cellular immunity with ASFV infection by swine leukocyte antigen (SLA)-peptide tetramers. Viruses 2021, 13, 2264. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Liang, Y.; Xu, S.; Weng, Z.; Gao, Q.; Huang, Z.; Zhang, G.; Gong, L. Interaction network of African swine fever virus structural protein p30 with host proteins. Front. Microbiol. 2022, 13, 971888. [Google Scholar] [CrossRef]
- Zhai, H.; Gao, Y.; Zhu, Y.; Hou, Q.; Wan, N.; Wang, T.; Li, S.; Zhao, D.; Qiu, H.J.; Li, Y. Anti-pA137R antibodies exacerbate the pathogenicity of African swine fever virus in pigs. J. Virol. 2025, 99, e0017225. [Google Scholar] [CrossRef]
- Wang, T.; Luo, R.; Zhang, J.; Lu, Z.; Li, L.F.; Zheng, Y.H.; Pan, L.; Lan, J.; Zhai, H.; Huang, S.; et al. The MGF300-2R protein of African swine fever virus is associated with viral pathogenicity by promoting the autophagic degradation of IKKα and IKKβ through the recruitment of TOLLIP. PLoS Pathog. 2023, 19, e1011580. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef]
- Dei Giudici, S.; Loi, F.; Ghisu, S.; Angioi, P.P.; Zinellu, S.; Fiori, M.S.; Carusillo, F.; Brundu, D.; Franzoni, G.; Zidda, G.M.; et al. The long-jumping of African swine fever, first genotype II notified in Sardinia, Italy. Viruses 2023, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, T.; Luo, R.; Lu, Z.; Lan, J.; Sun, Y.; Fu, Q.; Qiu, H.J. Genetic variations of African swine fever virus, major challenges and prospects. Viruses 2024, 16, 913. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, S.; Waghela, S.D.; Bray, J.; Sangewar, N.; Charendoff, C.; Martin, C.L.; Hassan, W.S.; Koynarski, T.; Gabbert, L.; Burrage, T.G.; et al. Adenovirus-vectored novel African swine fever virus antigens elicit robust immune responses in swine. PLoS ONE 2017, 12, e0177007. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, C.; Rcheulishvili, N.; Wang, Y.; Xiong, T.; Xie, F.; Wang, X.; Chen, R.; Wang, P.G.; He, Y. Development and efficacy of a novel mRNA cocktail for the delivery of African swine fever virus antigens and induction of immune responses. Microbiol. Spectr. 2025, 13, e0290924. [Google Scholar] [CrossRef]
- Taylor, R.P. T cells reinforce NK cell-mediated ADCC. Blood 2024, 143, 1786–1787. [Google Scholar] [CrossRef]
- Tanaka, M.; Ishige, A.; Yaguchi, M.; Matsumoto, T.; Shirouzu, M.; Yokoyama, S.; Ishikawa, F.; Kitabayashi, I.; Takemori, T.; Harada, M. Development of a simple new flow cytometric antibody-dependent cellular cytotoxicity (ADCC) assay with excellent sensitivity. J. Immunol. Methods 2019, 464, 74–86. [Google Scholar] [CrossRef]
- Clark, J.J.; Hoxie, I.; Adelsberg, D.C.; Sapse, I.A.; Andreata-Santos, R.; Yong, J.S.; Amanat, F.; Tcheou, J.; Raskin, A.; Singh, G.; et al. Protective effect and molecular mechanisms of human non-neutralizing cross-reactive spike antibodies elicited by SARS-CoV-2 mRNA vaccination. Cell Rep. 2024, 43, 114922. [Google Scholar] [CrossRef]
- Wöhner, M.; Nimmerjahn, F. Cytotoxic IgG; mechanisms, functions, and applications. Immunity 2025, 58, 1378–1395. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, S.; De Paolis, F.; Silvestris, D.A.; Baldari, S.; Salvatori, I.; Tullo, A.; Lanzilli, G.; Gurtner, A.; Ferri, A.; Valle, C.; et al. The CD64/CD28/CD3ζ chimeric receptor reprograms T cell metabolism and promotes T cell persistence and immune functions while triggering antibody-independent and antibody-dependent cytotoxicity. Exp. Hematol. Oncol. 2025, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Endo, Y.; Wu, W.J. Define critical parameters of trastuzumab-mediated ADCC assays via assay optimization processes, focusing on the impact of cryopreserved effector cells on assay performance. Cancers 2024, 16, 2367. [Google Scholar] [CrossRef]
- Noll, J.C.G.; Rani, R.; Butt, S.L.; Fernandes, M.H.V.; do Nascimento, G.M.; Martins, M.; Caserta, L.C.; Covaleda, L.; Diel, D.G. Identification of an immunodominant B cell epitope in African swine fever virus p30 protein and evidence of p30 antibody-mediated antibody dependent cellular cytotoxicity. Viruses 2024, 16, 758. [Google Scholar] [CrossRef] [PubMed]
- Mariuzza, R.A.; Singh, P.; Karade, S.S.; Shahid, S.; Sharma, V.K. Recognition of self and viral ligands by NK cell receptors. Immunol. Rev. 2025, 329, e13435. [Google Scholar] [CrossRef]
- Andrés, G.; García-Escudero, R.; Viñuela, E.; Salas, M.L.; Rodríguez, J.M. African swine fever virus structural protein pE120R is essential for virus transport from assembly sites to plasma membrane but not for infectivity. J. Virol. 2001, 75, 6758–6768. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]



| Strains | Accession Number | Hosts | Countries | Genotypes |
|---|---|---|---|---|
| Timor-Leste/2019/1 | MW396979.1 | Domestic pig | Timor-Leste | II |
| Georgia/2007/1 | FR682468.2 | Domestic pig | Georgia | II |
| Russia/Primorsky 2023 | PP348677.1 | Wild boar | Russia | II |
| China/HLJ/2018 | MK333180.1 | Domestic pig | China | II |
| China/Henan/2022 | OQ504954.1 | Domestic pig | China | I |
| Italy/Sardinia/2018 | MW736611.1 | Wild boar | Italy | I |
| China/Inner Mongolia/2022 | OQ504955.1 | Domestic pig | China | I |
| China/Jiangsu/2021 | OQ504956.1 | Domestic pig | China | I/II recombinant |
| Vietnam/VNUA/2023 | PP810980.1 | Domestic pig | Vietnam | I/II recombinant |
| Epitopes | Start–End Positions | Peptide Sequences | Scores |
|---|---|---|---|
| CD8+ T-cell | 142–150 | YTDIEMNRL | 0.45 |
| CD8+ T-cell | 48–56 | NADTMIPSF | 0.45 |
| CD8+ T-cell | 89–97 | AEEEDSNFL | 0.37 |
| CD4+ T-cell | 81–95 | TREFTSLVPDEADNK | 0.93 |
| CD4+ T-cell | 80–94 | DTREFTSLVPDEADN | 0.86 |
| CD4+ T-cell | 82–96 | REFTSLVPDEADNKP | 0.78 |
| CD4+ T-cell | 79–93 | HDTREFTSLVPDEAD | 0.68 |
| B-cell | 9–21 | QYLKEDSRDRTSI | None |
| B-cell | 42–57 | EEFEPIPDYDPTTSTS | None |
| B-cell | 87–109 | LVPDEADNKPEDDEESGAKPKKK | None |
| Primers | Sequences (5′-3′) |
|---|---|
| PLVX-IRES-ZsGreen-F | GAATTCGCCACCATGGCCTTCCTGTGGCTGCTGAGCTGCTGGGCCCTGCTGGGCACCACCTTCGGCGAC |
| PLVX-IRES-ZsGreen-R | TACAGACATAGAGATGAACCGACTTGGAAAGGGCGGTGGCGGTAGCGGCGGTGGCGGTAGCCACCACCACCACCACCACTAAGAATTC |
| E120R-V-F | ACCATGGCTTTCCTTTGGCTT |
| E120R-V-R | ATGTCGGTGTACTTGCTCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Lan, J.; Lu, Z.; Li, J.; Ma, C.; Luo, R.; Fu, Q.; Sun, Y.; Wang, T.; Qiu, H.-J. Antibody-Dependent Cellular Cytotoxicity Elicited by the Antibodies Against the E120R Protein of African Swine Fever Virus. Vaccines 2025, 13, 934. https://doi.org/10.3390/vaccines13090934
Chen S, Lan J, Lu Z, Li J, Ma C, Luo R, Fu Q, Sun Y, Wang T, Qiu H-J. Antibody-Dependent Cellular Cytotoxicity Elicited by the Antibodies Against the E120R Protein of African Swine Fever Virus. Vaccines. 2025; 13(9):934. https://doi.org/10.3390/vaccines13090934
Chicago/Turabian StyleChen, Shengmei, Jing Lan, Zhanhao Lu, Jia Li, Caoyuan Ma, Rui Luo, Qiang Fu, Yuan Sun, Tao Wang, and Hua-Ji Qiu. 2025. "Antibody-Dependent Cellular Cytotoxicity Elicited by the Antibodies Against the E120R Protein of African Swine Fever Virus" Vaccines 13, no. 9: 934. https://doi.org/10.3390/vaccines13090934
APA StyleChen, S., Lan, J., Lu, Z., Li, J., Ma, C., Luo, R., Fu, Q., Sun, Y., Wang, T., & Qiu, H.-J. (2025). Antibody-Dependent Cellular Cytotoxicity Elicited by the Antibodies Against the E120R Protein of African Swine Fever Virus. Vaccines, 13(9), 934. https://doi.org/10.3390/vaccines13090934







