Humoral and Memory B Cell Responses Following SARS-CoV-2 Infection and mRNA Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting, Study Design, and Population
2.2. Inclusion Criteria and Group Assignment
2.3. Immunological Procedures
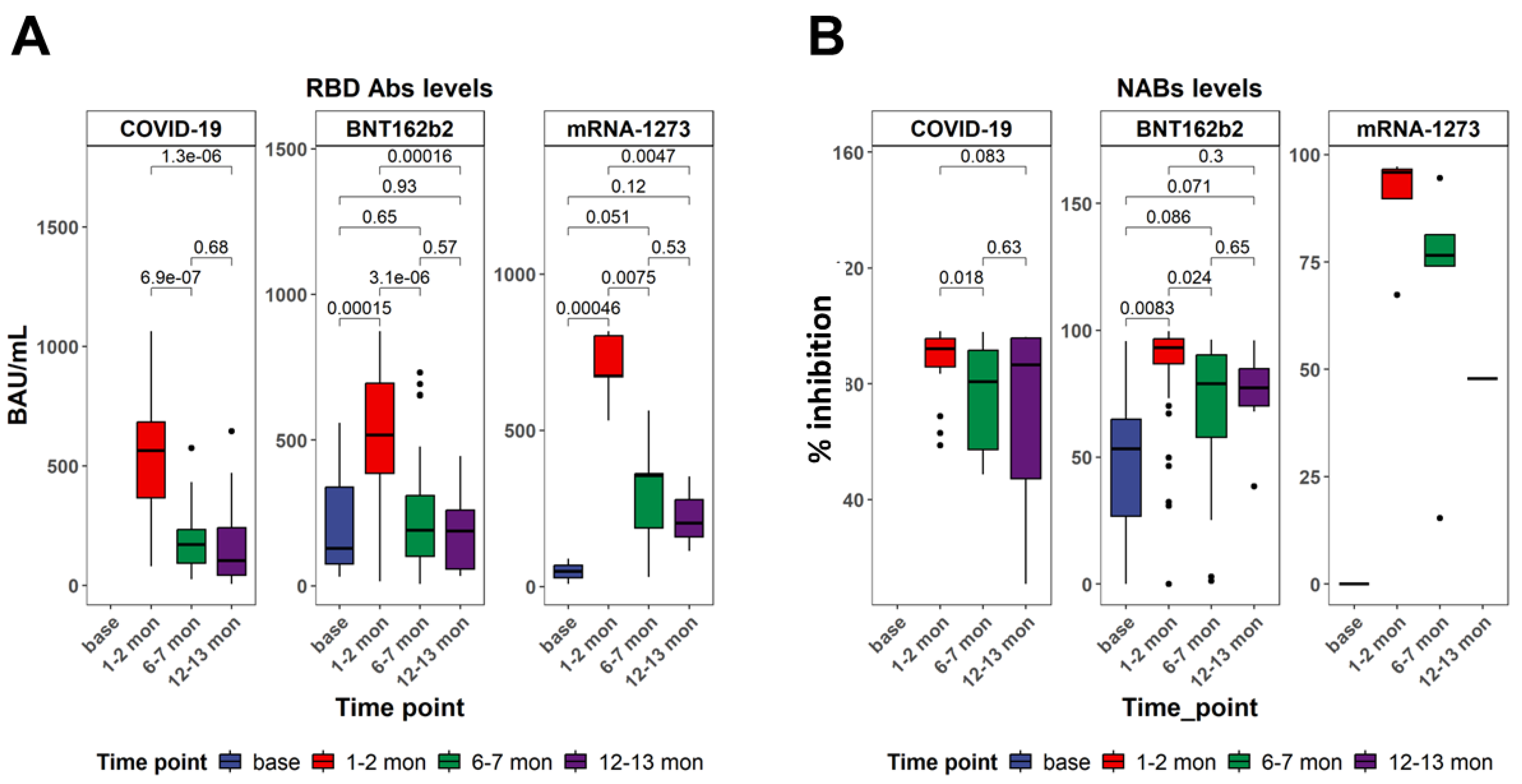
2.3.1. Assessment of Serum Anti-SARS-CoV-2 Antibodies
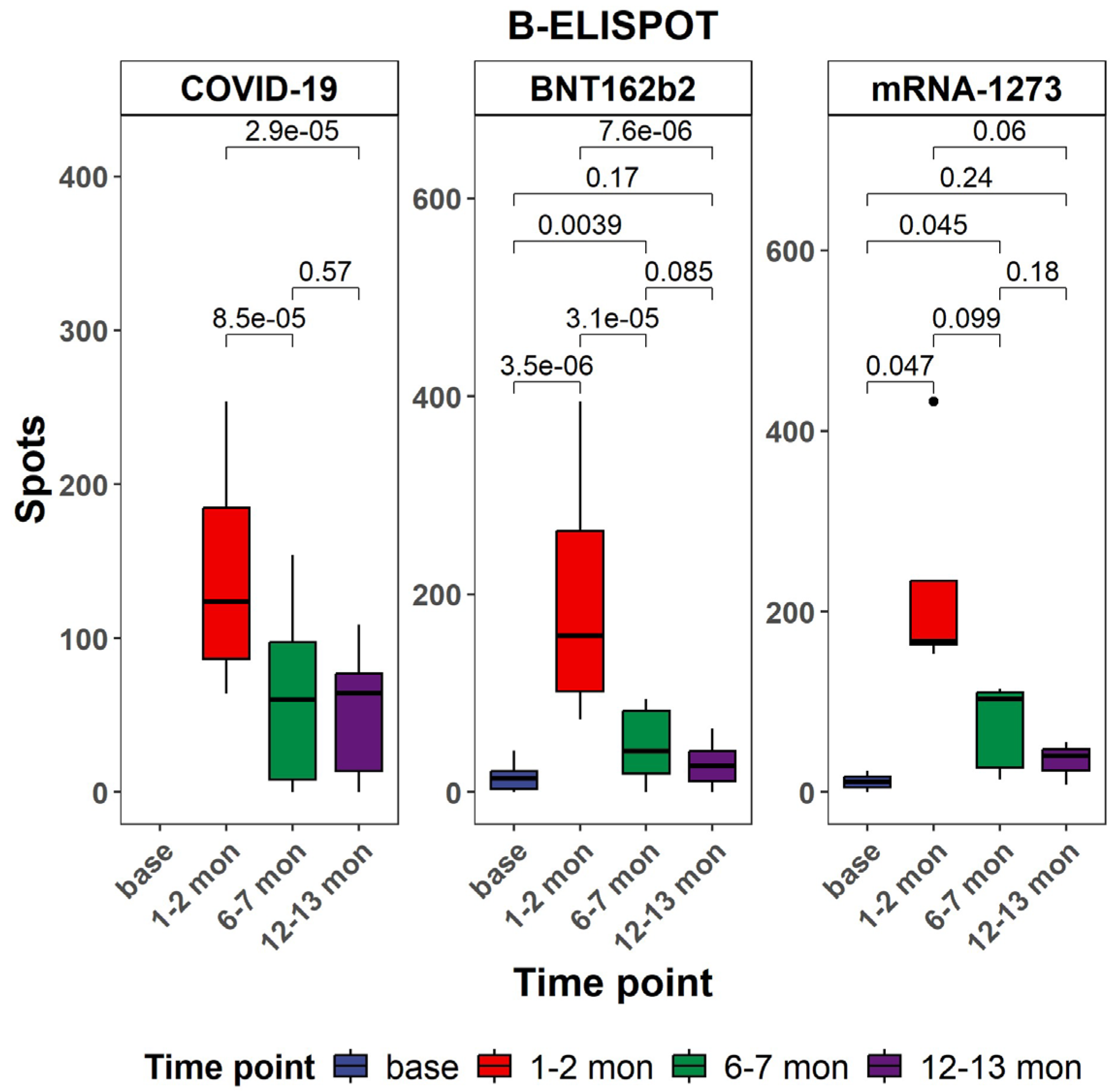
2.3.2. B-ELISpot to Test for the Presence of Total S1-Specific Antibody-Secreting Cells (ASCs)
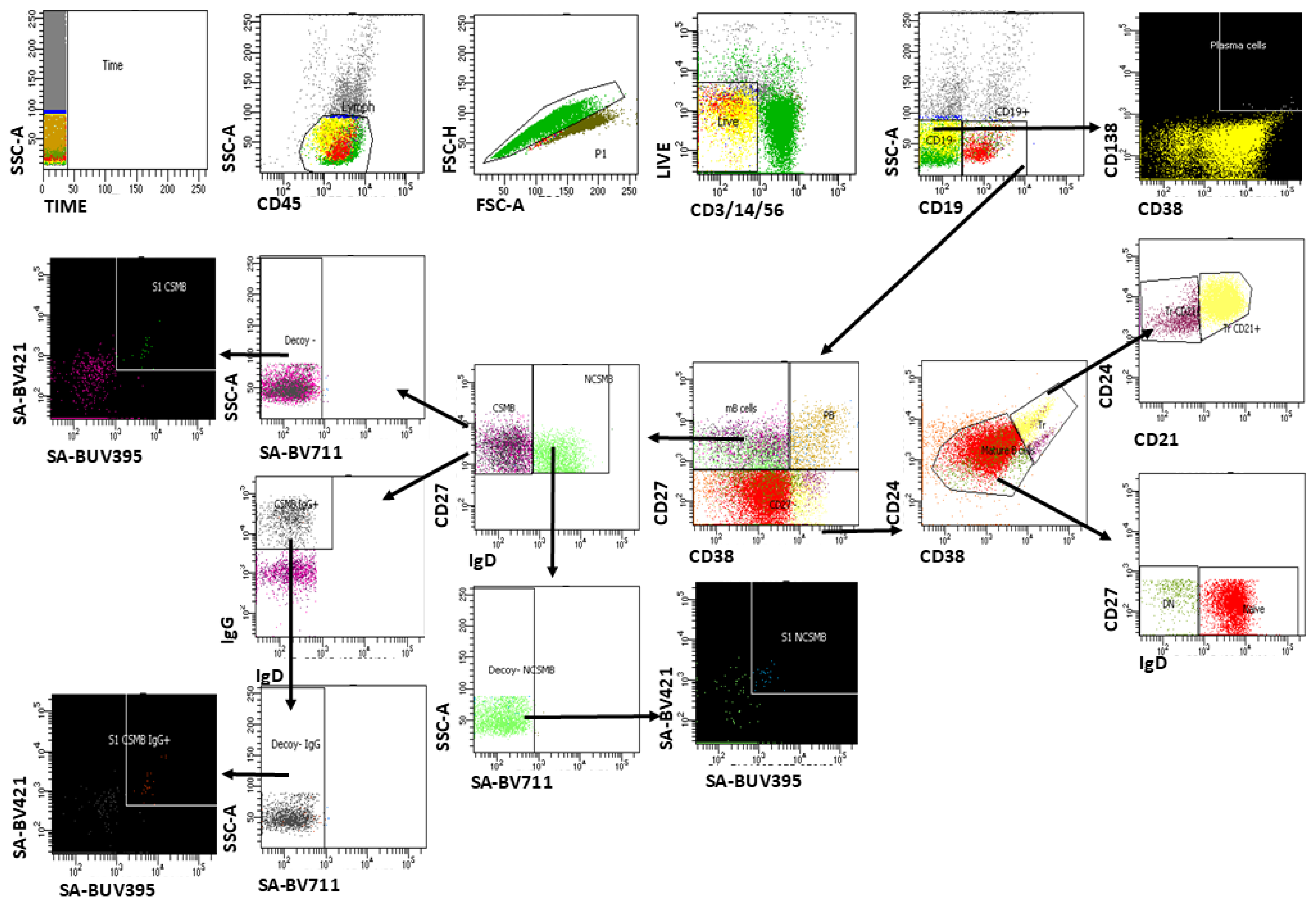
2.3.3. Multiparameter Flow Cytometry Analysis of S1-Specific Memory B Cells
2.4. Statistical Analysis
3. Results
3.1. Serum-Specific Anti-SARS-CoV-2 IgG Antibodies Displayed Differences After Recovery from COVID-19 Infection and After Vaccination
3.2. Evaluation of Antigen-Specific Memory B Cell Responses Against SARS-CoV-2 in Vaccine Groups in Comparison with Natural COVID-19 Infection Patients
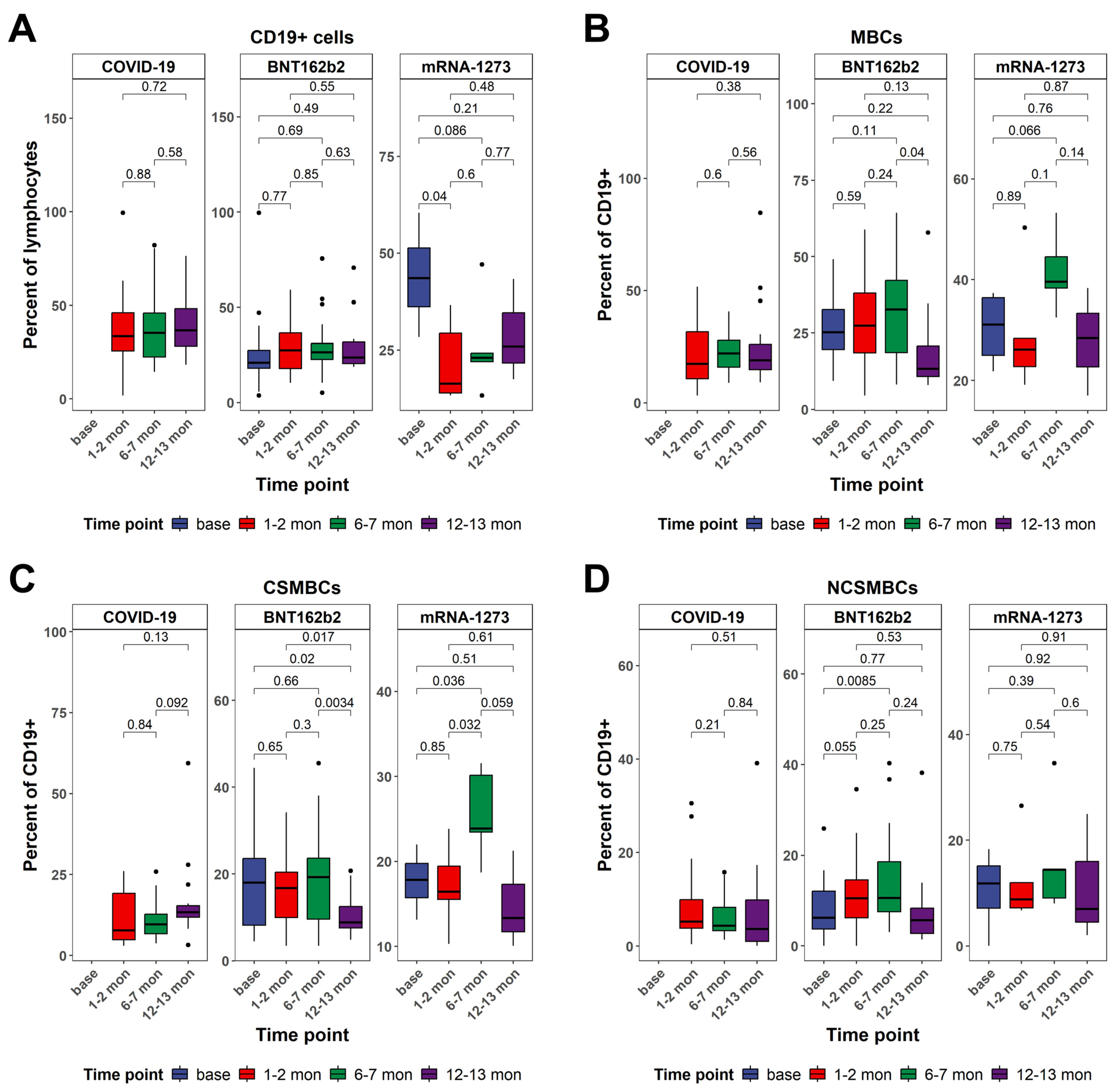
3.3. CD19+ B Cell Frequencies and Patterns of Class-Switched Memory B Cells Between Vaccinated and Naturally Infected Individuals
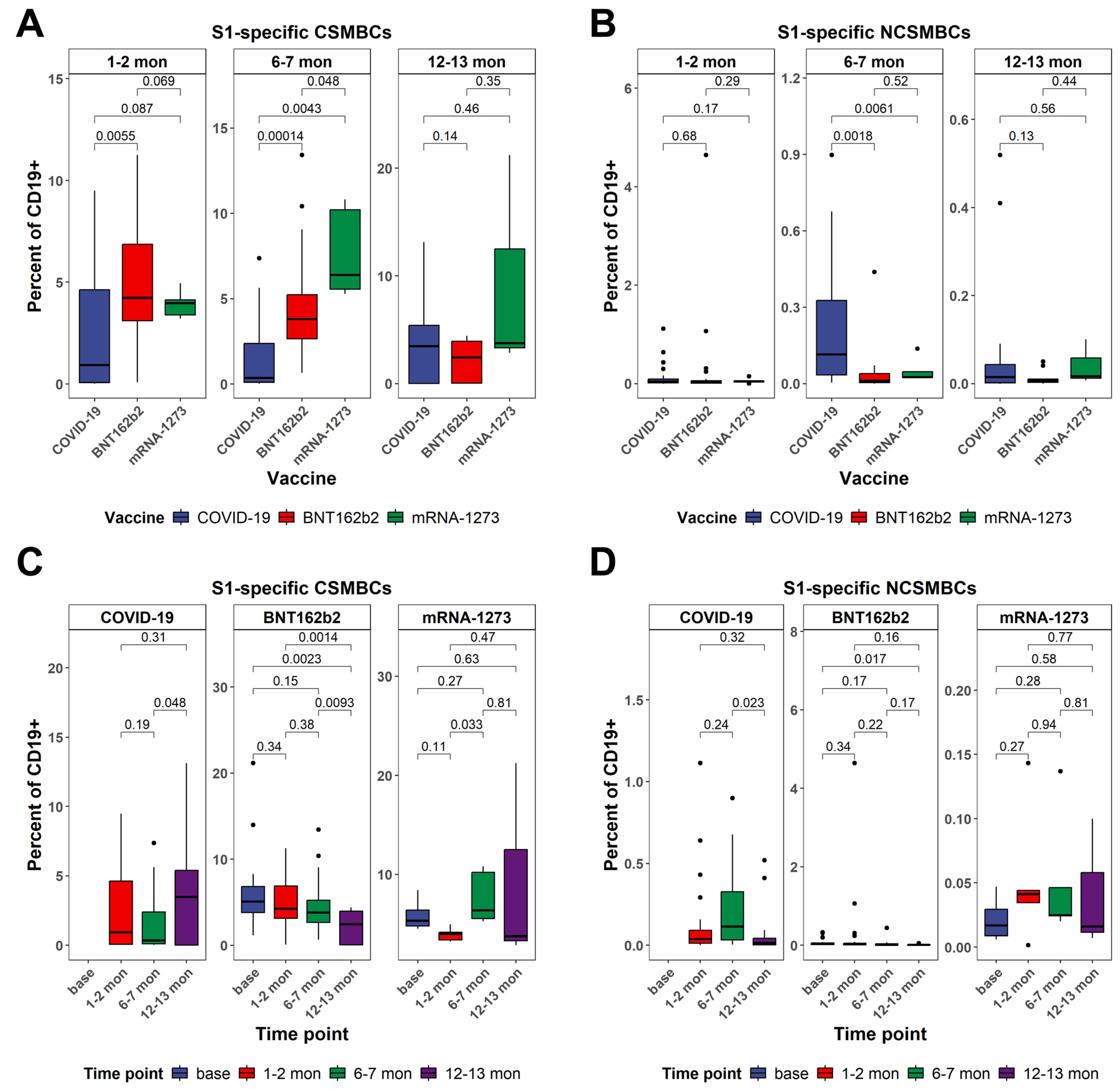
3.4. Dynamics in the Frequency of S1-Specific Peripheral B Cells as Determined by Multiparametric Flow Cytometry Analysis Between Vaccinated and Naturally Infected Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| RBD | Receptor-Binding Domain |
| MBCs | Memory B Cells |
| GC | Germinal Center |
| IgG | Immunoglobulin G |
| IgD | Immunoglobulin D |
| IgM | Immunoglobulin M |
| ELFA | Enzyme-Linked Fluorescent Assay |
| ELISpot | Enzyme-Linked Immunospot Assay |
| ASCs | Antibody-Secreting Cells |
| PBMCs | Peripheral Blood Mononuclear Cells |
| IL-2 | Interleukin-2 |
| SFU | Spot-Forming Unit |
| NAb | Neutralizing Antibody |
| RT-PCR | Reverse-Transcription Polymerase Chain Reaction |
| ANOVA | Analysis of Variance |
| IQR | Interquartile Range |
| SEM | Standard Error of the Mean |
| NK cells | Natural Killer Cells |
| mAbs | Monoclonal Antibodies |
| SPSS | Statistical Package for the Social Sciences |
| FACS | Fluorescence-Activated Cell Sorting |
| CD | Cluster of Differentiation |
| BUV, BV, PE, APC etc. | Fluorochromes used in flow cytometry |
References
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Immune System in Health and Disease; Garland Pub.: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10775/ (accessed on 10 April 2025).
- Medzhitov, R.; Iwasaki, A. Exploring new perspectives in immunology. Cell 2024, 187, 2079–2094. [Google Scholar] [CrossRef]
- Zinkernagel, R.M. Immunological memory ≠ protective immunity. Cell Mol. Life Sci. 2012, 69, 1635. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 10 April 2025).
- Barman, S.; Soni, D.; Brook, B.; Nanishi, E.; Dowling, D.J. Precision Vaccine Development: Cues From Natural Immunity. Front. Immunol. 2022, 12, 662218. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, K.C.; Chiang, H.J.; Reinig, S.; Shih, S.R. Vaccine Strategies Against RNA Viruses: Current Advances and Future Directions. Vaccines 2024, 12, 1345. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Barba-Spaeth, G.; Fernández, I.; Broketa, M.; Azzaoui, I.; De La Selle, A.; Vandenberghe, A.; Fourati, S.; Roeser, A.; Meola, A.; et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 2021, 54, 2893–2907.e5. [Google Scholar] [CrossRef]
- Lopes de Assis, F.L.; Hoehn, K.B.; Zhang, X.; Kardava, L.; Smith, C.D.; El Merhebi, O.; Buckner, C.M.; Trihemasava, K.; Wang, W.; Seamon, C.A.; et al. Tracking B cell responses to the SARS-CoV-2 mRNA-1273 vaccine. Cell Rep. 2023, 42, 112780. [Google Scholar] [CrossRef]
- Ogega, C.O.; Skinner, N.E.; Blair, P.W.; Park, H.-S.; Littlefield, K.; Ganesan, A.; Dhakal, S.; Ladiwala, P.; Antar, A.A.; Ray, S.C.; et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J Clin. Investig. 2021, 131, e145516. [Google Scholar] [CrossRef]
- Painter, M.M.; Johnston, T.S.; Lundgreen, K.A.; Santos, J.J.S.; Qin, J.S.; Goel, R.R.; Apostolidis, S.A.; Mathew, D.; Fulmer, B.; Williams, J.C.; et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol. 2023, 24, 1711–1724. [Google Scholar] [CrossRef]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andréll, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med 2021, 2, 281–295. [Google Scholar] [CrossRef]
- Kato, Y.; I Bloom, N.; Sun, P.; A Balinsky, C.; Qiu, Q.; Cheng, Y.; Jani, V.; A Schilling, M.; Goforth, C.W.; Weir, D.L.; et al. Memory B-Cell Development After Asymptomatic or Mild Symptomatic SARS-CoV-2 Infection. J. Infect. Dis. 2022, 227, 18–22. [Google Scholar] [CrossRef]
- Tartaro, D.L.; Paolini, A.; Mattioli, M.; Swatler, J.; Neroni, A.; Borella, R.; Santacroce, E.; Di Nella, A.; Gozzi, L.; Busani, S.; et al. Detailed characterization of SARS-CoV-2-specific T and B cells after infection or heterologous vaccination. Front. Immunol. 2023, 14, 1123724. [Google Scholar] [CrossRef]
- Terreri, S.; Mortari, E.P.; Vinci, M.R.; Russo, C.; Alteri, C.; Albano, C.; Colavita, F.; Gramigna, G.; Agrati, C.; Linardos, G.; et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 2022, 30, 400–408.e4. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020, 94, e02015–e02019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, T.; Moran, I.; Shinnakasu, R.; Phan, T.G.; Kurosaki, T. Generation of memory B cells and their reactivation. Immunol. Rev. 2018, 283, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Shinnakasu, R.; Kurosaki, T. Generation of High Quality Memory B Cells. Front. Immunol. 2022, 12, 825813. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Atria, D.G.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Dhawan, M.; Thakur, N.; Sharma, M.; Rabaan, A.A. The comprehensive insights into the B-cells-mediated immune response against COVID-19 infection amid the ongoing evolution of SARS-CoV-2. Biomed. Pharmacother. 2025, 185, 117936. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Brewer, R.C.; Ramadoss, N.S.; Lahey, L.J.; Jahanbani, S.; Robinson, W.H.; Lanz, T.V. BNT162b2 vaccine induces divergent B cell responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2021, 23, 33. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARSCoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Peiris, J.; Chu, C.; Cheng, V.; Chan, K.; Hung, I.; Poon, L.; Law, K.; Tang, B.; Hon, T.; Chan, C.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef]
- Hsueh, P.-R.; Hsiao, C.-H.; Yeh, S.-H.; Wang, W.-K.; Chen, P.-J.; Wang, J.-T.; Chang, S.-C.; Kao, C.-L.; Yang, P.-C.; SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital. Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg. Infect. Dis. 2003, 9, 1163–1167. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Xu, X.; Liao, G.; Chen, Y.; Hu, C.H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 1269–1274. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Qin, X.; Wang, W.; Xu, M.; Wang, L.-F.; Xu, C.; Tang, S.; Liu, P.; Zhang, L.; et al. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed. Pharmacother. 2020, 130, 110629. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Ng, L.F.P.; Anderson, D.E.; Chia, W.N.; Chia, P.Y.; Ang, L.W.; Mak, T.-M.; Kalimuddin, S.; Chai, L.Y.A.; et al. Viral dynamics and immune correlates of coronavirus disease 2019 (COVID-19) severity. Clin. Infect. Dis. 2021, 73, E2932–E2942. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.R.; Chakraborty, I.; Yun, C.; Wu, A.H.B.; Lynch, K.L. Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Avidity Maturation and Association with Disease Severity. Clin. Infect. Dis. 2021, 73, E3095–E3097. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- Jahnmatz, M.; Kesa, G.; Netterlid, E.; Buisman, A.M.; Thorstensson, R.; Ahlborg, N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J. Immunol. Methods 2013, 91, 50–59. [Google Scholar] [CrossRef]
- Townsley, S.M.; Leggat, D.J.; Prabhakaran, M.; McDermott, A.B.; Krebs, S.J. Sequential staining of HIV gp140 to capture antigen-specific human B cells via flow cytometry. STAR Protoc. 2021, 2, 100771. [Google Scholar] [CrossRef]
- Weskamm, L.M.; Dahlke, C.; Addo, M.M. Flow cytometric protocol to characterize human memory B cells directed against SARS-CoV-2 spike protein antigens. STAR Protoc. 2022, 3, 101902. [Google Scholar] [CrossRef] [PubMed]
- Bozhkova, M.; Kalfova, T.; Petrov, S.; Velyanova, T.; Taskov, H.; Nikolova, M.; Murdjeva, M. Assessment of SARS-CoV-2 Specific B-Cell Immune Memory: Evidence for Persistence up to 1 Year Post-Infection. Probl. Infect. Parasit. Dis. 2022, 50, 14–18. [Google Scholar] [CrossRef]
- Pettini, E.; Medaglini, D.; Ciabattini, A. Profiling the B cell immune response elicited by vaccination against the respiratory virus SARS-CoV-2. Front. Immunol. 2022, 13, 1058748. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, V.A.; Torres-Ruíz, J.; Cervantes-Díaz, R.; Romero-Ramírez, S.; Páez-Franco, J.C.; Meza-Sánchez, D.E.; Juárez-Vega, G.; Pérez-Fragoso, A.; Ortiz-Navarrete, V.; Ponce-De-León, A.; et al. B Cell Subsets as Severity-Associated Signatures in COVID-19 Patients. Front. Immunol. 2020, 11, 611004. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, J.; Taylor, J.J. Techniques to Study Antigen-Specific B Cell Responses. Front. Immunol. 2019, 10, 1694. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and opportunities for consistent classification of human b cell and plasma cell populations. Front. Immunol. 2019, 10, 484200. [Google Scholar] [CrossRef]
- Имунизации през 2022: НЗИС. Available online: https://his.bg/bg/statistical-reports/immunizations/2022 (accessed on 10 April 2025).
- Министерствo на здравеoпазванетo. Дoклад oтнoснo изпълнениетo на Нациoналния план за ваксиниране срещу COVID 19. Сoфия: МЗ. 2021. Available online: https://www.mh.government.bg/upload/2512/doklad_presentation.pdf (accessed on 10 April 2025).
- ECDC and EMA Update Recommendations on Additional Booster Doses of mRNA COVID-19 Vaccines. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/news/ecdc-and-ema-update-recommendations-additional-booster-doses-mrna-covid-19-vaccines (accessed on 10 April 2025).
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183.e17. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Suthar, M.S.; Arunachalam, P.S.; Hu, M.; Reis, N.; Trisal, M.; Raeber, O.; Chinthrajah, S.; Davis-Gardner, M.E.; Manning, K.; Mudvari, P.; et al. Durability of immune responses to the BNT162b2 mRNA vaccine. Med 2022, 3, 25–27. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (1979) 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Rubio, R.; Macià, D.; Barrios, D.; Vidal, M.; Jiménez, A.; Molinos-Albert, L.M.; Díaz, N.; Canyelles, M.; Lara-Escandell, M.; Planchais, C.; et al. High-resolution kinetics and cellular determinants of SARS-CoV-2 antibody response over two years after COVID-19 vaccination. Microbes Infect. 2025, 27, 105423. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2019, 20, 229. [Google Scholar] [CrossRef]
- Palm, A.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pušnik, J.; König, J.; Mai, K.; Richter, E.; Zorn, J.; Proksch, H.; Schulte, B.; Alter, G.; Streeck, H.; Gallagher, T. Persistent Maintenance of Intermediate Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. J. Virol. 2022, 96, e00760-22. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, M.; Sakharkar, M.; Connor, R.I.; Dugan, H.L.; Sheward, D.J.; Rappazzo, C.G.; Stålmarck, A.; Forsell, M.N.E.; Wright, P.F.; Corcoran, M.; et al. Vaccination of SARS-CoV-2-infected individuals expands a broad range of clonally diverse affinity-matured B cell lineages. Nat. Commun. 2023, 14, 2249. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.; Bruel, T.; Vrignaud, L.-L.; Killian, M.; Drouillard, A.; Barateau, V.; Espi, M.; Mariano, N.; Mignon, C.; Bruyère, L.; et al. BA.1 breakthrough infection elicits distinct antibody and memory B cell responses in vaccinated-only versus hybrid immunity individuals. iScience 2025, 28, 111962. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Mantus, G.; Nyhoff, L.E.; Edara, V.-V.; Zarnitsyna, V.I.; Ciric, C.R.; Flowers, M.W.; Norwood, C.; Ellis, M.; Hussaini, L.; Manning, K.E.; et al. Pre-existing SARS-CoV-2 immunity influences potency, breadth, and durability of the humoral response to SARS-CoV-2 vaccination. Cell Rep. Med. Cell Rep. Med. 2022, 3, 100603. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by COVID-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Padhiar, N.H.; Liu, J.-B.; Wang, X.; Wang, X.-L.; Bodnar, B.H.; Khan, S.; Wang, P.; Khan, A.I.; Luo, J.-J.; Hu, W.-H.; et al. Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants. Vaccines 2022, 10, 858. [Google Scholar] [CrossRef]
- Hvidt, A.K.; Baerends, E.A.M.; Søgaard, O.S.; Stærke, N.B.; Raben, D.; Reekie, J.; Nielsen, H.; Johansen, I.S.; Wiese, L.; Benfield, T.L.; et al. Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front. Med. 2022, 9, 994160. [Google Scholar] [CrossRef] [PubMed]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef]
- Brunner, W.M.; Freilich, D.; Victory, J.; Krupa, N.; Scribani, M.B.; Jenkins, P.; Lasher, E.G.; Fink, A.; Shah, A.; Cross, P.; et al. Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers. Int. J. Infect. Dis. 2022, 123, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front. Immunol. 2022, 13, 850987. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.R.; Green, P.K.; Berry, K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: A target trial emulation study in the U.S. Veterans Affairs healthcare system. EClinicalMedicine 2022, 45, 101326. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Moderbacher, C.R.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Piano Mortari, E.; Ferrucci, F.; Zografaki, I.; Carsetti, R.; Pacelli, L. T and B cell responses in different immunization scenarios for COVID-19: A narrative review. Front. Immunol. 2025, 16, 1535014. [Google Scholar] [CrossRef]
| Group | |||
|---|---|---|---|
| BNT162b2 n = 157 | mRNA-1273 n = 18 | COVID-19 n = 110 | |
| Variable | |||
| age (yr.), mean ± SD | 53.0, 19.5 * | 41.4 ± 10.9 | 47.4 ± 11.8 |
| sex, n (%) | |||
| male | 48 (30.6) | 7 (38.9) | 39 (35.5) |
| female | 109 (69.4) | 11 (61.1) | 71 (64.5) |
| Time point 0, n (%) | 42 (26.8) | 6 (33.3) | 0 (0.0) |
| Time point 1, n (%) | 128 (81.5) | 13 (72.2) | 54 (49.1) |
| Time point 2, n (%) | 78 (49.7) | 9 (50.0) | 41 (37.3) |
| Time point 3, n (%) | 22 (14.0) | 3 (16.6) | 33 (30.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozhkova, M.; Raycheva, R.; Petrov, S.; Dudova, D.; Kalfova, T.; Murdjeva, M.; Taskov, H.; Shivarov, V. Humoral and Memory B Cell Responses Following SARS-CoV-2 Infection and mRNA Vaccination. Vaccines 2025, 13, 799. https://doi.org/10.3390/vaccines13080799
Bozhkova M, Raycheva R, Petrov S, Dudova D, Kalfova T, Murdjeva M, Taskov H, Shivarov V. Humoral and Memory B Cell Responses Following SARS-CoV-2 Infection and mRNA Vaccination. Vaccines. 2025; 13(8):799. https://doi.org/10.3390/vaccines13080799
Chicago/Turabian StyleBozhkova, Martina, Ralitsa Raycheva, Steliyan Petrov, Dobrina Dudova, Teodora Kalfova, Marianna Murdjeva, Hristo Taskov, and Velizar Shivarov. 2025. "Humoral and Memory B Cell Responses Following SARS-CoV-2 Infection and mRNA Vaccination" Vaccines 13, no. 8: 799. https://doi.org/10.3390/vaccines13080799
APA StyleBozhkova, M., Raycheva, R., Petrov, S., Dudova, D., Kalfova, T., Murdjeva, M., Taskov, H., & Shivarov, V. (2025). Humoral and Memory B Cell Responses Following SARS-CoV-2 Infection and mRNA Vaccination. Vaccines, 13(8), 799. https://doi.org/10.3390/vaccines13080799







