Protection Against Transplacental Transmission of a Highly Virulent Classical Swine Fever Virus Two Weeks After Single-Dose FlagT4G Vaccination in Pregnant Sows
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Experimental Design
2.3. Evaluation of Humoral Response Against CSFV E2 and Neutralizing Antibodies
2.4. ELISA for IFN-α Detection in Serum Samples
2.5. Detection of CSFV RNA
3. Results
3.1. FlagT4G Provides Clinical Protection Against CSFV Challenge in Pregnant Sows at 14 Days After Single Vaccination
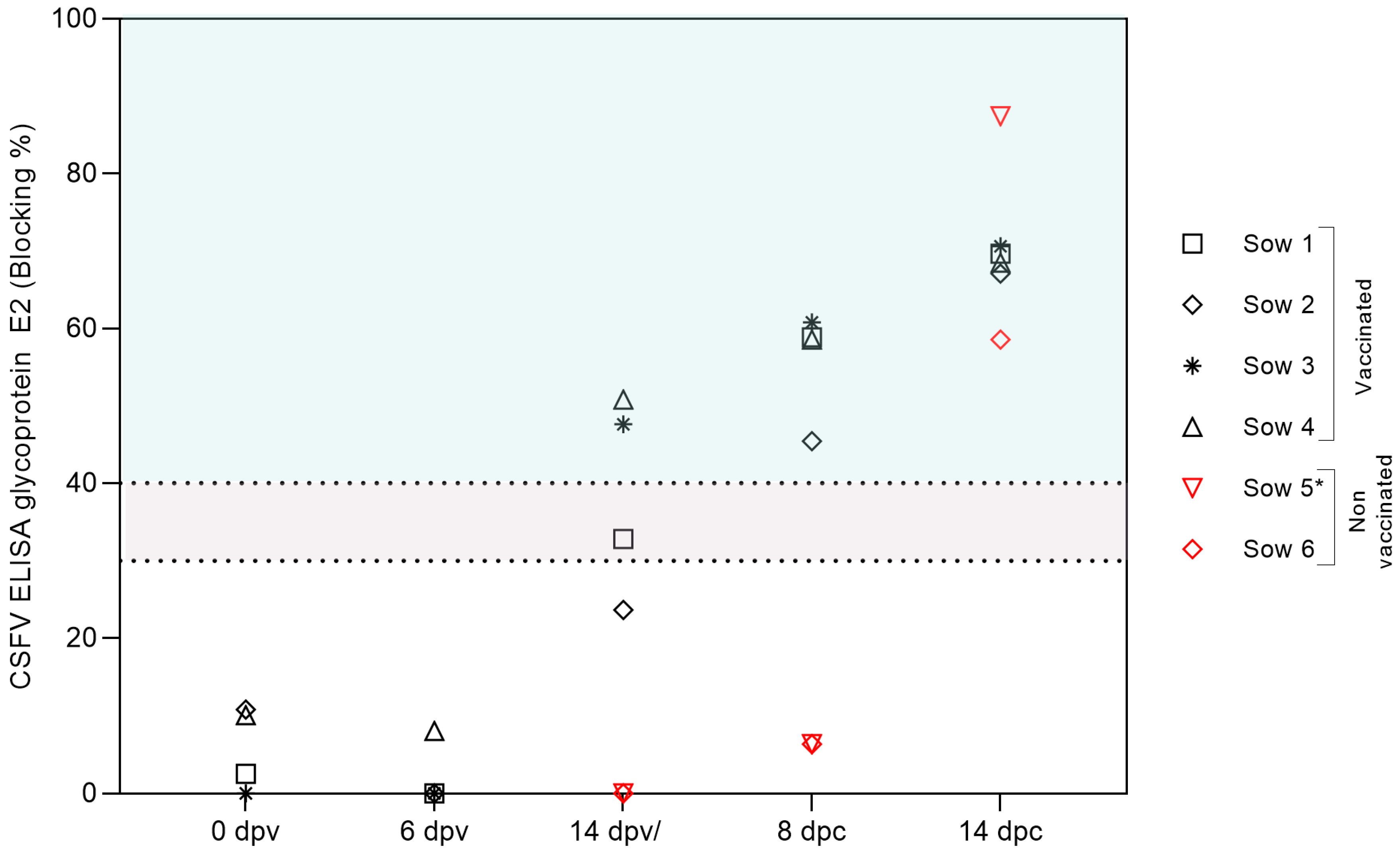
3.2. Robust Humoral Immune Response Evidenced by E2-Specific and Neutralizing Antibody Production in FlagT4G-Vaccinated Pregnant Sows
3.3. Absence of Viremia and Viral Shedding in Pregnant Sows Two Weeks After Single-Dose FlagT4G Vaccination
3.4. Complete Early Protection of Fetuses Against Highly Virulent CSFV Challenge Following Single-Dose FlagT4G Vaccination in Pregnant Sows
3.5. Lack of Humoral and Type I Interferon Responses in Fetuses from FlagT4G-Vaccinated Sows Following CSFV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moennig, V. The Control of Classical Swine Fever in Wild Boar. Front. Microbiol. 2015, 6, 1211. [Google Scholar] [CrossRef]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever—An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Tautz, N.; Tews, B.A.; Meyers, G. The Molecular Biology of Pestiviruses. Adv. Virus Res. 2015, 93, 47–160. [Google Scholar] [CrossRef]
- Rümenapf, T.; Unger, G.; Strauss, J.H.; Thiel, H.J. Processing of the Envelope Glycoproteins of Pestiviruses. J. Virol. 1993, 67, 3288–3294. [Google Scholar] [CrossRef]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical Signs and Epidemiology of Classical Swine Fever: A Review of New Knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T. Vaccinology of Classical Swine Fever: From Lab to Field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, Y.; Zhang, G. Research Progress and Challenges in Vaccine Development against Classical Swine Fever Virus. Viruses 2021, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical Swine Fever Vaccines—State-of-the-Art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef]
- Suradhat, S.; Damrongwatanapokin, S.; Thanawongnuwech, R. Factors Critical for Successful Vaccination against Classical Swine Fever in Endemic Areas. Vet. Microbiol. 2007, 119, 1–9. [Google Scholar] [CrossRef]
- Graham, S.P.; Everett, H.E.; Haines, F.J.; Johns, H.L.; Sosan, O.A.; Salguero, F.J.; Clifford, D.J.; Steinbach, F.; Drew, T.W.; Crooke, H.R. Challenge of Pigs with Classical Swine Fever Viruses after C-Strain Vaccination Reveals Remarkably Rapid Protection and Insights into Early Immunity. PLoS ONE 2012, 7, e29310. [Google Scholar] [CrossRef]
- Eblé, P.L.; Geurts, Y.; Quak, S.; Moonen-Leusen, H.W.; Blome, S.; Hofmann, M.A.; Koenen, F.; Beer, M.; Loeffen, W.L.A. Efficacy of Chimeric Pestivirus Vaccine Candidates against Classical Swine Fever: Protection and DIVA Characteristics. Vet. Microbiol. 2013, 162, 437–446. [Google Scholar] [CrossRef]
- Ahrens, U. Efficacy of the Classical Swine Fever (CSF) Marker Vaccine Porcilis® Pesti in Pregnant Sows. Vet. Microbiol. 2000, 77, 83–97. [Google Scholar] [CrossRef]
- Depner, K.R.; Bouma, A.; Koenen, F.; Klinkenberg, D.; Lange, E.; de Smit, H.; Vanderhallen, H. Classical Swine Fever (CSF) Marker Vaccine. Vet. Microbiol. 2001, 83, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pedroso, M.; Sordo-Puga, Y.; Sosa-Teste, I.; Rodriguez-Molto, M.P.; Naranjo-Valdés, P.; Sardina-González, T.; Santana-Rodríguez, E.; Montero-Espinosa, C.; Frías-Laporeaux, M.T.; Fuentes-Rodríguez, Y.; et al. Novel Chimeric E2CD154 Subunit Vaccine Is Safe and Confers Long Lasting Protection against Classical Swine Fever Virus. Vet. Immunol. Immunopathol. 2021, 234, 110222. [Google Scholar] [CrossRef]
- Suárez, M.; Sordo, Y.; Prieto, Y.; Rodríguez, M.P.; Méndez, L.; Rodríguez, E.M.; Rodríguez-Mallon, A.; Lorenzo, E.; Santana, E.; González, N.; et al. A Single Dose of the Novel Chimeric Subunit Vaccine E2-CD154 Confers Early Full Protection against Classical Swine Fever Virus. Vaccine 2017, 35, 4437–4443. [Google Scholar] [CrossRef]
- Sordo-Puga, Y.; Suárez-Pedroso, M.; Naranjo-Valdéz, P.; Pérez-Pérez, D.; Santana-Rodríguez, E.; Sardinas-Gonzalez, T.; Mendez-Orta, M.K.; Duarte-Cano, C.A.; Estrada-Garcia, M.P.; Rodríguez-Moltó, M.P. Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus. Vaccines 2021, 9, 167. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Sordo, Y.; Pérez-Simó, M.; Suárez, M.; Canturri, A.; Rodriguez, M.P.; Frías-Lepoureau, M.T.; Domingo, M.; Estrada, M.P.; Ganges, L. Efficacy of E2 Glycoprotein Fused to Porcine CD154 as a Novel Chimeric Subunit Vaccine to Prevent Classical Swine Fever Virus Vertical Transmission in Pregnant Sows. Vet. Microbiol. 2017, 205, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, F.; Renzullo, S.; Faust, A.; Beer, M.; Kaden, V.; Hofmann, M.A. Chimeric Pestiviruses: Candidates for Live-Attenuated Classical Swine Fever Marker Vaccines. J. Gen. Virol. 2007, 88, 2247–2258. [Google Scholar] [CrossRef]
- Postel, A.; Becher, P. Genetically Distinct Pestiviruses Pave the Way to Improved Classical Swine Fever Marker Vaccine Candidates Based on the Chimeric Pestivirus Concept. Emerg. Microbes Infect. 2020, 9, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Wernike, K.; Reimann, I.; König, P.; Moß, C.; Beer, M. A Decade of Research into Classical Swine Fever Marker Vaccine CP7_E2alf (Suvaxyn® CSF Marker): A Review of Vaccine Properties. Vet. Res. 2017, 48, 51. [Google Scholar] [CrossRef]
- Henke, J.; Carlson, J.; Zani, L.; Leidenberger, S.; Schwaiger, T.; Schlottau, K.; Teifke, J.P.; Schröder, C.; Beer, M.; Blome, S. Protection against Transplacental Transmission of Moderately Virulent Classical Swine Fever Virus Using Live Marker Vaccine “CP7_E2alf. ” Vaccine 2018, 36, 4181–4187. [Google Scholar] [CrossRef]
- Holinka, L.G.; O’Donnell, V.; Risatti, G.R.; Azzinaro, P.; Arzt, J.; Stenfeldt, C.; Velazquez-Salinas, L.; Carlson, J.; Gladue, D.P.; Borca, M.V. Early Protection Events in Swine Immunized with an Experimental Live Attenuated Classical Swine Fever Marker Vaccine, FlagT4G. PLoS ONE 2017, 12, e0177433. [Google Scholar] [CrossRef]
- Bohórquez, J.A.; Defaus, S.; Rosell, R.; Pérez-Simó, M.; Alberch, M.; Gladue, D.P.; Borca, M.V.; Andreu, D.; Ganges, L. Development of a Dendrimeric Peptide-Based Approach for the Differentiation of Animals Vaccinated with Flagt4g against Classical Swine Fever from Infected Pigs. Viruses 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Coronado, L.; Muñoz-Aguilera, A.; Cantero, G.; Martínez, P.; Alberch, M.; Rosell, R.; Gladue, D.P.; Borca, M.V.; Ganges, L. FlagT4G Vaccine Prevents Transplacental Transmission of Highly Virulent Classical Swine Fever Virus after Single Vaccination in Pregnant Sows. Vaccines 2024, 12, 832. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Perera, C.L.; Rodríguez, L.J.P.; Frias-Lepoureau, M.T.; Becher, P. Classical Swine Fever Virus Isolates from Cuba Form a New Subgenotype 1.4. Vet. Microbiol. 2013, 161, 334–338. [Google Scholar] [CrossRef]
- Wensvoort, G.; Terpstra, C.; Boonstra, J.; Bloemraad, M.; Van Zaane, D. Production of Monoclonal Antibodies against Swine Fever Virus and Their Use in Laboratory Diagnosis. Vet. Microbiol. 1986, 12, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Terpstra, C.; Bloemraad, M.; Gielkens, A.L.J. The Neutralizing Peroxidase-Linked Assay for Detection of Antibody against Swine Fever Virus. Vet. Microbiol. 1984, 9, 113–120. [Google Scholar] [CrossRef]
- Terpstra, C.; Wensvoort, G. The Protective Value of Vaccine-Induced Neutralising Antibody Titres in Swine Fever. Vet. Microbiol. 1988, 16, 123–128. [Google Scholar] [CrossRef]
- Diaz de Arce, H.; Artursson, K.; L’Haridon, R.; Perers, A.; La Bonnardiere, C.; Alm, G.V. A Sensitive Immunoassay for Porcine Interferon-α. Vet. Immunol. Immunopathol. 1992, 30, 319–327. [Google Scholar] [CrossRef]
- Guzylack-Piriou, L.; Balmelli, C.; McCullough, K.C.; Summerfield, A. Type-A CpG Oligonucleotides Activate Exclusively Porcine Natural Interferon-producing Cells to Secrete Interferon-α, Tumour Necrosis Factor-α and Interleukin-12. Immunology 2004, 112, 28–37. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a Real-Time RT-PCR Assay for Sensitive and Specific Detection of Classical Swine Fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Pérez-Simó, M.; Colom-Cadena, A.; Cabezón, O.; Bohórquez, J.A.; Rosell, R.; Pérez, L.J.; Marco, I.; Lavín, S.; Domingo, M.; et al. Classical Swine Fever Virus vs. Classical Swine Fever Virus: The Superinfection Exclusion Phenomenon in Experimentally Infected Wild Boar. PLoS ONE 2016, 11, e0149469. [Google Scholar] [CrossRef]
- Lowings, P.; Ibata, G.; Needham, J.; Paton, D. Classical Swine Fever Virus Diversity and Evolution. J. Gen. Virol. 1996, 77, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids 1999, 41, 95–98. [Google Scholar]
- World Organisation for Animal Health (WOAH). Chapter 3.9.3: Classical Swine Fever Virus (Infection with Classical Swine Fever Virus). In WOAH Terrestrial Manual; WOAH: Paris, France, 2022. [Google Scholar]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, Diagnosis and Control of Classical Swine Fever: Recent Developments and Future Challenges. Transbound. Emerg. Dis. 2018, 65, 248–261. [Google Scholar] [CrossRef]
- Acosta, A.; Cardenas, N.C.; Imbacuan, C.; Lentz, H.H.K.; Dietze, K.; Amaku, M.; Burbano, A.; Gonçalves, V.S.P.; Ferreira, F. Modelling Control Strategies against Classical Swine Fever: Influence of Traders and Markets Using Static and Temporal Networks in Ecuador. Prev. Vet. Med. 2022, 205, 105683. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-N.; Chen, Y.-H. Marker Vaccine Strategies and Candidate CSFV Marker Vaccines. Vaccine 2007, 25, 205–230. [Google Scholar] [CrossRef]
- Swanson, I.M.; Haralambieva, I.H.; Rasche, M.M.; Ovsyannikova, I.G.; Kennedy, R.B. Frequencies of SARS-CoV-2 Spike Protein-Specific Memory B Cells in Human PBMCs, Quantified by ELISPOT Assay. In Handbook of ELISPOT; Springer: Berlin/Heidelberg, Germany, 2024; pp. 153–166. [Google Scholar]
- Piriou, L.; Chevallier, S.; Hutet, E.; Charley, B.; Le Potiera, M.-F.; Albina, E. Humoral and Cell-Mediated Immune Responses of d/d Histocompatible Pigs against Classical Swine Fever (CSF) Virus. Vet. Res. 2003, 34, 389–404. [Google Scholar] [CrossRef]
- Weskamm, L.M.; Dahlke, C.; Addo, M.M. Flow Cytometric Protocol to Characterize Human Memory B Cells Directed against SARS-CoV-2 Spike Protein Antigens. STAR Protoc. 2022, 3, 101902. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; Velazquez-Salinas, L.; Valladares, A.; Rai, A.; Burton, L.; Sastre, L.; Silva, E.; Risatti, G.R.; Ganges, L.; Borca, M.V. Assessment of the Reversion to Virulence and Protective Efficacy in Pigs Receiving the Live Attenuated Classical Swine Fever Recombinant Vaccine Candidate FlagT4G. Vaccines 2025, 13, 544. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronado, L.; Cobos, À.; Muñoz-Aguilera, A.; Puente-Marin, S.; Guevara, G.; Riquelme, C.; Heredia, S.; Borca, M.V.; Ganges, L. Protection Against Transplacental Transmission of a Highly Virulent Classical Swine Fever Virus Two Weeks After Single-Dose FlagT4G Vaccination in Pregnant Sows. Vaccines 2025, 13, 803. https://doi.org/10.3390/vaccines13080803
Coronado L, Cobos À, Muñoz-Aguilera A, Puente-Marin S, Guevara G, Riquelme C, Heredia S, Borca MV, Ganges L. Protection Against Transplacental Transmission of a Highly Virulent Classical Swine Fever Virus Two Weeks After Single-Dose FlagT4G Vaccination in Pregnant Sows. Vaccines. 2025; 13(8):803. https://doi.org/10.3390/vaccines13080803
Chicago/Turabian StyleCoronado, Liani, Àlex Cobos, Adriana Muñoz-Aguilera, Sara Puente-Marin, Gemma Guevara, Cristina Riquelme, Saray Heredia, Manuel V. Borca, and Llilianne Ganges. 2025. "Protection Against Transplacental Transmission of a Highly Virulent Classical Swine Fever Virus Two Weeks After Single-Dose FlagT4G Vaccination in Pregnant Sows" Vaccines 13, no. 8: 803. https://doi.org/10.3390/vaccines13080803
APA StyleCoronado, L., Cobos, À., Muñoz-Aguilera, A., Puente-Marin, S., Guevara, G., Riquelme, C., Heredia, S., Borca, M. V., & Ganges, L. (2025). Protection Against Transplacental Transmission of a Highly Virulent Classical Swine Fever Virus Two Weeks After Single-Dose FlagT4G Vaccination in Pregnant Sows. Vaccines, 13(8), 803. https://doi.org/10.3390/vaccines13080803





