Polymorphism in IFNλ Can Impact the Immune/Inflammatory Response to COVID-19 Vaccination in Older CMV-Seropositive Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Recruitment of Participants
2.3. Selection Criteria
2.4. Ethical Aspects
2.5. Biological Sample Collection
2.5.1. Determination of Specific IgM and IgG for CMV Antigens
2.5.2. Determination of Specific IgG for SARS-CoV-2 Antigens
2.5.3. Analysis of the Neutralizing Antibodies
2.5.4. Determination of Circulating Cytokine Concentrations
2.5.5. Determination of Viral Load of CMV
2.5.6. Analysis of the Polymorphism in the IL28B Gene
2.5.7. Immunophenotypic Characterization of Monocyte Subtypes and T Cells
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Volunteer Group
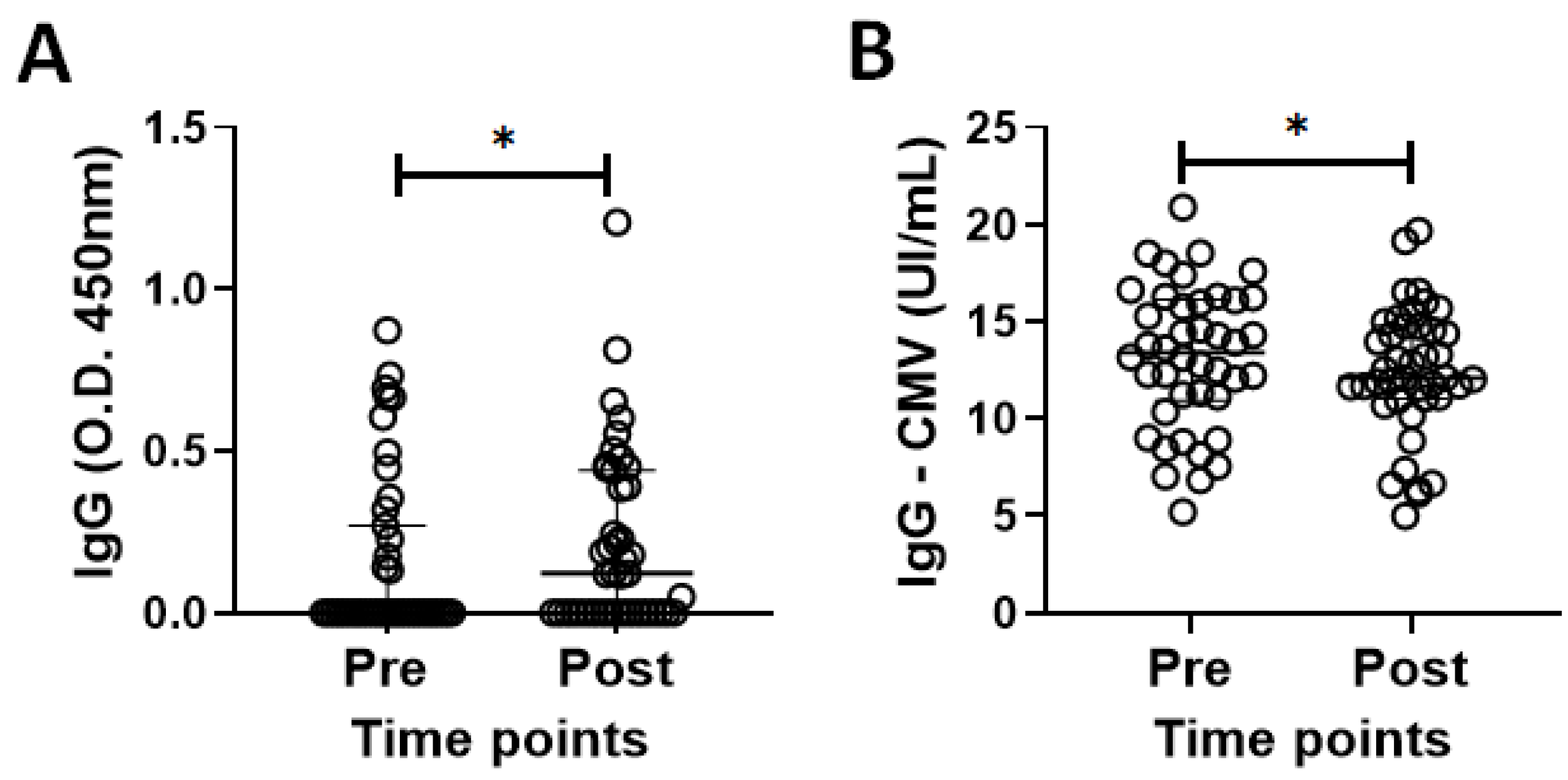
3.2. Specific IgG for the CMV and SARS-CoV-2 Antigens in the Volunteer Cohort
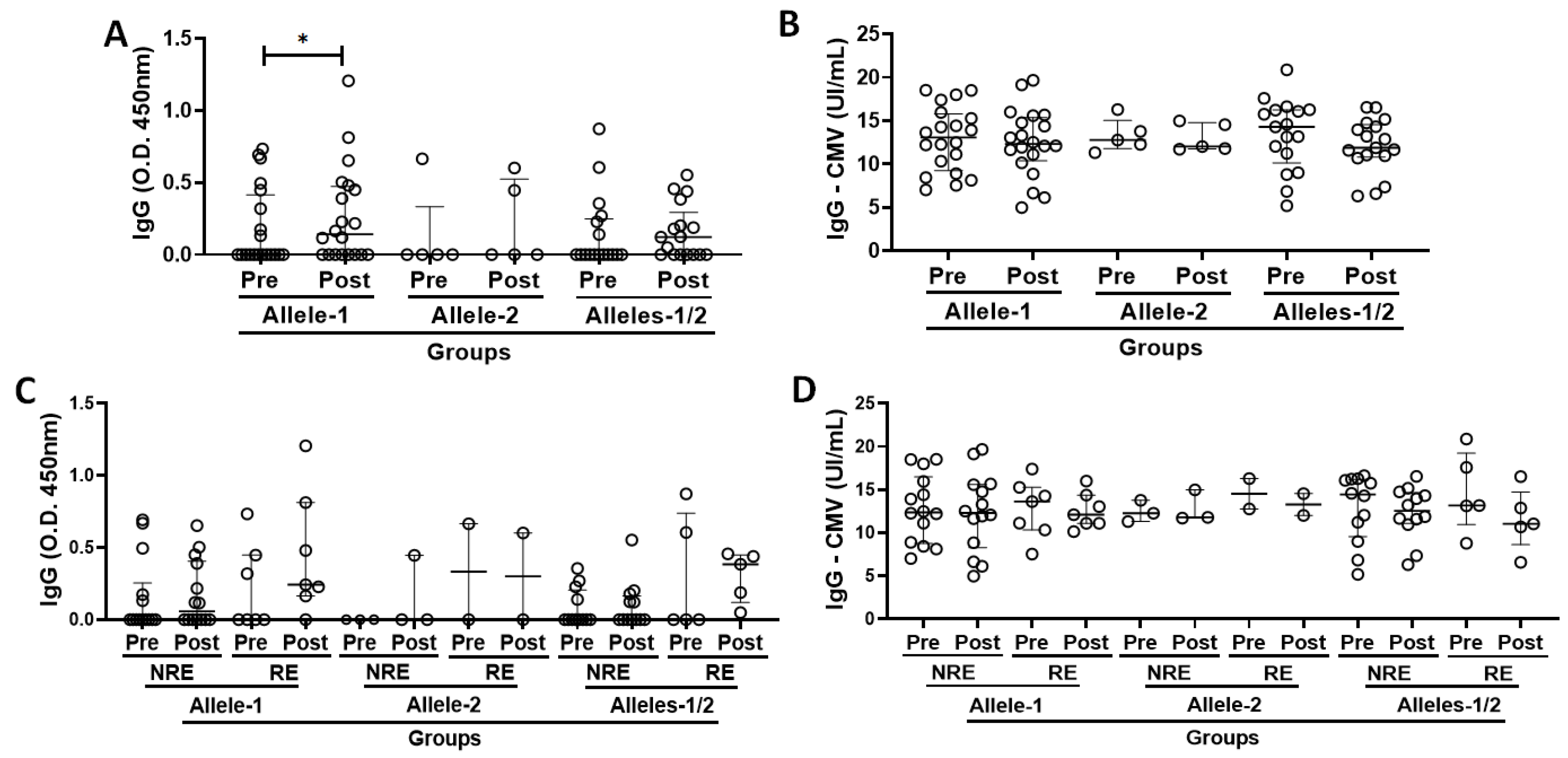
3.3. Specific IgG for the SARS-CoV-2 and CMV Antigens in the Volunteer Cohort Grouped According to Allelic Discrimination
3.4. Immunophenotyping of T Cells and Monocyte Subtypes in the Subject Cohort Grouped According to Allelic Differentiation
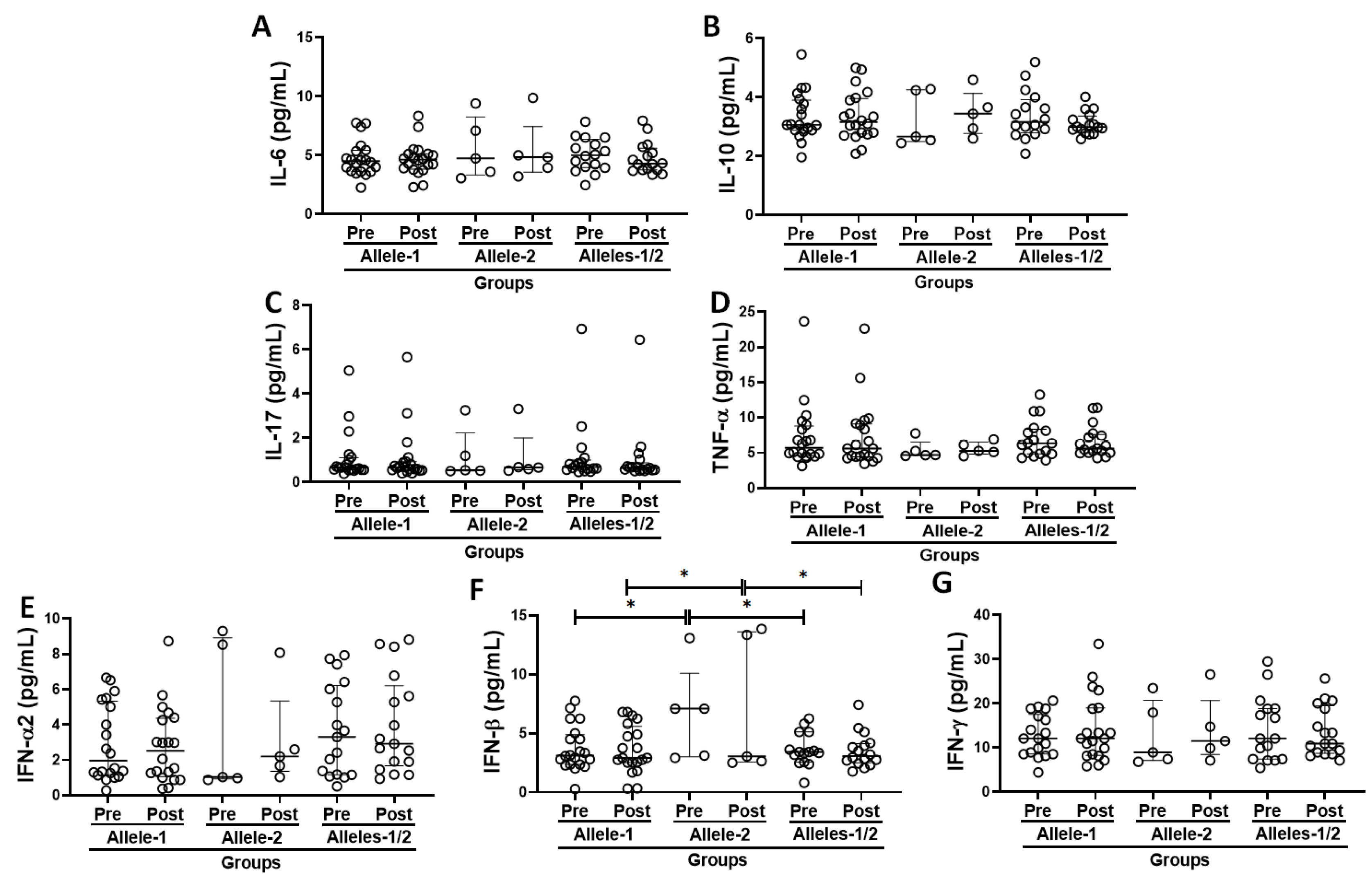
3.5. Systemic Inflammatory Status in the Volunteer Cohort Grouped Based on the Allelic Discrimination
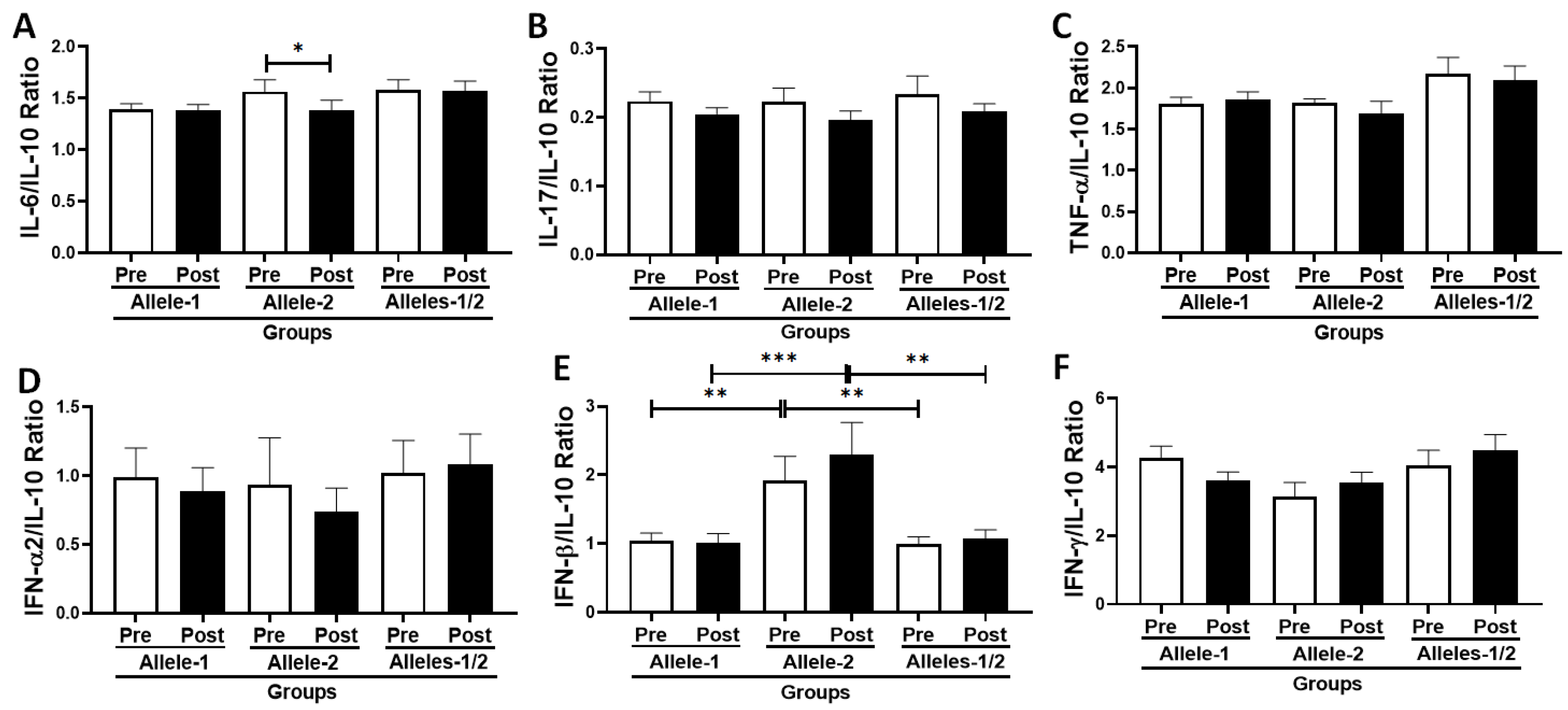
3.6. Analysis of the Ratio Between Pro- and Anti-Inflammatory Cytokines in the Volunteer Cohort According to Allelic Discrimination
3.7. Correlation Analysis Between Immune/Inflammatory Parameters Assessed in the Volunteer Cohort Grouped Based on the Allelic Discrimination
3.8. Multivariate Linear Regression Analysis Between Immune/Inflammatory Parameters Assessed in the Volunteer Cohort Grouped Based on the Allelic Discrimination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, L.; Zhang, C.; Dong, J.; Zhao, L.; Li, Y.; Sun, J. Oral Microbiome and SARS-CoV-2: Beware of Lung Co-infection. Front. Microbiol. 2020, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Monti, D.; Sansoni, P.; Cossarizza, A. The immunology of exceptional individuals: The lesson of centenarians. Immunol. Today 1995, 16, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.J.; Shukla, S.; Kumar, S.; Mishra, P. Immunosenescence and Inflamm-Aging: Clinical Interventions and the Potential for Reversal of Aging. Cureus 2024, 16, e53297. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Chambers, E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef]
- Kovaiou, R.D.; Herndler-Brandstetter, D.; Grubeck-Loebenstein, B. Age-related changes in immunity: Implications for vaccination in the elderly. Expert. Rev. Mol. Med. 2007, 9, 1–17. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef]
- Staras, S.A.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef]
- Aiello, A.; Ligotti, M.E.; Garnica, M.; Accardi, G.; Calabrò, A.; Pojero, F.; Arasanz, H.; Bocanegra, A.; Blanco, E.; Chocarro, L.; et al. How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells. Int. J. Mol. Sci. 2022, 23, 9880. [Google Scholar] [CrossRef]
- Hesson, J.; Fudge, N.; Grant, M. Cytomegalovirus Immunity, Inflammation and Cognitive Abilities in the Elderly. Viruses 2021, 13, 2321. [Google Scholar] [CrossRef]
- Schmaltz, H.N.; Fried, L.P.; Xue, Q.L.; Walston, J.; Leng, S.X.; Semba, R.D. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J. Am. Geriatr. Soc. 2005, 53, 747–754. [Google Scholar] [CrossRef]
- Davies, E.L.; Noor, M.; Lim, E.Y.; Houldcroft, C.J.; Okecha, G.; Atkinson, C.; Reeves, M.B.; Jackson, S.E.; Wills, M.R. HCMV carriage in the elderly diminishes anti-viral functionality of the adaptive immune response resulting in virus replication at peripheral sites. Front. Immunol. 2022, 13, 1083230. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Interferon at the crossroads of SARS-CoV-2 infection and COVID-19 disease. J. Biol. Chem. 2023, 299, 104960. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Santer, D.M.; O’Shea, D.; Tyrrell, D.L.; Houghton, M. The impact of the interferon-lambda family on the innate and adaptive immune response to viral infections. Emerg. Microbes Infect. 2014, 3, e51. [Google Scholar] [CrossRef]
- Sezgin, E.; An, P.; Winkler, C.A. Host Genetics of Cytomegalovirus Pathogenesis. Front. Genet. 2019, 10, 616. [Google Scholar] [CrossRef]
- Zahid, W.; Farooqui, N.; Zahid, N.; Ahmed, K.; Anwar, M.F.; Rizwan-Ul-Hasan, S.; Hussain, A.R.; Sarría-Santamera, A.; Abidi, S.H. Association of Interferon Lambda 3 and 4 Gene SNPs and Their Expression with COVID-19 Disease Severity: A Cross-Sectional Study. Infect. Drug Resist. 2023, 16, 6619–6628. [Google Scholar] [CrossRef]
- Nardy, A.; Camargo, C.T.S.; Oliveira, Y.F.C.; Silva, F.C.D.; Almeida, M.S.; Monteiro, F.R.; Silva, B.R.; Amaral, J.B.D.; Oliveira, D.B.L.; Durigon, E.L.; et al. Assessment of the Interferon-Lambda-3 Polymorphism in the Antibody Response to COVID-19 in Older Adults Seropositive for CMV. Vaccines 2023, 11, 480. [Google Scholar] [CrossRef]
- Petrini, C. Helsinki 50 years on. Clin. Ter. 2014, 165, 179–181. [Google Scholar] [CrossRef]
- Novoa, P.C. What changes in research ethics in Brazil: Resolution no. 466/12 of the National Health Council. Einstein 2014, 12, vii–x. [Google Scholar] [CrossRef]
- Dos Santos, J.M.B.; do Amaral, J.B.; França, C.N.; Monteiro, F.R.; Alvares-Saraiva, A.M.; Kalil, S.; Durigon, E.L.; Oliveira, D.B.L.; Rodrigues, S.S.; Heller, D.; et al. Distinct Immunological Profiles Help in the Maintenance of Salivary Secretory IgA Production in Mild Symptoms COVID-19 Patients. Front. Immunol. 2022, 13, 890887. [Google Scholar] [CrossRef]
- Wendel, S.; Kutner, J.M.; Machado, R.; Fontão-Wendel, R.; Bub, C.; Fachini, R.; Yokoyama, A.; Candelaria, G.; Sakashita, A.; Achkar, R.; et al. Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program. Transfusion 2020, 60, 2938–2951. [Google Scholar] [CrossRef]
- Charvet, B.; Brunel, J.; Pierquin, J.; Iampietro, M.; Decimo, D.; Queruel, N.; Lucas, A.; Encabo-Berzosa, M.D.M.; Arenaz, I.; Marmolejo, T.P.; et al. SARS-CoV-2 awakens ancient retroviral genes and the expression of proinflammatory HERV-W envelope protein in COVID-19 patients. iScience 2023, 26, 106604. [Google Scholar] [CrossRef]
- Kuo, C.P.; Wu, C.L.; Ho, H.T.; Chen, C.G.; Liu, S.I.; Lu, Y.T. Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy. Clin. Microbiol. Infect. 2008, 14, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I.; et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013, 45, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Felismino, E.S.; Santos, J.M.B.; Rossi, M.; Santos, C.A.F.; Durigon, E.L.; Oliveira, D.B.L.; Thomazelli, L.M.; Monteiro, F.R.; Sperandio, A.; Apostólico, J.S.; et al. Better Response to Influenza Virus Vaccination in Physically Trained Older Adults Is Associated with Reductions of Cytomegalovirus-Specific Immunoglobulins as Well as Improvements in the Inflammatory and CD8+ T-Cell Profiles. Front. Immunol. 2021, 12, 713763. [Google Scholar] [CrossRef] [PubMed]
- Merani, S.; Pawelec, G.; Kuchel, G.A.; McElhaney, J.E. Impact of Aging and Cytomegalovirus on Immunological Response to Influenza Vaccination and Infection. Front Immunol. 2017, 8, 784. [Google Scholar] [CrossRef]
- Silva, B.R.; Monteiro, F.R.; Cezário, K.; Amaral, J.B.D.; Paixão, V.; Almeida, E.B.; Santos, C.A.F.D.; Amirato, G.R.; Oliveira, D.B.L.; Durigon, E.L.; et al. Older Adults Who Maintained a Regular Physical Exercise Routine before the Pandemic Show Better Immune Response to Vaccination for COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 1939. [Google Scholar] [CrossRef]
- De Matos, S.B.; Meyer, R.; Lima, F.W. Seroprevalence and serum profile of cytomegalovirus infection among patients with hematologic disorders in Bahia State, Brazil. J. Med. Virol. 2011, 83, 298–304. [Google Scholar] [CrossRef]
- Rugwizangoga, B.; Andersson, M.E.; Kabayiza, J.C.; Nilsson, M.S.; Ármannsdóttir, B.; Aurelius, J.; Nilsson, S.; Hellstrand, K.; Lindh, M.; Martner, A. IFNL4 Genotypes Predict Clearance of RNA Viruses in Rwandan Children with Upper Respiratory Tract Infections. Front. Cell Infect. Microbiol. 2019, 9, 340. [Google Scholar] [CrossRef]
- Roohi, A.; Soroosh, P. May interferon λ be a novel therapeutic approach against COVID-19? Med. Hypotheses 2021, 146, 110351. [Google Scholar] [CrossRef]
- Bravo, D.; Solano, C.; Giménez, E.; Remigia, M.J.; Corrales, I.; Amat, P.; Navarro, D. Effect of the IL28B Rs12979860 C/T polymorphism on the incidence and features of active cytomegalovirus infection in allogeneic stem cell transplant patients. J. Med. Virol. 2014, 86, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.C.; Varelias, A.; Reddy, A.; Barone, S.M.; Olver, S.D.; Chilson, K.; Onstad, L.E.; Ensbey, K.S.; Henden, A.S.; Samson, L.; et al. CMV exposure drives long-term CD57+ CD4 memory T-cell inflation following allogeneic stem cell transplant. Blood 2021, 138, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Chanouzas, D.; Sagmeister, M.; Faustini, S.; Nightingale, P.; Richter, A.; Ferro, C.J.; Morgan, M.D.; Moss, P.; Harper, L. Subclinical Reactivation of Cytomegalovirus Drives CD4+CD28null T-Cell Expansion and Impaired Immune Response to Pneumococcal Vaccination in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Infect. Dis. 2019, 219, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.; Anand, S.; Graber, B.; Zeng, Q.; Velazquez, V.M.; Boddeda, S.R.; Fitch, J.R.; Minz, R.W.; Minz, M.; Sharma, A.; et al. Murine cytomegalovirus promotes renal allograft inflammation via Th1/17 cells and IL-17A. Am. J. Transplant. 2022, 22, 2306–2322. [Google Scholar] [CrossRef]
- Roberts, E.T.; Haan, M.N.; Dowd, J.B.; Aiello, A.E. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol. 2010, 172, 363–371. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef]
- Puissant-Lubrano, B.; Apoil, P.A.; Guedj, K.; Congy-Jolivet, N.; Roubinet, F.; Guyonnet, S.; Sourdet, S.; Nourhashemi, F.; Blancher, A. Distinct effect of age, sex, and CMV seropositivity on dendritic cells and monocytes in human blood. Immunol. Cell Biol. 2018, 96, 114–120. [Google Scholar] [CrossRef]
- De Pablo-Bernal, R.S.; Cañizares, J.; Rosado, I.; Galvá, M.I.; Alvarez-Ríos, A.I.; Carrillo-Vico, A.; Ferrando-Martínez, S.; Muñoz-Fernández, M.Á.; Rafii-El-Idrissi Benhnia, M.; Pacheco, Y.M.; et al. Monocyte Phenotype and Polyfunctionality Are Associated with Elevated Soluble Inflammatory Markers, Cytomegalovirus Infection, and Functional and Cognitive Decline in Elderly Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 610–618. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, Y.; Li, F.; Hao, Y.; Kong, Y.; Chen, C.; Hao, X.; Han, D.; Li, G.; Wang, Z.; et al. Phenotypic and functional alterations of monocyte subsets with aging. Immun. Ageing 2022, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Shimamura, M.; Musgrove, L.C.; Dennis, E.A.; Bimczok, D.; Novak, L.; Ballestas, M.; Fenton, A.; Dandekar, S.; Britt, W.J.; et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J. Immunol. 2014, 193, 5604–5612. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Mou, W.; Su, C.; Chen, X.; Zhang, H.; Cao, B.; Li, X.; Wu, D.; Ni, X.; Gui, J.; et al. Increase in Peripheral Blood Intermediate Monocytes is Associated with the Development of Recent-Onset Type 1 Diabetes Mellitus in Children. Int. J. Biol. Sci. 2017, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Annibali, O.; Piccioni, L.; Tomarchio, V.; Circhetta, E.; Sarlo, C.; Franceschini, L.; Cantonetti, M.; Rizzo, E.; Angeletti, S.; Tirindelli, M.C.; et al. Impact of IFN lambda 3/4 single nucleotide polymorphisms on the cytomegalovirus reactivation in autologous stem cell transplant patients. PLoS ONE 2018, 13, e0200221. [Google Scholar] [CrossRef]
- Corrales, I.; Solano, C.; Amat, P.; Giménez, E.; de la Cámara, R.; Nieto, J.; López, J.; García-Noblejas, A.; Piñana, J.L.; Navarro, D. IL28B genetic variation and cytomegalovirus-specific T-cell immunity in allogeneic stem cell transplant recipients. J. Med. Virol. 2017, 89, 685–695. [Google Scholar] [CrossRef]
- Weltevrede, M.; Eilers, R.; de Melker, H.E.; van Baarle, D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: A systematic review. Exp. Gerontol. 2016, 77, 87–95. [Google Scholar] [CrossRef]
- Giacconi, R.; Cardelli, M.; Piacenza, F.; Pierpaoli, E.; Farnocchia, E.; Di Rosa, M.; Bonfigli, A.R.; Casoli, T.; Marchegiani, F.; Marcheselli, F.; et al. Effect of Cytomegalovirus Reactivation on Inflammatory Status and Mortality of Older COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 6832. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, E.C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef]
- Delannoy, A.S.; Hober, D.; Bouzidi, A.; Wattre, P. Role of interferon alpha (IFN-alpha) and interferon gamma (IFN-gamma) in the control of the infection of monocyte-like cells with human cytomegalovirus (HCMV). Microbiol. Immunol. 1999, 43, 1087–1096. [Google Scholar] [CrossRef]
- Kropp, K.A.; Robertson, K.A.; Sing, G.; Rodriguez-Martin, S.; Blanc, M.; Lacaze, P.; Hassim, M.F.; Khondoker, M.R.; Busche, A.; Dickinson, P.; et al. Reversible inhibition of murine cytomegalovirus replication by gamma interferon (IFN-γ) in primary macrophages involves a primed type I IFN-signaling subnetwork for full establishment of an immediate-early antiviral state. J. Virol. 2011, 85, 10286–10299. [Google Scholar] [CrossRef]
- Shindo, H.; Maekawa, S.; Komase, K.; Miura, M.; Kadokura, M.; Sueki, R.; Komatsu, N.; Shindo, K.; Amemiya, F.; Nakayama, Y.; et al. IL-28B (IFN-λ3) and IFN-α synergistically inhibit HCV replication. J. Viral Hepat. 2013, 20, 281–289. [Google Scholar] [CrossRef]
- Da Silva, A.M.V.; Alvarado-Arnez, L.E.; Azamor, T.; Batista-Silva, L.R.; Leal-Calvo, T.; Bezerra, O.C.L.; Ribeiro-Alves, M.; Kehdy, F.S.G.; Neves, P.C.D.C.; Bayma, C.; et al. Interferon-lambda 3 and 4 Polymorphisms Increase Sustained Virological Responses and Regulate Innate Immunity in Antiviral Therapy with Pegylated Interferon-Alpha. Front. Cell Infect. Microbiol. 2021, 11, 656393. [Google Scholar] [CrossRef]
- Tong, M.Z.; Sng, J.D.; Carney, M.; Cooper, L.; Brown, S.; Lineburg, K.E.; Chew, K.Y.; Collins, N.; Ignacio, K.; Airey, M.; et al. Elevated BMI reduces the humoral response to SARS-CoV-2 infection. Clin. Transl. Immunol. 2023, 12, e1476. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Bell, M.R.; Marcy, J.; Kutzler, M.; Haddad, E.K. The impact of immuno-aging on SARS-CoV-2 vaccine development. Geroscience 2021, 43, 31–51. [Google Scholar] [CrossRef]
- Poloni, C.; Szyf, M.; Cheishvili, D.; Tsoukas, C.M. Are the Healthy Vulnerable? Cytomegalovirus Seropositivity in Healthy Adults Is Associated with Accelerated Epigenetic Age and Immune Dysregulation. J. Infect. Dis. 2022, 225, 443–452. [Google Scholar] [CrossRef]
- Bueno, S.M.; Abarca, K.; González, P.A.; Gálvez, N.M.S.; Soto, J.A.; Duarte, L.F.; Schultz, B.M.; Pacheco, G.A.; González, L.A.; Vázquez, Y.; et al. Safety and Immunogenicity of an Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Subgroup of Healthy Adults in Chile. Clin. Infect. Dis. 2022, 75, e792–e804. [Google Scholar] [CrossRef]

| Parameters | Volunteers | p Value | |||
|---|---|---|---|---|---|
| Total (n = 42) | Allele-1 (C/C) (n = 20) | Allele-2 (T/T) (n = 05) | Alleles-1/2 (C/T) (n = 17) | ||
| Age (years) | 73.7 ± 4.5 | 73.9 ± 4.9 | 72.2 ± 4.4 | 74.0 ± 4.0 | 0.722 |
| Older men (n) | 12 | 4 | 2 | 6 | 0.439 |
| Older women (n) | 30 | 16 | 3 | 11 | |
| Ratio M–W | 1:2.58 | 1:4.25 | 1:1.5 | 1:1.83 | |
| Height (m) | 1.58 ± 0.10 | 1.56 ± 0.10 | 1.61 ± 0.10 | 1.59 ± 0.12 | 0.488 |
| Weight (kg) | 64.4 ± 12.7 | 63.6 ± 12.0 | 62.9 ± 14.3 | 65.8 ± 13.6 | 0.839 |
| BMI * (kg/m2) | 25.7 ± 4.4 | 26.1 ± 5.0 | 23.8 ± 3.7 | 25.7 ± 3.8 | 0.579 |
| Allele-1 group (C/C) | |||||
| Parameters | Pre-vaccination | Parameters | Post-vaccination | ||
| rho-value | p-value | rho-value | p-value | ||
| IgG-COVID-19 X BMI * | −0.450 | 0.040 | IgG-CMV X IFN-γ | 0.554 | 0.009 |
| IgG-CMV X IL-17 | 0.558 | 0.008 | IgG-CMV X IL-6/IL-10 | 0.475 | 0.029 |
| IgG-CMV X IFN-γ | 0.534 | 0.012 | IgG-CMV X IFN-γ/IL-10 | 0.697 | <0.001 |
| IgG-CMV X IL-17/IL-10 | 0.510 | 0.018 | IgG-CMV X senescent CD4+ T cells | 0.655 | <0.001 |
| IgG-CMV X IFN-γ/IL-10 | 0.606 | 0.003 | IgG-CMV X senescent CD8+ T cells | 0.675 | <0.001 |
| Allele-2 group (T/T) | |||||
| Parameters | Pre-vaccination | Parameters | Post-vaccination | ||
| rho-value | p-value | rho-value | p-value | ||
| IgG-COVID-19 X TNF-α | −0.995 | <0.001 | IgG-COVID-19 X IL-10 | 0.878 | 0.048 |
| IgG-COVID-19 X IFN-α2 | 0.996 | <0.001 | IgG-COVID-19 X senescent CD8+ T cells | −0.886 | 0.045 |
| IgG-COVID-19 X IFN-γ | 0.918 | 0.027 | |||
| IgG-COVID-19 X IL-17 | −0.968 | 0.006 | |||
| IgG-COVID-19 X TNF-α/IL-10 | −0.999 | <0.001 | |||
| IgG-COVID-19 X IFN-α2/IL-10 | 0.998 | <0.001 | |||
| IgG-COVID-19 X IFN-γ/IL-10 | 0.922 | 0.025 | |||
| IgG-COVID-19 X IL-17/IL-10 | −0.990 | 0.001 | |||
| Alleles-1/2 group (C/T) | |||||
| Parameters | Pre-vaccination | Parameters | Post-vaccination | ||
| rho-value | p-value | rho-value | p-value | ||
| IgG-COVID-19 X Intermediate | −0.528 | 0.029 | IgG-COVID-19 X IL-10 | 0.513 | 0.035 |
| IgG-COVID-19 X IFN-γ | 0.567 | 0.017 | |||
| IgG-Specific for SARS-CoV-2 Antigen Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Allele-1 group (C/C) | |||||||
| Pre-vaccination | Post-vaccination | |||||||
| β-value | 95% CI * | p-value | R2 | β-value | 95% CI * | p-value | R2 | |
| BMI ** | −0.03636 | −0.014 to 0.042 | 0.0433 | 0.5496 | −0.03849 | −0.067 to −0.0097 | 0.0124 | 0.1686 |
| IL-17 | Ns | Ns | Ns | Ns | −0.1911 | −0.359 to −0.023 | 0.0301 | 0.6288 |
| IL-17/IL-10 | Ns | Ns | Ns | Ns | −0.6557 | −1.272 to −0.039 | 0.0392 | 0.5796 |
| IFN-γ | Ns | Ns | Ns | Ns | 0.00445 | 0.002 to 0.007 | 0.0049 | 0.8339 |
| IFN-γ/IL-10 | Ns | Ns | Ns | Ns | 0.01261 | 0.004 to 0.022 | 0.0102 | 0.6984 |
| Parameters | Allele-2 group (T/T) | |||||||
| Pre-vaccination | Post-vaccination | |||||||
| β-value | 95% CI * | p-value | R2 | β-value | 95% CI * | p-value | R2 | |
| IgG-CMV | −0.0354 | −0.033 to −0.038 | 0.0001 | 0.9998 | −0.2331 | −0.273 to −0.193 | 0.0003 | 0.9997 |
| Non classic | Ns | Ns | Ns | Ns | 6.734 | 3.774 to 9.69 | 0.0054 | 0.9996 |
| Parameters | Alleles-1/2 group (C/T) | |||||||
| Pre-vaccination | Post-vaccination | |||||||
| β-value | 95% CI * | p-value | R2 | β-value | 95% CI * | p-value | R2 | |
| Age | Ns | Ns | Ns | Ns | −0.02826 | −0.059 to −0.002 | 0.0379 | 0.2423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardy, A.; Monteiro, F.R.; Silva, B.R.; do Amaral, J.B.; Leal Oliveira, D.B.; Cândido, É.D.d.O.; Luiz Durigon, E.; Aguiar, A.S.; Scagion, G.P.; Chalup, V.N.; et al. Polymorphism in IFNλ Can Impact the Immune/Inflammatory Response to COVID-19 Vaccination in Older CMV-Seropositive Adults. Vaccines 2025, 13, 785. https://doi.org/10.3390/vaccines13080785
Nardy A, Monteiro FR, Silva BR, do Amaral JB, Leal Oliveira DB, Cândido ÉDdO, Luiz Durigon E, Aguiar AS, Scagion GP, Chalup VN, et al. Polymorphism in IFNλ Can Impact the Immune/Inflammatory Response to COVID-19 Vaccination in Older CMV-Seropositive Adults. Vaccines. 2025; 13(8):785. https://doi.org/10.3390/vaccines13080785
Chicago/Turabian StyleNardy, Ariane, Fernanda Rodrigues Monteiro, Brenda Rodrigues Silva, Jônatas Bussador do Amaral, Danielle Bruna Leal Oliveira, Érika Donizetti de Oliveira Cândido, Edison Luiz Durigon, Andressa Simões Aguiar, Guilherme Pereira Scagion, Vanessa Nascimento Chalup, and et al. 2025. "Polymorphism in IFNλ Can Impact the Immune/Inflammatory Response to COVID-19 Vaccination in Older CMV-Seropositive Adults" Vaccines 13, no. 8: 785. https://doi.org/10.3390/vaccines13080785
APA StyleNardy, A., Monteiro, F. R., Silva, B. R., do Amaral, J. B., Leal Oliveira, D. B., Cândido, É. D. d. O., Luiz Durigon, E., Aguiar, A. S., Scagion, G. P., Chalup, V. N., Furtado, G. E., Shio, M. T., França, C. N., Nali, L. H. d. S., & Bachi, A. L. L. (2025). Polymorphism in IFNλ Can Impact the Immune/Inflammatory Response to COVID-19 Vaccination in Older CMV-Seropositive Adults. Vaccines, 13(8), 785. https://doi.org/10.3390/vaccines13080785





