Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Animals, and Ethics Statements
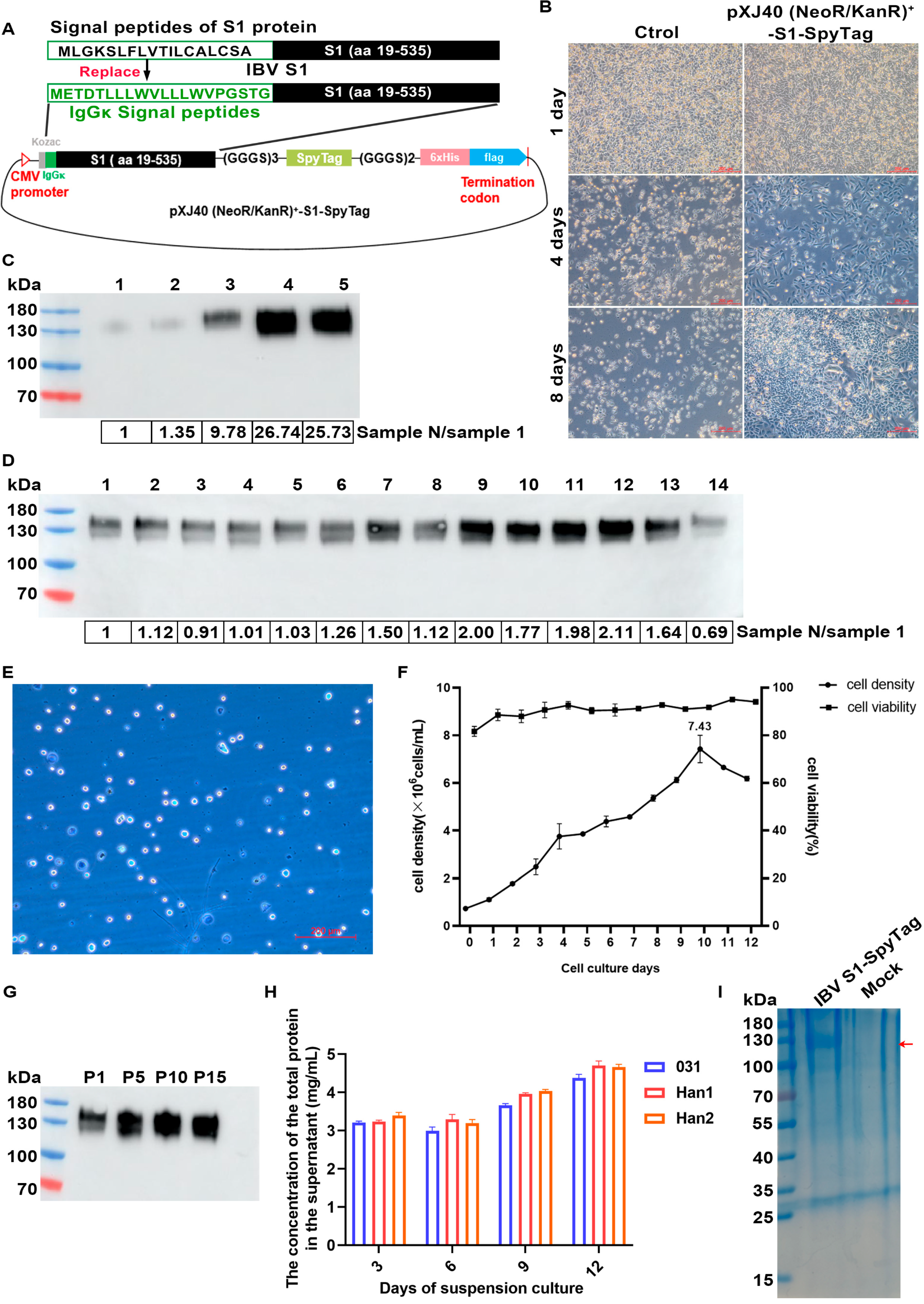
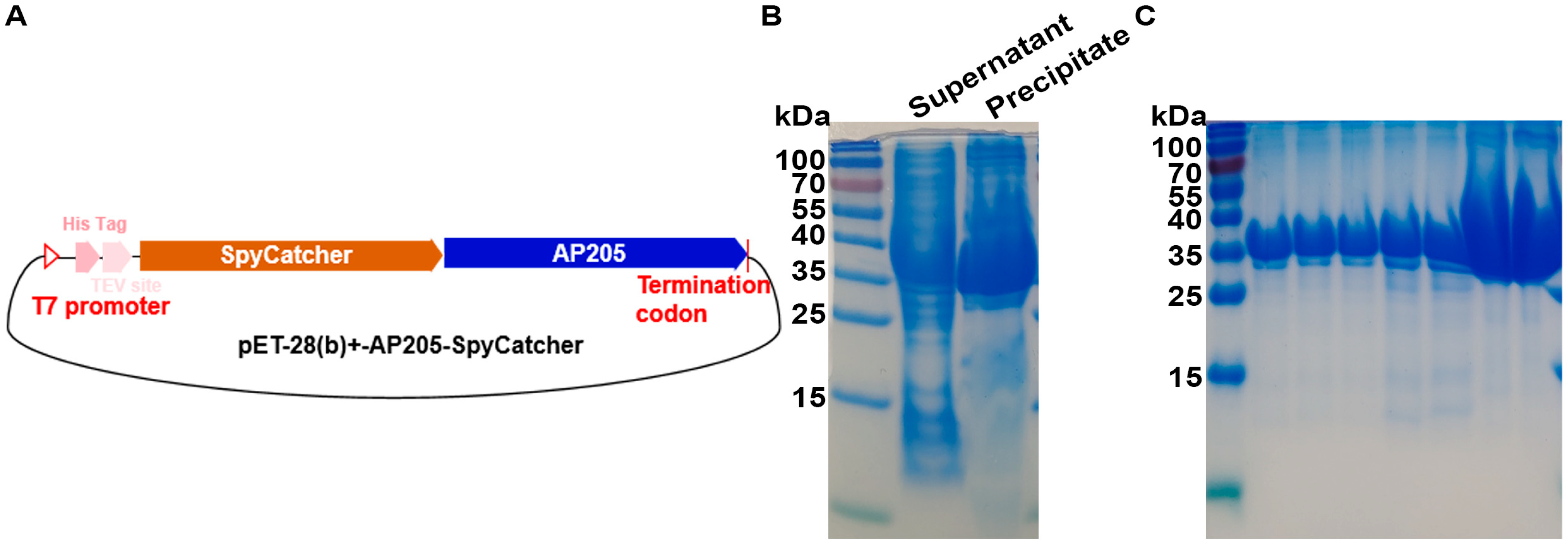
2.2. Cloning, Expression, and Purification of Recombinant S1-SpyTag and AP205-SpyCatcher Proteins
2.3. Generation and Verification of nAP205-S1
2.4. Vaccine Preparations
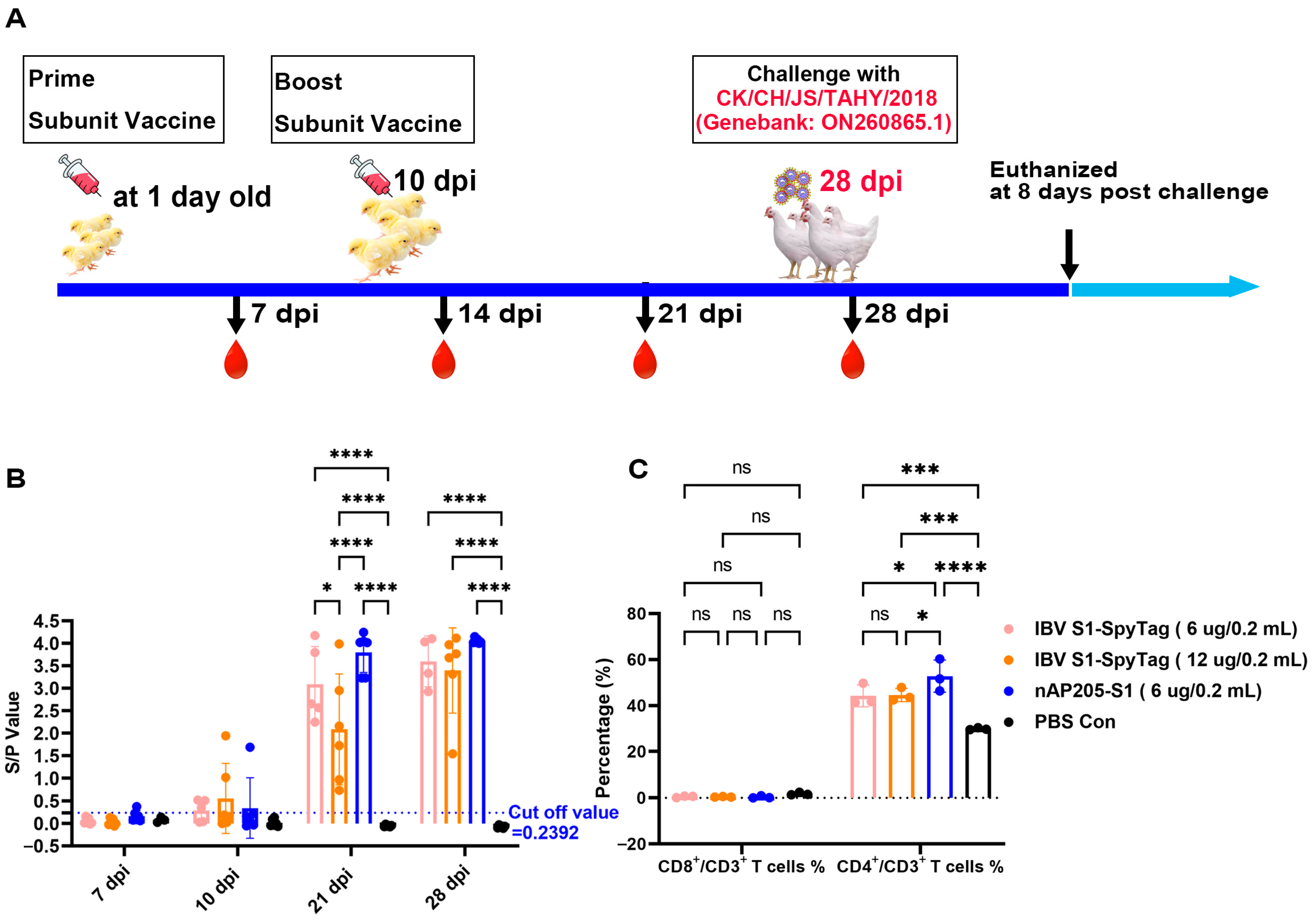
2.5. Immunization of Chickens and Virus Challenges
2.6. Antibody Detection and Cellular Immunoassay
2.7. Statistical Analysis
3. Results
3.1. Expression and Purification of IBV S1-SpyTag
3.2. Expression and Purification of Acinetobacter Phage AP205 Capsid Protein AP205-SpyCatcher
3.3. Generation of nAP205-S1 Vaccine Preparations
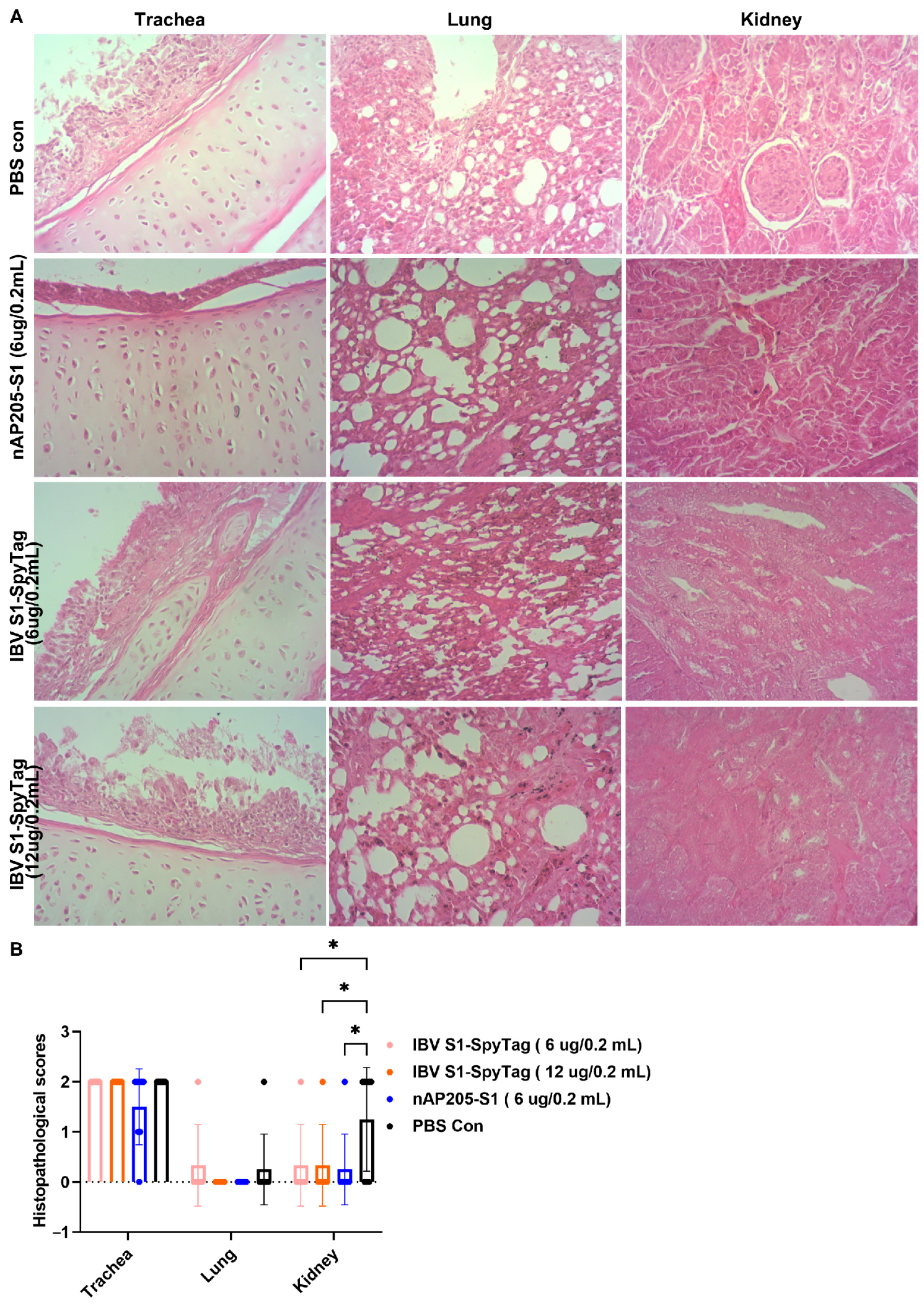
3.4. Immunizations of SPF Chickens with nAP205-S1 Vaccine Candidate
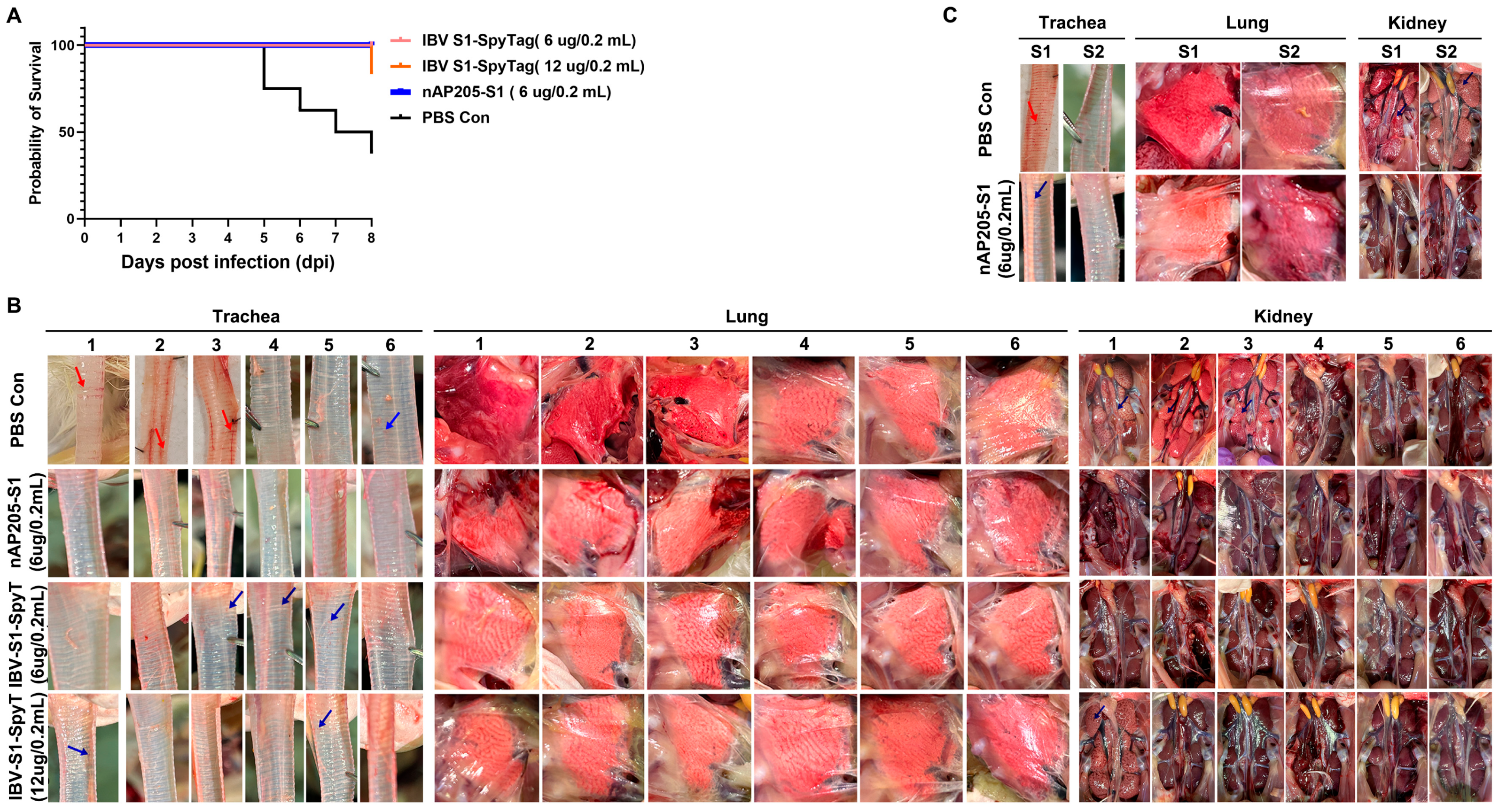
3.5. Protective Efficacy Against Challenges with Virulent Strains CK/CH/JS/TAHY in Chickens Immunized with nAP205-S1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Grunewald, M.; Perlman, S. Coronaviruses: An Updated Overview of Their Replication and Pathogenesis. Methods Mol. Biol. 2020, 2203, 1–29. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol 2019, 73, 529–557. [Google Scholar] [CrossRef]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet. Sci. 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef]
- Xiong, T.; Xie, H.; Li, L.; Liang, S.; Huang, M.; Yu, C.; Zhuang, T.; Liang, X.; Liu, D.; Chen, R. Prevalence, Genotype Diversity, and Distinct Pathogenicity of 205 Gammacoronavirus Infectious Bronchitis Virus Isolates in China during 2019–2023. Viruses 2024, 16, 930. [Google Scholar] [CrossRef] [PubMed]
- Houta, M.H.; Hassan, K.E.; El-Sawah, A.A.; Elkady, M.F.; Kilany, W.H.; Ali, A.; Abdel-Moneim, A.S. The emergence, evolution and spread of infectious bronchitis virus genotype GI-23. Arch. Virol. 2021, 166, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, D.; Bai, Y.; Huang, W.; Gao, S.; Wu, X.; Wang, Y.; Ren, J.; He, J.; Jin, L.; et al. Genetic and pathogenic characterization of new infectious bronchitis virus strains in the GVI-1 and GI-19 lineages isolated in central China. J. Integr. Agric. 2024, 23, 2407–2420. [Google Scholar] [CrossRef]
- Han, Z.; Xu, X.; Li, H.; Zhang, L.; Hou, Y.; Liu, S. Replication and vaccine protection of multiple infectious bronchitis virus strains in pheasants (Phasianus colchicus). Infect. Genet. Evol. 2021, 93, 104980. [Google Scholar] [CrossRef]
- Sives, S.; Keep, S.; Bickerton, E.; Vervelde, L. Revealing Novel-Strain-Specific and Shared Epitopes of Infectious Bronchitis Virus Spike Glycoprotein Using Chemical Linkage of Peptides onto Scaffolds Precision Epitope Mapping. Viruses 2023, 15, 2279. [Google Scholar] [CrossRef]
- Kang, H.J.; Coulibaly, F.; Clow, F.; Proft, T.; Baker, E.N. Stabilizing isopeptide bonds revealed in gram-positive bacterial pilus structure. Science 2007, 318, 1625–1628. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Brune, K.D.; Leneghan, D.B.; Brian, I.J.; Ishizuka, A.S.; Bachmann, M.F.; Draper, S.J.; Biswas, S.; Howarth, M. Plug-and-Display: Decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci. Rep. 2016, 6, 19234. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Thrane, S.; Janitzek, C.M.; Nielsen, M.A.; Theander, T.G.; Theisen, M.; Salanti, A.; Sander, A.F. Improving the malaria transmission-blocking activity of a Plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display. Vaccine 2017, 35, 3726–3732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomedicine 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Keeble, A.H.; Howarth, M. Power to the protein: Enhancing and combining activities using the Spy toolbox. Chem. Sci. 2020, 11, 7281–7291. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, C.; Kellmann, S.J.; Putyrski, M.; Cavada, M.; Hanuschka, H.; Knappik, A.; Ylera, F. Periplasmic expression of SpyTagged antibody fragments enables rapid modular antibody assembly. Cell Chem. Biol. 2021, 28, 813–824. [Google Scholar] [CrossRef]
- Alam, M.K.; Gonzalez, C.; Hill, W.; El-Sayed, A.; Fonge, H.; Barreto, K.; Geyer, C.R. Synthetic Modular Antibody Construction by Using the SpyTag/SpyCatcher Protein-Ligase System. Chembiochem 2017, 18, 2217–2221. [Google Scholar] [CrossRef]
- Xu, T.; Xiong, T.; Xie, W.; Wu, J.; Liu, X.; Li, G.; Lv, Y.; Li, L.; Yang, Z.; Wang, H.; et al. Construction and Evaluation of the Immunogenicity and Protective Efficacy of Recombinant Replication-Deficient Human Adenovirus-5 Expressing Genotype VII Newcastle Disease Virus F Protein and Infectious Bursal Disease Virus VP2 Protein. Vaccines 2023, 11, 1051. [Google Scholar] [CrossRef]
- Sjaak, D.W.J.; Cook, J.K.; Heijden, H.M. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef]
- Ganguly, J.; Zongming, F.; James, M.; Pan, Y.; Ruano, J.; Dahle, M.; Li, X. Fluorescent-protein co-expression to select CHO cells expressing high quantities of vaccine antigens. Biotechnol. J. 2024, 19, e2300671. [Google Scholar] [CrossRef]
- Sanchez-Martinez, Z.V.; Alpuche-Lazcano, S.P.; Stuible, M.; Durocher, Y. CHO cells for virus-like particle and subunit vaccine manufacturing. Vaccine 2024, 42, 2530–2542. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Li, Q.; Ma, K.; Lin, Y.; Feng, H.; Wang, T. Increase recombinant antibody yields through optimizing vector design and production process in CHO cells. Appl. Microbiol. Biotechnol. 2022, 106, 4963–4975. [Google Scholar] [CrossRef]
- Yamada, Y.; Liu, D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009, 83, 8744–8758. [Google Scholar] [CrossRef]
- Zheng, J.; Yamada, Y.; Fung, T.S.; Huang, M.; Chia, R.; Liu, D.X. Identification of N-linked glycosylation sites in the spike protein and their functional impact on the replication and infectivity of coronavirus infectious bronchitis virus in cell culture. Virology 2018, 513, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Eldemery, F.; Joiner, K.S.; Toro, H.; Santen, V.L. Protection against infectious bronchitis virus by spike ectodomain subunit vaccine. Vaccine 2017, 35, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Sepúlveda, D.; Vardia, I.; Skov, J.; Goksøyr, L.; Sander, A.F.; Lorenzen, N. High immunogenicity of virus-like particles (VLPs) decorated with Aeromonas salmonicida VapA antigen in rainbow trout. Front. Immunol. 2023, 14, 1139206. [Google Scholar] [CrossRef]
- Stander, J.; Chabeda, A.; Rybicki, E.P.; Meyers, A.E. A Plant-Produced Virus-Like Particle Displaying Envelope Protein Domain III Elicits an Immune Response Against West Nile Virus in Mice. Front. Plant Sci. 2021, 12, 738619. [Google Scholar] [CrossRef]
- Yenkoidiok-Douti, L.; Williams, A.E.; Canepa, G.E.; Molina-Cruz, A.; Barillas-Mury, C. Engineering a Virus-Like Particle as an Antigenic Platform for a Pfs47-Targeted Malaria Transmission-Blocking Vaccine. Sci. Rep. 2019, 9, 16833. [Google Scholar] [CrossRef]
- Yao, G.; Min, H.; Yu, X.; Liu, F.; Cui, L.; Cao, Y. A nanoparticle vaccine displaying the ookinete PSOP25 antigen elicits transmission-blocking antibody response against Plasmodium berghei. Parasit. Vectors 2023, 16, 403. [Google Scholar] [CrossRef]
- Janitzek, C.M.; Matondo, S.; Thrane, S.; Nielsen, M.A.; Kavishe, R.; Mwakalinga, S.B.; Theander, T.G.; Salanti, A.; Sander, A.F. Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses. Malar. J. 2016, 15, 545. [Google Scholar] [CrossRef]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007, 25, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, T.; Sun, Y.; Qiao, H.; Hu, N.; Li, X.; Wang, W.; Zhang, L.; Cong, Y. Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens. Vaccines 2023, 11, 1292. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.P.; He, Y.N.; Fan, W.S.; Dong, Z.H.; Zhang, L.H.; Sun, X.K.; Song, L.L.; Wei, T.C.; Mo, M.L.; et al. Protection against Virulent Infectious Bronchitis Virus Challenge Conferred by a Recombinant Baculovirus Co-Expressing S1 and N Proteins. Viruses 2018, 10, 347. [Google Scholar] [CrossRef]
- Qin, Y.; Tu, K.; Teng, Q.; Feng, D.; Zhao, Y.; Zhang, G. Identification of Novel T-Cell Epitopes on Infectious Bronchitis Virus N Protein and Development of a Multi-epitope Vaccine. J. Virol. 2021, 95, e66721. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]
- Shao, G.; Fu, J.; Pan, Y.; Gong, S.; Song, C.; Chen, S.; Feng, K.; Zhang, X.; Xie, Q. Development of a recombinant infectious bronchitis virus vaccine expressing infectious laryngotracheitis virus multiple epitopes. Poult. Sci. 2025, 104, 104578. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, T.; Lyu, Y.; Li, H.; Xu, T.; Wu, S.; Yang, Z.; Jing, M.; Xu, F.; Liu, D.; Chen, R. Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus. Vaccines 2025, 13, 802. https://doi.org/10.3390/vaccines13080802
Xiong T, Lyu Y, Li H, Xu T, Wu S, Yang Z, Jing M, Xu F, Liu D, Chen R. Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus. Vaccines. 2025; 13(8):802. https://doi.org/10.3390/vaccines13080802
Chicago/Turabian StyleXiong, Ting, Yanfen Lyu, Hongmei Li, Ting Xu, Shuting Wu, Zekun Yang, Mengyao Jing, Fei Xu, Dingxiang Liu, and Ruiai Chen. 2025. "Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus" Vaccines 13, no. 8: 802. https://doi.org/10.3390/vaccines13080802
APA StyleXiong, T., Lyu, Y., Li, H., Xu, T., Wu, S., Yang, Z., Jing, M., Xu, F., Liu, D., & Chen, R. (2025). Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus. Vaccines, 13(8), 802. https://doi.org/10.3390/vaccines13080802




