Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Safety Parameters
2.3. Statistical Analysis
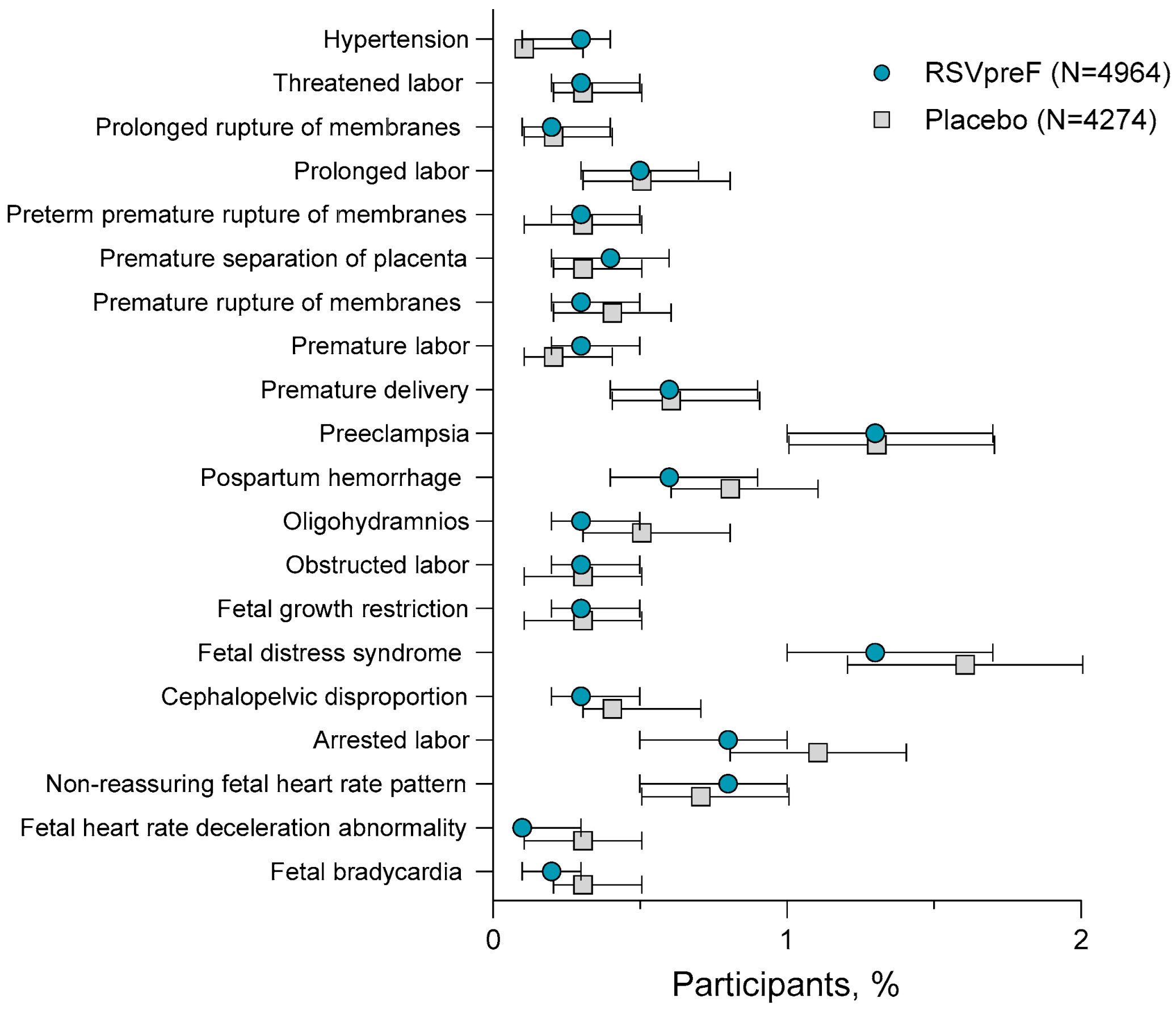
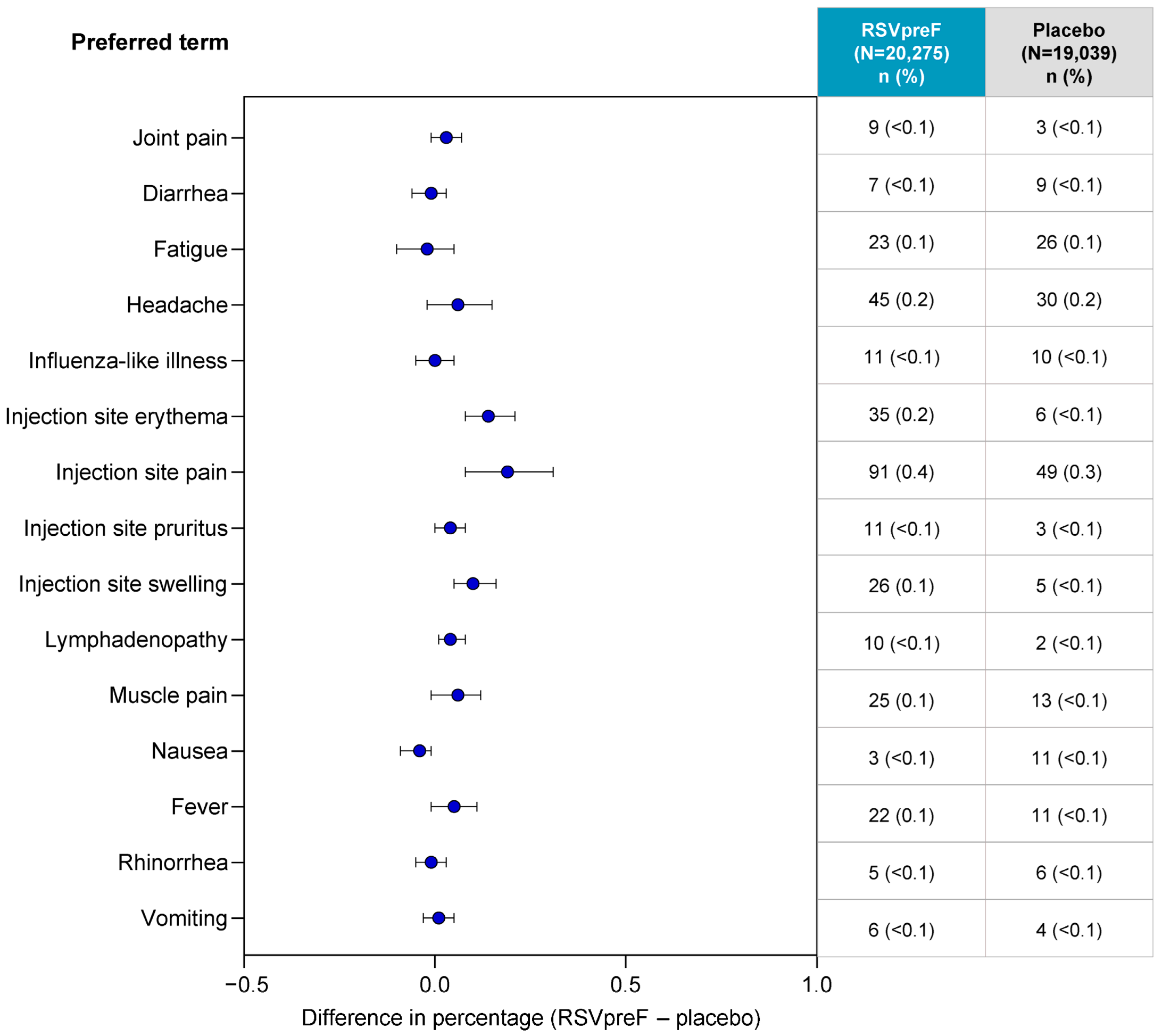
3. Results
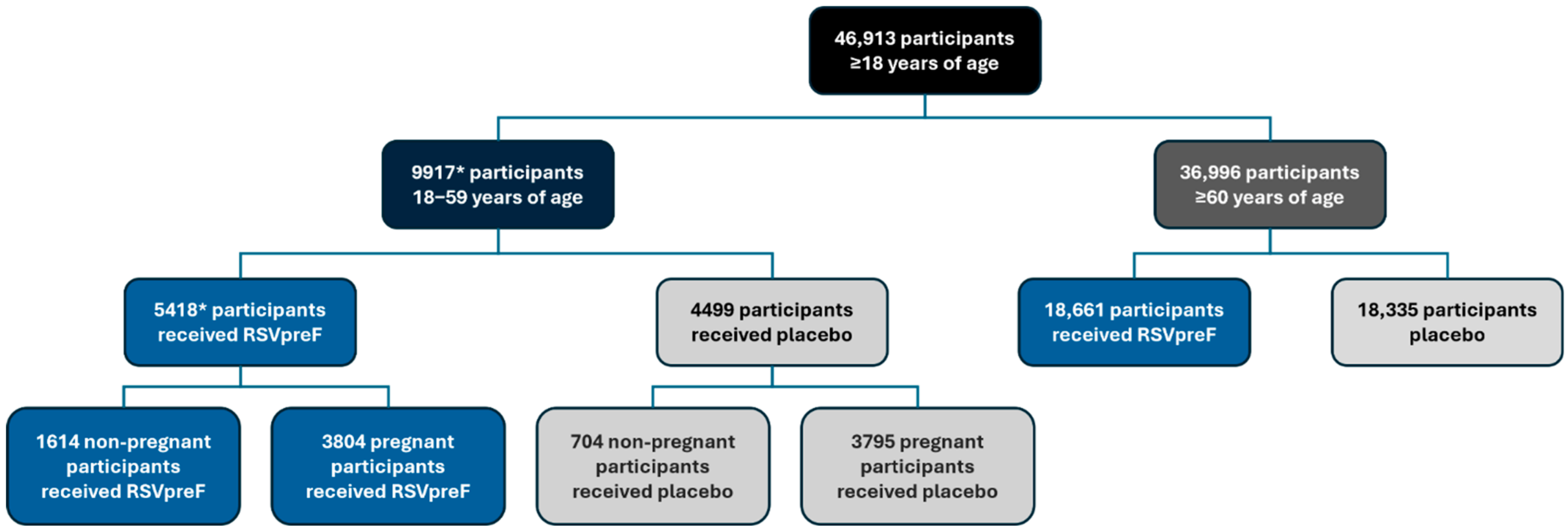
3.1. Populations
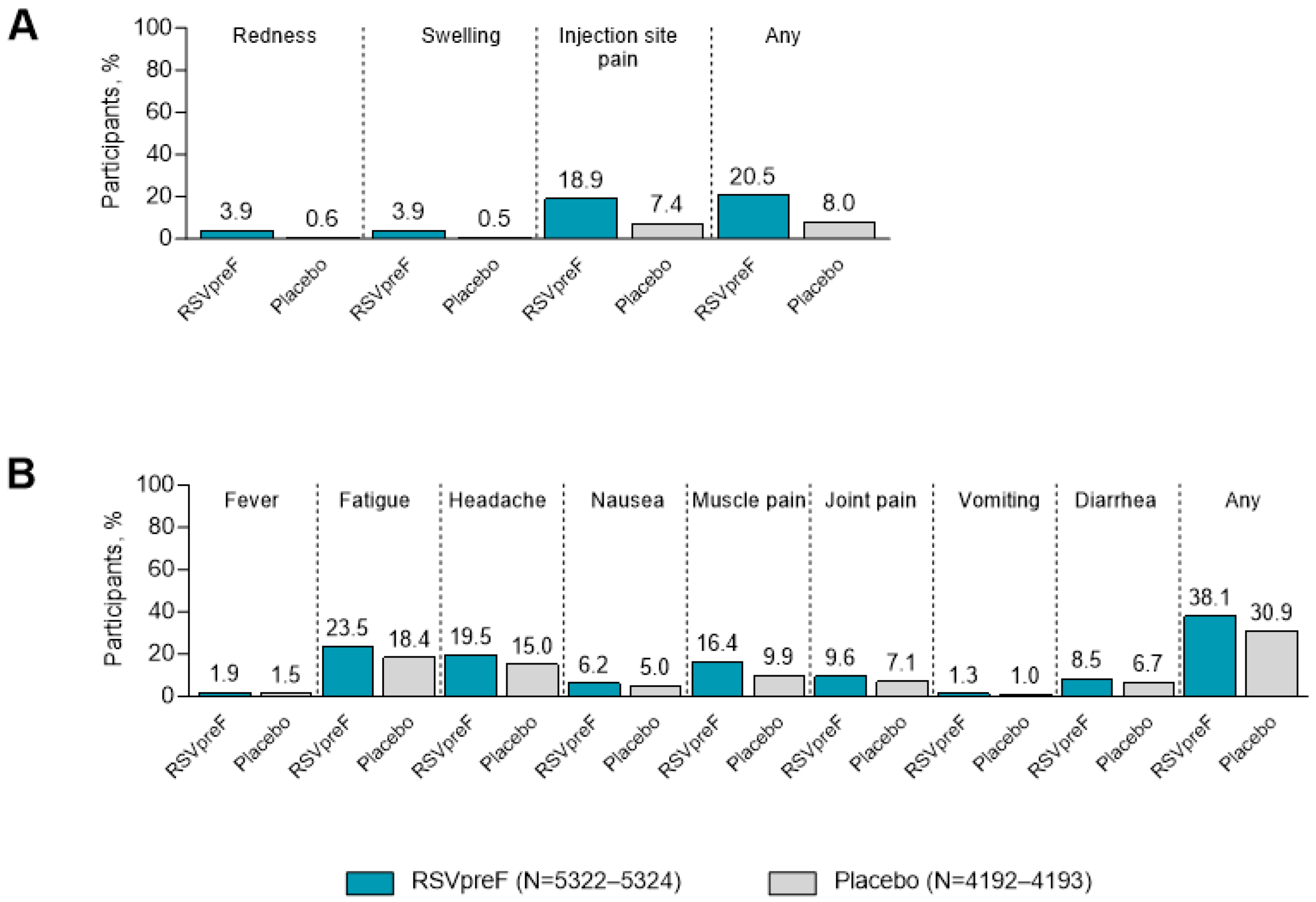
3.2. Local Reactions and Systemic Events Among ≥18-Year-Olds
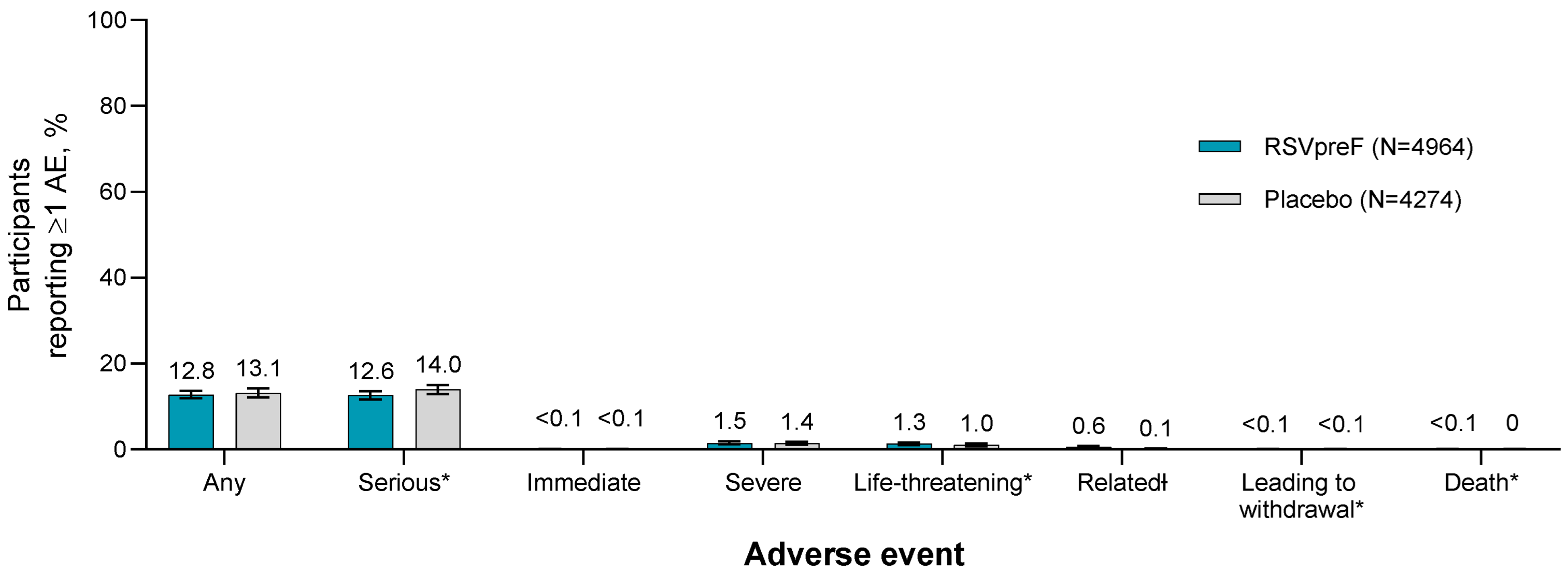
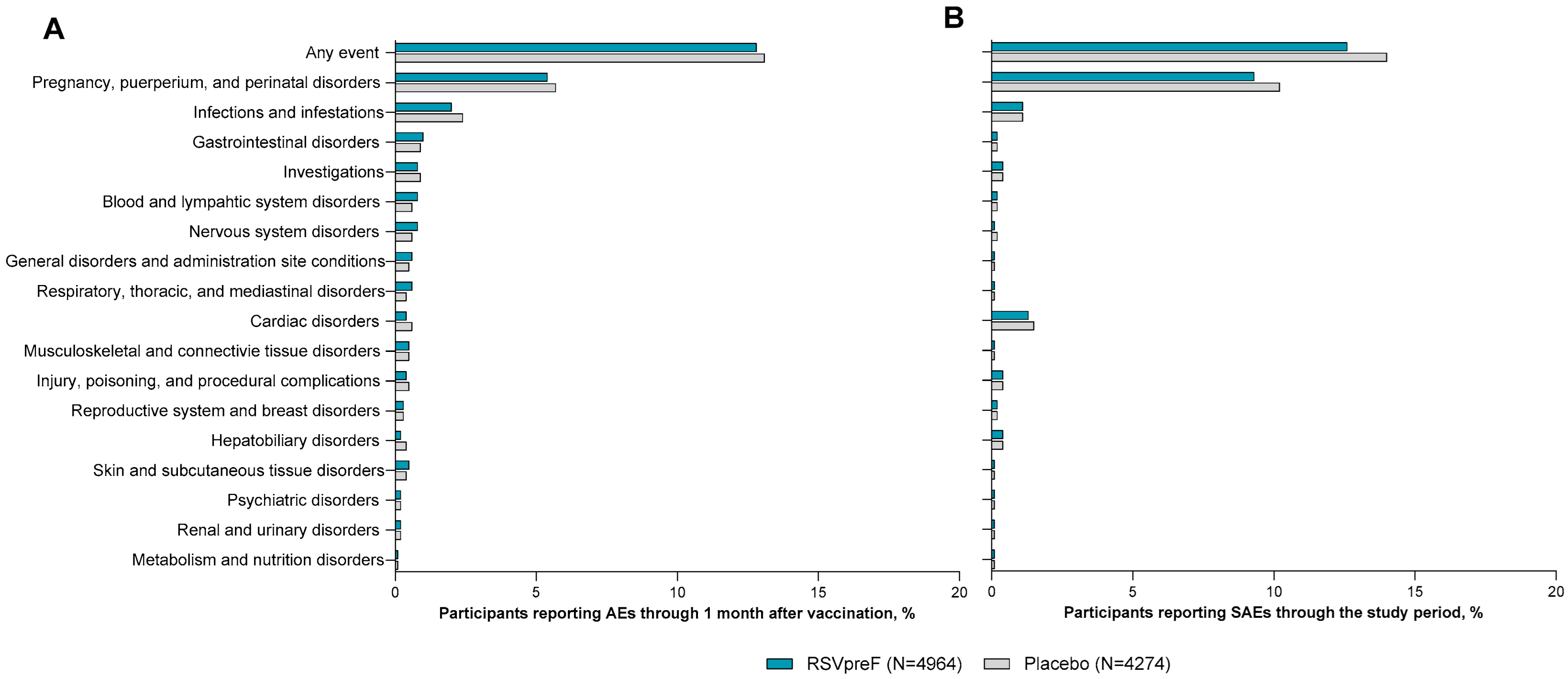
3.3. Adverse Drug Reactions Among ≥18-Year-Olds
3.4. Adverse Events Among 18- Through 59-Year-Olds
3.5. Related Adverse Events Among ≥18-Year-Olds
3.6. Post-Marketing Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen-Van-Tam, J.S.; O’Leary, M.; Martin, E.T.; Heijnen, E.; Callendret, B.; Fleischhackl, R.; Comeaux, C.; Tran, T.M.P.; Weber, K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022, 31, 220105. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory syncytial virus and associations with cardiovascular disease in adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.M.; Khan, F.; Schmitt, H.J.; Agosti, Y.; Jodar, L.; Simoes, E.A.F.; Swerdlow, D.L. Respiratory syncytial virus-associated hospitalization rates among US infants: A systematic review and meta-analysis. J. Infect. Dis. 2022, 225, 1100–1111. [Google Scholar] [CrossRef]
- Doty, B.; Ghaswalla, P.; Bohn, R.L.; Stoszek, S.K.; Panozzo, C.A. Incidence of RSV in adults: A comprehensive review of observational studies and critical gaps in information. J. Infect. Dis. 2024, 230, e1182–e1201. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. RSV in Infants and Young Children. Available online: https://www.cdc.gov/rsv/infants-young-children/index.html (accessed on 28 April 2025).
- Centers for Disease Control and Prevention. RSV in Older Adults. Available online: https://www.cdc.gov/rsv/older-adults/ (accessed on 28 April 2025).
- D’Ambrosio, F.; Lomazzi, M.; Moore, M.; Maida, A.; Ricciardi, R.; Munno, L.; Lettieri, M.; De Vito, E.; Ricciardi, W.; Calabrò, G.E. Addressing the underestimated burden of RSV in older adults in Europe: Epidemiology, surveillance gaps, and public health implications. Vaccines 2025, 13, 510. [Google Scholar] [CrossRef]
- Tin Tin Htar, M.; Yerramalla, M.S.; Moïsi, J.C.; Swerdlow, D.L. The burden of respiratory syncytial virus in adults: A systematic review and meta-analysis. Epidemiol. Infect. 2020, 148, e48. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Surveillance of RSV. Available online: https://www.cdc.gov/rsv/php/surveillance/index.html#cdc_survey_profile_surveys_used-rsv-burden-estimates (accessed on 16 July 2025).
- Howa, A.C.; Zhu, Y.; Wyatt, D.; Markus, T.; Chappell, J.D.; Halasa, N.; Trabue, C.H.; Schaffner, W.; Grijalva, C.G.; Talbot, H.K. Estimating the undetected burden of respiratory syncytial virus hospitalizations in adults through capture-recapture methods. Influenza Other Respir. Viruses 2024, 18, e13299. [Google Scholar] [CrossRef]
- Kim, T.; Choi, S.H. Epidemiology and disease burden of respiratory syncytial virus infection in adults. Infect. Chemother. 2024, 56, 1–12. [Google Scholar] [CrossRef]
- ABRYSVO® (RSVpreF). Full Prescribing Information; Pfizer Inc.: New York, NY, USA, 2025. [Google Scholar]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simoes, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Perez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef]
- Walsh, E.E.; Eiras, D.; Woodside, J.; Jiang, Q.; Patton, M.; Marc, G.P.; Llapur, C.; Ramet, M.; Fukushima, Y.; Hussen, N.; et al. Efficacy, immunogenicity, and safety of the bivalent RSV prefusion F (RSVpreF) vaccine in older adults over 2 RSV seasons. Clin. Infect. Dis. 2025, ciaf061. [Google Scholar] [CrossRef]
- European Medicines Agency. Abrysvo (Respiratory Syncytial Virus Vaccine (Bivalent, Recombinant)). Available online: https://www.ema.europa.eu/en/documents/overview/abrysvo-epar-medicine-overview_en.pdf (accessed on 22 April 2025).
- Arexvy (Respiratory Syncytial Virus Vaccine, Adjuvanted). In Full Prescribing Information; GlaxoSmithKline: Durham, NC, USA, 2025.
- mRESVIA (Respiratory Syncytial Virus Vaccine). In Full Prescribing Information; Moderna US, Inc.: Princeton, NJ, USA, 2024.
- Shaw, A. RSV Vaccination in Adults: Work Group Interpretations. In Proceedings of the Meeting of the Advisory Committee on Immunization Practices (ACIP) Respiratory Syncytial Virus (RSV) Vaccines, Adults, Virtually, 16 April 2025; Available online: https://www.cdc.gov/acip/downloads/slides-2025-04-15-16/01-Shaw-Adult-RSV-508.pdf (accessed on 18 July 2025).
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q.; et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J. Infect. Dis. 2022, 225, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.T.; Zareba, A.M.; Fitz-Patrick, D.; Essink, B.J.; Scott, D.A.; Swanson, K.A.; Chelani, D.; Radley, D.; Cooper, D.; Jansen, K.U.; et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F vaccine when coadministered with a tetanus, diphtheria, and acellular pertussis vaccine. J. Infect. Dis. 2022, 225, 2077–2086. [Google Scholar] [CrossRef]
- Schmoele-Thoma, B.; Zareba, A.M.; Jiang, Q.; Maddur, M.S.; Danaf, R.; Mann, A.; Eze, K.; Fok-Seang, J.; Kabir, G.; Catchpole, A.; et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2022, 386, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Aliabadi, N.; Munjal, I.; Jiang, Q.; Feng, Y.; Brock, L.G.; Cooper, D.; Anderson, A.S.; Swanson, K.A.; Gruber, W.C.; et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18–49 years of age: Results of a randomized phase 3 study. Vaccine 2024, 42, 3172–3179. [Google Scholar] [CrossRef]
- Davis, M.; Towner, W.; DeHaan, E.; Jiang, Q.; Li, W.; Rahman, F.; Patton, M.; Wyper, H.; Lino, M.M.; Sarwar, U.N.; et al. Bivalent RSVpreF vaccine in adults 18 to <60 years old with high-risk conditions. Clin. Infect. Dis. 2025, 80, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Center, K.J.; Tita, A.T.N.; Swanson, K.A.; Radley, D.; Houghton, J.; McGrory, S.B.; Gomme, E.; Anderson, M.; Roberts, J.P.; et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 2022, 386, 1615–1626. [Google Scholar] [CrossRef]
- Simões, E.A.F.; Pahud, B.A.; Madhi, S.A.; Kampmann, B.; Shittu, E.; Radley, D.; Llapur, C.; Baker, J.; Pérez Marc, G.; Barnabas, S.L.; et al. Efficacy, safety, and immunogenicity of the MATISSE (Maternal Immunization Study for Safety and Efficacy) maternal respiratory syncytial virus prefusion F protein vaccine trial. Obstet. Gynecol. 2025, 145, 157–167. [Google Scholar] [CrossRef]
- Department of Health and Human Services; US Food and Drug Administration. Meta-Analyses of Randomized Controlled Clinical Trials to Evaluate the Safety of Human Drugs or Biological Products: Guidance for Industry. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM625241.pdf (accessed on 19 November 2018).
- Food and Drug Administration. Guidance for Industry: Adverse Reactions Section of Labeling for Human Prescription Drug and Biological Products. Available online: https://www.fda.gov/media/72139/download (accessed on 30 April 2025).
- Uppsala Monitoring Centre. Pharmacovigilance Communications Glossary. Available online: https://who-umc.org/pharmacovigilance-communications/glossary/ (accessed on 29 April 2025).
- European Medicines Agency. Abrysvo Summary of Product Charactersitcs. Available online: https://www.ema.europa.eu/en/documents/product-information/abrysvo-epar-product-information_en.pdf (accessed on 8 May 2025).
- Newcombe, R.G. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat. Med. 1998, 17, 873–890. [Google Scholar] [CrossRef]
- Kohl, K.S.; Bonhoeffer, J.; Braun, M.M.; Chen, R.T.; Duclos, P.; Heijbel, H.; Heininger, U.; Loupi, E.; Marcy, S.M.; The Brighton, C. The Brighton collaboration: Creating a global standard for case definitions (and guidelines) for adverse events following immunization. In Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology); Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005. [Google Scholar]
- Britton, A.; Roper, L.E.; Kotton, C.N.; Hutton, D.W.; Fleming-Dutra, K.E.; Godfrey, M.; Ortega-Sanchez, I.R.; Broder, K.R.; Talbot, H.K.; Long, S.S.; et al. Use of respiratory syncytial virus vaccines in adults aged ≥60 years: Updated recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 696–702. [Google Scholar] [CrossRef]
- Njue, A.; Nuabor, W.; Lyall, M.; Margulis, A.; Mauskopf, J.; Curcio, D.; Kurosky, S.; Gessner, B.D.; Begier, E. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect. Dis. 2023, 10, ofad513. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Symptoms and Care of RSV. Available online: https://www.cdc.gov/rsv/symptoms/index.html (accessed on 29 April 2025).
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect. Dis. 2019, 6, 2049936119865798. [Google Scholar] [CrossRef]
- Wyffels, V.; Kariburyo, F.; Gavart, S.; Fleischhackl, R.; Yuce, H. A real-world analysis of patient characteristics and predictors of hospitalization among US Medicare beneficiaries with respiratory syncytial virus infection. Adv. Ther. 2020, 37, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current state of respiratory syncytial virus disease and management. Infect. Dis. Ther. 2021, 10, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of respiratory syncytial virus infection in older adults: Limitations of current data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef]
- Kenmoe, S.; Nair, H. The disease burden of respiratory syncytial virus in older adults. Curr. Opin. Infect. Dis. 2024, 37, 129–136. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Judy, J.; Tran, D.; Yacisin, K.; Kurosky, S.K.; Begier, E. Low levels of RSV testing among adults hospitalized for lower respiratory tract infection in the United States. Infect. Dis. Ther. 2023, 12, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: A systematic literature review and meta-analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef]
- Li, Y.; Kenmoe, S.; Beiger, E.; Wahi-Singh, P.; Lianga, C.; Wahi-Singh, B.; Sato, R.; Gessner, B.; Nair, H. Hospitalisation Burden Estimates of Respiratory Syncytial Virus (RSV) with Adjustment for Case Underascertainment in Adults Aged 18 to 64 Years in High-Income Countries. In Proceedings of the RSVVW 24. 8th ReSViNET Conference, Mumbai, India, 13–16 February 2024. [Google Scholar]
- Li, Y.; Kulkarni, D.; Begier, E.; Wahi-Singh, P.; Wahi-Singh, B.; Gessner, B.; Nair, H. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: A systematic review and modelling study. Infect. Dis. Ther. 2023, 12, 1137–1149. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Possible Side Effects from Vaccines. Available online: https://www.cdc.gov/vaccines/basics/possible-side-effects.html#:~:text=Influenza%20(inactivated)%20vaccine%20side%20effects&text=Soreness%2C%20redness%2C%20and%20swelling%20where,vaccine%20(the%20flu%20shot) (accessed on 30 April 2025).
- Li, Y.; Li, J.; Dang, Y.; Chen, Y.; Tao, C. Adverse events of COVID-19 vaccines in the United States: Temporal and spatial analysis. JMIR Public. Health Surveill. 2024, 10, e51007. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Key Facts About Seasonal Flu Vaccine. Available online: https://www.cdc.gov/flu/vaccines/keyfacts.html (accessed on 29 April 2025).
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin. Infect. Dis. 2024, 78, 1732–1744. [Google Scholar] [CrossRef]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and immunogenicity of an mRNA-based RSV vaccine including a 12-month booster in a phase 1 clinical trial in healthy older adults. J. Infect. Dis. 2024, 230, e647–e656. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Chan, E.W.W.; Leung, M.T.Y.; Lau, L.K.W.; Leung, J.; Lum, D.; Wong, R.S.; Li, X.; Chui, C.S.L.; Wan, E.Y.F.; Wong, C.K.H.; et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: A prospective cohort study. Int. J. Infect. Dis. 2022, 116, 47–50. [Google Scholar] [CrossRef]
- Nachtigall, I.; Bonsignore, M.; Hohenstein, S.; Bollmann, A.; Günther, R.; Kodde, C.; Englisch, M.; Ahmad-Nejad, P.; Schröder, A.; Glenz, C.; et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: A survey among health care workers. BMC Infect. Dis. 2022, 22, 291. [Google Scholar] [CrossRef]
- Kiely, M.; Tadount, F.; Lo, E.; Sadarangani, M.; Wei, S.Q.; Rafferty, E.; Quach, C.; MacDonald, S.E. Sex differences in adverse events following seasonal influenza vaccines: A meta-analysis of randomised controlled trials. J. Epidemiol. Community Health 2023, 77, 791–801. [Google Scholar] [CrossRef]
- Anjidani, N.; Shahpari, R.; Kafi, H.; Petrovsky, N.; Barati, S. Effects of age and gender on immunogenicity and reactogenicity of SpikoGen recombinant spike protein vaccine: A post-hoc analysis. Sci. Rep. 2024, 14, 22631. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Balzano-Nogueira, L.; Lleo, A.; Conesa, A. Transcriptional differences for COVID-19 disease map genes between males and females indicate a different basal immunophenotype relevant to the disease. Genes 2020, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Protein subunit RSV vaccines (GSK Arexvy and Pfizer Abrysvo) in older adults. 2024. Available online: https://www.cdc.gov/acip/grade/protein-subunit-rsv-vaccines-older-adults.html (accessed on 30 July 2025).
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barre syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef]
- Yang, B.; Lian, Y.; Liu, Y.; Wu, B.Y.; Duan, R.S. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J. Neuroimmunol. 2016, 293, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.D.; Park, S.; Lee, S.; Jang, W.; Park, J.; Lee, K.; Lee, J.; Kang, J.; Udeh, R.; Rahmati, M.; et al. Global burden of vaccine-associated Guillain-Barre syndrome over 170 countries from 1967 to 2023. Sci. Rep. 2024, 14, 24561. [Google Scholar] [CrossRef]
- Goud, R.; Lufkin, B.; Duffy, J.; Whitaker, B.; Wong, H.L.; Liao, J.; Lo, A.C.; Parulekar, S.; Agger, P.; Anderson, S.A.; et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in Medicare beneficiaries. JAMA Intern. Med. 2021, 181, 1623–1630. [Google Scholar] [CrossRef]
- Lucas, S.; Ailani, J.; Smith, T.R.; Abdrabboh, A.; Xue, F.; Navetta, M.S. Pharmacovigilance: Reporting requirements throughout a product’s lifecycle. Ther. Adv. Drug Saf. 2022, 13, 20420986221125006. [Google Scholar] [CrossRef] [PubMed]
- Hodel, K.V.S.; Fiuza, B.S.D.; Conceição, R.S.; Aleluia, A.C.M.; Pitanga, T.N.; Fonseca, L.; Valente, C.O.; Minafra-Rezende, C.S.; Machado, B.A.S. Pharmacovigilance in vaccines: Importance, main aspects, perspectives, and challenges—A narrative review. Pharmaceuticals 2024, 17, 807. [Google Scholar] [CrossRef] [PubMed]
- Araja, D.; Krumina, A.; Nora-Krukle, Z.; Berkis, U.; Murovska, M. Vaccine vigilance system: Considerations on the effectiveness of vigilance data use in COVID-19 vaccination. Vaccines 2022, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.G.; David, M.P.; Zima, J.; Baril, L.; Dubin, G.; Arellano, F.; Struyf, F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol. Drug Saf. 2014, 23, 466–479. [Google Scholar] [CrossRef]
- Maurer, G.; Buerger, V.; Larcher-Senn, J.; Erlsbacher, F.; Dubischar, K.; Eder-Lingelbach, S.; Jaramillo, J.C. Pooled safety evaluation for a new single-shot live-attenuated chikungunya vaccine. J. Travel. Med. 2024, 31, taae133. [Google Scholar] [CrossRef]
- Smith, K.; Hegazy, K.; Cai, M.R.; McKnight, I.; Rousculp, M.D.; Alves, K. Safety of the NVX-CoV2373 COVID-19 vaccine in randomized placebo-controlled clinical trials. Vaccine 2023, 41, 3930–3936. [Google Scholar] [CrossRef]
- Gailhardou, S.; Skipetrova, A.; Dayan, G.H.; Jezorwski, J.; Saville, M.; Van der Vliet, D.; Wartel, T.A. Safety overview of a recombinant live-attenuated tetravalent dengue vaccine: Pooled analysis of data from 18 clinical trials. PLoS Negl. Trop. Dis. 2016, 10, e0004821. [Google Scholar] [CrossRef] [PubMed]
- Hồ, N.T.; Hughes, S.G.; Ta, V.T.; Phan, L.T.; Đỗ, Q.; Nguyễn, T.V.; Phạm, A.T.V.; Thị Ngọc Đặng, M.; Nguyễn, L.V.; Trịnh, Q.V.; et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: Pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat. Commun. 2024, 15, 4081. [Google Scholar] [CrossRef] [PubMed]
| Study Name/Number (NCT Number) | Design | Location | Population | Number of Participants ≥18 Years of Age | |
|---|---|---|---|---|---|
| Total Randomized | Included in Safety Analysis | ||||
| C3671001 (NCT03529773) [20,21] | Phase 1/2 randomized, placebo-controlled, observer-blind, dose-finding first-in-human study | United States | Healthy male and non-pregnant female participants 50–85 years of age | 617 | 291 * |
| C3671004 (NCT04071158) [22] | Phase 2b randomized, placebo-controlled, multi-center, observer-blind, noninferiority study | United States | Healthy non-pregnant female participants 18–49 years of age receiving concomitant Tdap | 427 | 421 |
| WI257521 (NCT04785612) [23] | Phase 2a, randomized, placebo-controlled, single-center, double-blind, exploratory human challenge study | United Kingdom | Healthy male and non-pregnant female participants 19–50 years of age | 70 | 70 |
| RENOIR (NCT05035212) [15] | Phase 3, randomized, placebo-controlled, multi-center, double-blind study | Argentina, Canada, Finland, Japan, Netherlands, South Africa, United States | Healthy † male and non-pregnant participants 59–97 years of age | 36,862 | 36,862 |
| C3671014 (NCT05096208) [24] | Phase 3, randomized, placebo-controlled, multi-center, double-blind, lot consistency study | United States | Healthy male and non-pregnant female participants 18–49 years of age | 993 | 992 |
| MONET Substudy A (NCT05842967) [25] | Phase 3, randomized, placebo-controlled, multi-center, double-blind study | United States | Healthy male and non-pregnant female participants 18–59 years of age and at high risk of severe RSV disease | 681 | 678 |
| C3671003 (NCT04032093) [26] | Phase 2b, observer-blinded, randomized, placebo-controlled, dose-finding and proof-of-concept study | Argentina, Chile, South Africa, United States | Healthy pregnant female participants 18–49 years of age | 232 | 232 |
| MATISSE (NCT04424316) [14,27] | Phase 3, multi-center, double-blinded, placebo-controlled trial | Argentina, Australia, Brazil, Canada, Chile, Denmark, Finland, Gambia, Japan, Mexico, Netherlands, New Zealand, Philippines, Republic of Korea, South Africa, Spain, Taiwan, United States | Healthy pregnant female participants 14–47 years of age | 7420 | 7367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilangovan, K.; Radley, D.; Patton, M.; Shittu, E.; Lino, M.M.; Goulas, C.; Swanson, K.A.; Anderson, A.S.; Gurtman, A.; Munjal, I. Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine. Vaccines 2025, 13, 827. https://doi.org/10.3390/vaccines13080827
Ilangovan K, Radley D, Patton M, Shittu E, Lino MM, Goulas C, Swanson KA, Anderson AS, Gurtman A, Munjal I. Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine. Vaccines. 2025; 13(8):827. https://doi.org/10.3390/vaccines13080827
Chicago/Turabian StyleIlangovan, Kumar, David Radley, Michael Patton, Emma Shittu, Maria Maddalena Lino, Christos Goulas, Kena A. Swanson, Annaliesa S. Anderson, Alejandra Gurtman, and Iona Munjal. 2025. "Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine" Vaccines 13, no. 8: 827. https://doi.org/10.3390/vaccines13080827
APA StyleIlangovan, K., Radley, D., Patton, M., Shittu, E., Lino, M. M., Goulas, C., Swanson, K. A., Anderson, A. S., Gurtman, A., & Munjal, I. (2025). Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine. Vaccines, 13(8), 827. https://doi.org/10.3390/vaccines13080827







