A Narrative Review of College Meningococcal Vaccination Mandates Across the United States
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
4.1. Patterns of Vaccination Requirements and Coverage
4.2. Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACHA | American College Health Association |
| ACIP | Advisory Committee on Immunization Practices |
| IMD | Invasive meningococcal disease |
| MenACWY | Meningococcal serogroups A/C/W/Y |
| MenB | Meningococcal serogroup B |
Appendix A
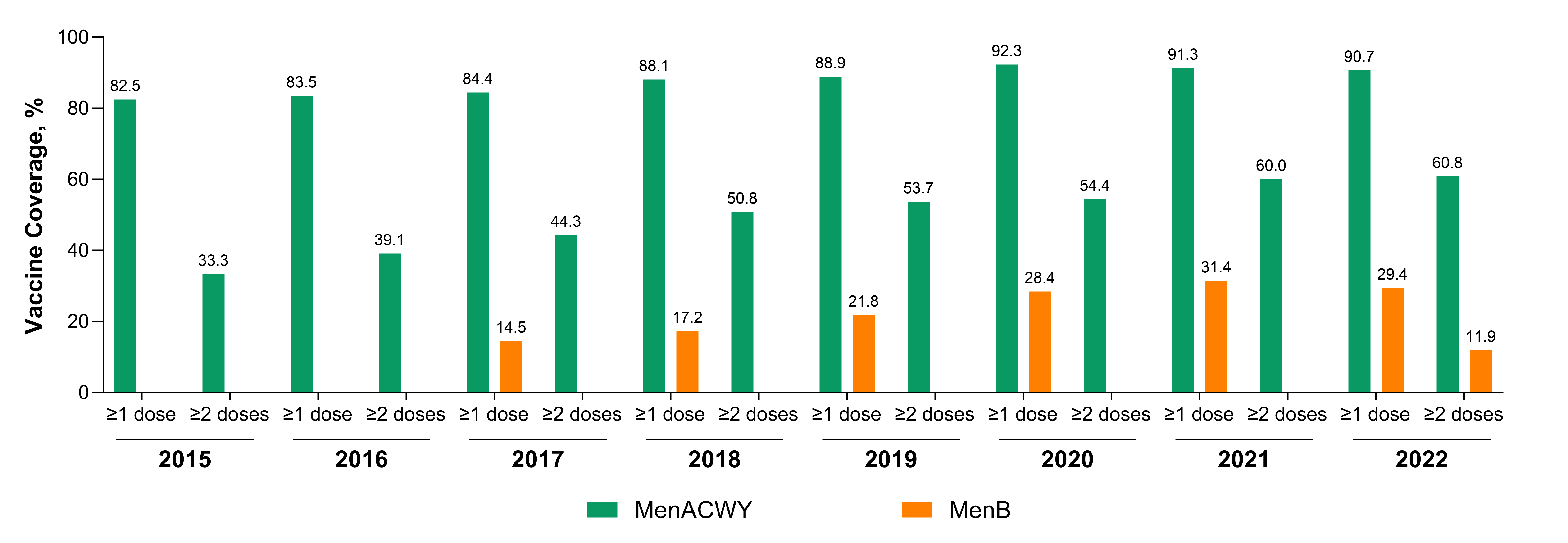
References
- Rosenstein, N.E.; Perkins, B.A.; Stephens, D.S.; Popovic, T.; Hughes, J.M. Meningococcal disease. N. Engl. J. Med. 2001, 344, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, S.; Duffy, J.; McNamara, L. Meningococcal disease. In Epidemiology and Prevention of Vaccine-Preventable Diseases, Hall, E., Wodi, A.P., Hamborsky, J., Schillie, S., Eds.; 14th ed.; Public Health Foundation: Washington, DC, USA, 2021. [Google Scholar]
- World Health Organization. Meningitis. Available online: https://www.who.int/news-room/fact-sheets/detail/meningitis (accessed on 14 February 2023).
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Watle, S.V.; Caugant, D.A.; Tunheim, G.; Bekkevold, T.; Laake, I.; Brynildsrud, O.B.; Naess, L.M. Meningococcal carriage in Norwegian teenagers: Strain characterisation and assessment of risk factors. Epidemiol. Infect. 2020, 148, e80. [Google Scholar] [CrossRef] [PubMed]
- Deghmane, A.E.; Taha, S.; Taha, M.K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: A narrative review. Infect. Dis. 2022, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2019. Available online: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf (accessed on 24 January 2023).
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2020. Available online: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2020.pdf (accessed on 9 April 2025).
- Centers for Disease Control and Prevention. Enhanced Meninigococcal Disease Surveillance Report. 2021. Available online: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2021.pdf (accessed on 22 June 2023).
- Pardo de Santayana, C.; Tin Tin Htar, M.; Findlow, J.; Balmer, P. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: A literature review. Epidemiol. Infect. 2023, 151, e57. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, S.A.; Joseph, S.J.; Blain, A.; Wang, X.; Hariri, S.; MacNeil, J.R. Meningococcal disease among college-aged young adults: 2014-2016. Pediatrics 2019, 143, e20182130. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Dempsey, A.F.; Srivastava, A.; Isturiz, R.E. US college students are at increased risk for serogroup B meningococcal disease. J. Pediatr. Infect. Dis. Soc. 2020, 9, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Recommended Child and Adolescent Immunization Schedule for Ages 18 Years or Younger: United States 2023. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/past/2023-child.pdf (accessed on 21 July 2025).
- US Food and Drug Administration. PENBRAYA Prescribing Information, Updated 11/2024. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/penbraya (accessed on 28 April 2025).
- Pfizer Inc. FDA Approves Penbraya™, the First and Only Vaccine for the Prevention of the Five Most Common Serogroups Causing Meningococcal Disease in Adolescents. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-approves-penbrayatm-first-and-only-vaccine-prevention (accessed on 6 June 2025).
- Peterson, J.; Drazan, D.; Czajka, H.; Maguire, J.; Pregaldien, J.L.; Seppa, I.; Maansson, R.; O’Neill, R.; Balmer, P.; Jodar, L.; et al. Immunogenicity and safety of a pentavalent meningococcal ABCWY vaccine in adolescents and young adults: An observer-blind, active-controlled, randomised trial. Lancet Infect. Dis. 2023, 23, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Meeting of the Advisory Committee on Immunization Practices (ACIP)-October 25-26, 2023. Available online: https://www.cdc.gov/acip/downloads/minutes/summary-2023-10-25-26-508.pdf (accessed on 20 July 2025).
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; DeSisto, C.L.; Stokley, S.; Singleton, J.A. Vaccination coverage among adolescents aged 13-17 years-National Immunization Survey-Teen, United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Meningococcal Vaccination for Preteens and Teens. Available online: https://www.cdc.gov/meningococcal/vaccines/preteens-teens.html?CDC_AAref_Val=https://www.cdc.gov/vaccines/vpd/mening/public/adolescent-vaccine.html (accessed on 21 July 2025).
- La, E.M.; Garbinsky, D.; Hunter, S.; Poston, S.; Novy, P.; Ghaswalla, P. Meningococcal B vaccination coverage among older adolescents in the United States. Vaccine 2021, 39, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Snedecor, S.J.; Balmer, P.; Srivastava, A. Potential public health impact of a Neisseria meningitidis A, B, C, W, and Y pentavalent vaccine in the United States. Postgrad. Med. 2022, 134, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Fergie, J.; Presa, J.; Peyrani, P. Rationale for the development of a pentavalent meningococcal vaccine: A US-focused review. Infect. Dis. Ther. 2022, 11, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Immunize.org. State Meningococcal ACWY (MenACWY) Vaccine Requirements for Colleges and Universities. Available online: https://www.immunize.org/laws/menin.asp (accessed on 3 November 2023).
- Ohio Department of Health. Meningococcal Disease and College Students. Available online: https://odh.ohio.gov/wps/wcm/connect/gov/8ef50ea6-58ad-4f94-b552-e0b0ec6d6d4d/Meningococcal-Disease-Meningitis.pdf?MOD=AJPERES (accessed on 21 July 2025).
- Ohio Department of Health. Recommended Vaccines for College Students. Available online: https://odh.ohio.gov/know-our-programs/Immunization/Recommended-Vaccines-College-Students/Recommended-Vaccines-College-Students (accessed on 14 November 2023).
- National Conference of State Legislatures. State Vaccine Requirements for College Entry. Available online: https://www.ncsl.org/health/state-vaccine-requirements-for-college-entry (accessed on 3 November 2023).
- Council of the District of Columbia. Notice D. C. Law 3-20 “Immunization of School Students Act of 1979”. Available online: https://code.dccouncil.gov/us/dc/council/laws/docs/3-20.pdf (accessed on 15 November 2023).
- West Virginia Department of Health and Human Resources. Recommendations of the West Virginia Bureau for Public Health: Immunizations for West Virginia College, University, Community and Technical School Students. Available online: https://oeps.wv.gov/immunizations/Documents/school/college_requirements.pdf (accessed on 15 November 2023).
- American Society for Meningitis Prevention. Meningitis B Cases & School Vaccination Mandates Tracker. Available online: https://meningitisprevention.org/menbtracker (accessed on 21 July 2025).
- Alabama Public Health. Immunization. Available online: https://www.alabamapublichealth.gov/immunization/ (accessed on 3 November 2023).
- Alaska Department of Health. Meningococcal Disease. Available online: https://health.alaska.gov/dph/Epi/id/Pages/Meningococcal.aspx#:~:text=Most%20cases%20are%20caused%20by,see%20below%20for%20more%20details (accessed on 3 November 2023).
- National Vaccine Information Center. Alaska State Vaccine Requirements. Available online: https://www.nvic.org/law-policy-state/vaccine-laws-alaska (accessed on 2 November 2023).
- Arizona Department of Health Services. Arizona Immunization Program. Available online: https://www.azdhs.gov/preparedness/epidemiology-disease-control/immunization/index.php#college-info (accessed on 3 November 2023).
- The Arizona Partnership for Immunization. Off to College? Available online: https://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/immunization/school-childcare/tapi-az-off-to-college.pdf (accessed on 3 November 2023).
- Arkansas State Board of Health. Rules and Regulations Pertaining to Immunization Requirements. Available online: https://www.healthy.arkansas.gov/images/uploads/rules/ImmunizationRequirements.pdf (accessed on 3 November 2023).
- Arkansas Department of Health. Immunizations. Available online: https://healthy.arkansas.gov/programs-services/community-family-child-health/immunizations/ (accessed on 21 July 2025).
- Centers for Disease Control and Prevention. Meningococcal ACWY Vaccine: What You Need to Know. Available online: https://www.cdc.gov/vaccines/hcp/current-vis/downloads/mening.pdf (accessed on 21 July 2025).
- California Department of Public Health. Immunization Recommendations and Screening Requirements for California Colleges & Universities. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/School/college.aspx (accessed on 21 July 2025).
- LexisNexis. C.R.S. 23-5-128. Available online: https://advance.lexis.com/documentpage/?pdmfid=1000516&crid=52555103-e7f0-43c2-a57f-77e505c244ce&nodeid=AAXAABAABAAOABL&nodepath=%2fROOT%2fAAX%2fAAXAAB%2fAAXAABAAB%2fAAXAABAABAAO%2fAAXAABAABAAOABL&level=5&haschildren=&populated=false&title=23-5-128.+Meningococcal+disease+-+information+-+immunity+-+definitions&config=014FJAAyNGJkY2Y4Zi1mNjgyLTRkN2YtYmE4OS03NTYzNzYzOTg0OGEKAFBvZENhdGFsb2d592qv2Kywlf8caKqYROP5&pddocfullpath=%2fshared%2fdocument%2fstatutes-legislation%2furn%3acontentItem%3a61P5-WTV1-DYDC-J3VB-00008-00&ecomp=L38_9kk&prid=0ec356a6-343e-4cdd-9b03-54b881538165 (accessed on 3 November 2023).
- Colorado Department of Public Health & Environment. School-Required Vaccines. Available online: https://cdphe.colorado.gov/schoolrequiredvaccines (accessed on 3 November 2023).
- Fergie, J.; Howard, A.; Huang, L.; Srivastava, A. Implementation experience with meningococcal serogroup B vaccines in the United States. Pediatr. Infect. Dis. J. 2021, 40, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Connecticut General Assembly. Title 10A State System of Higher Education, Chapter 185b. Available online: https://www.cga.ct.gov/current/pub/chap_185b.htm#sec_10a-155b (accessed on 3 November 2023).
- State of Connecticut. DPH Immunizations. Available online: https://portal.ct.gov/immunization/Public-Landing-Page/Vaccine-Information?language=en_US (accessed on 3 November 2023).
- The Delaware Code Online. Title 16. Health and Safety—Regulatory Provisions Concerning Public Health—Chapter 5. Contagious Diseases Generally. Available online: https://delcode.delaware.gov/title16/c005/sc01/index.html (accessed on 28 November 2023).
- Florida Legislature. The 2023 Florida Statutes. Available online: https://www.flsenate.gov/Laws/Statutes/2023/1006.69 (accessed on 21 July 2025).
- Florida Department of Health. Meningococcal Disease. Available online: https://www.floridahealth.gov/diseases-and-conditions/meningococcal-disease/index.html#:~:text=For%20adolescents%20who%20receive%20the,not%20need%20a%20booster%20dose (accessed on 3 November 2023).
- Justia US Law. 2022 Georgia Code Title 31—Health Chapter 12—Control of Hazardous Conditions, Preventable Diseases, and Metabolic Disorders § 31-12-3.2. Meningococcal Disease; Vaccinations; Disclosures. Available online: https://law.justia.com/codes/georgia/2022/title-31/chapter-12/section-31-12-3-2/ (accessed on 23 November 2023).
- Board of Regents of the University System of Georgia. Immunization Requirements and Recommendations for University System of Georgia Students. Available online: https://dph.georgia.gov/sites/dph.georgia.gov/files/Immunizations/IPC-Imm-Req-and-Rec-for-University-GA.pdf (accessed on 21 July 2025).
- Hawaii Department of Health. Important Notice to Post-Secondary School Students: Health Requirements Effective; 2020. Available online: https://health.hawaii.gov/docd/files/2019/08/VaxToSchool_PostSecStudenttNotice.pdf (accessed on 3 November 2023).
- Department of Health. Amendment and Compilation of Chapter 11-157 Hawaii Administrative Rules. Available online: https://health.hawaii.gov/opppd/files/2019/08/11-157-includes-Exhibit-A-and-Exhibit-B.pdf (accessed on 3 November 2023).
- State of Hawaii, Disease Outbreak Control Division. Meningococcal Disease. Available online: https://health.hawaii.gov/docd/disease_listing/meningococcal/ (accessed on 3 November 2023).
- Idaho Department of Health & Welfare. Immunizations. Available online: https://healthandwelfare.idaho.gov/services-programs/children-families/child-and-adolescent-immunization (accessed on 3 November 2023).
- CWI College of Western Idaho. Student Handbook: 2.2 Vaccinations. Available online: https://cwi.edu/student-handbook/22-vaccinations (accessed on 3 November 2023).
- University of Idaho. Immunization Policy. Available online: https://www.uidaho.edu/vandal-health-clinic/immunization#:~:text=Vaccination%20Information%20for%20Idaho%20Post,admission%20or%20enrollment%20for%20classes (accessed on 3 November 2023).
- Illinois General Assembly. Administrative Code. Available online: https://www.ilga.gov/commission/jcar/admincode/077/077006940B01000R.html (accessed on 3 November 2023).
- Indiana Department of Education. Immunizations. Available online: https://www.in.gov/doe/students/school-safety-and-wellness/health/immunizations/ (accessed on 3 November 2023).
- Indiana General Assembly. Title 21. Higher Education. Available online: https://iga.in.gov/laws/2020/ic/titles/21#21-40-5-2 (accessed on 3 November 2023).
- Purdue University. State Immunization Requirements for Enrolled Students. Available online: https://www.purdue.edu/push/immunization/ (accessed on 30 November 2023).
- Iowa Department of Public Health. Immunization Law and You. Available online: https://publications.iowa.gov/43492/1/idph_immunizationlaw.pdf (accessed on 21 July 2025).
- University of Iowa. Immunizations. Available online: https://studenthealth.uiowa.edu/immunizations (accessed on 9 November 2023).
- Kansas State Legislature. 2024 Statute. Available online: https://www.kslegislature.org/li/b2023_24/statute/076_000_0000_chapter/076_007_0000_article/076_007_0061a_section/076_007_0061a_k/ (accessed on 21 July 2025).
- Team Kentucky. Immunization Branch. Available online: https://www.chfs.ky.gov/agencies/dph/dehp/Pages/immunization.aspx (accessed on 9 November 2023).
- Louisiana State Legislature. RS 17:170.1. Available online: https://www.legis.la.gov/legis/Law.aspx?d=409695 (accessed on 9 November 2023).
- Louisiana Department of Health. Immunization Program. Available online: https://ldh.la.gov/immunization-program (accessed on 21 July 2025).
- Maine Center for Disease Control and Prevention. School Vaccine Law. Post-Secondary School. Available online: https://www.maine.gov/dhhs/mecdc/diseases-conditions/immunization/school-vaccine-law (accessed on 21 July 2025).
- Maine Immunization Program. Meningococcal B Vaccine Campaign. Available online: https://www.maine.gov/dhhs/mecdc/infectious-disease/immunization/providers/documents/Meningococcal%20B%20Vaccine%20Campaign.pdf (accessed on 10 November 2023).
- Maine Immunization Program. Meningococcal Vaccines and Disease Prevention. Available online: https://content.govdelivery.com/accounts/MEHHS/bulletins/3333150 (accessed on 21 July 2025).
- Maryland General Assembly. Statute 18–102. Available online: https://mgaleg.maryland.gov/mgawebsite/Laws/StatuteText?article=ghg§ion=18-102&enactments=false (accessed on 9 November 2023).
- Maryland Department of Health. 2020 Recommended Childhood Immunization Schedule. Available online: https://health.maryland.gov/phpa/OIDEOR/IMMUN/Shared%20Documents/Current_Recommended_Childhood-Adolescent_Immunization_Schedule_Final.pdf (accessed on 9 November 2023).
- Commonwealth of Massachusetts. Section 15D. Available online: https://malegislature.gov/Laws/GeneralLaws/PartI/TitleXII/Chapter76/Section15D (accessed on 9 November 2023).
- Commonwealth of Massachusetts. School Immunizations. Available online: https://www.mass.gov/info-details/school-immunizations (accessed on 10 November 2023).
- Michigan Department of Health & Human Services. Meningococcal Disease Information and Investigation Guidelines. Available online: https://www.michigan.gov/-/media/Project/Websites/mdhhs/Folder2/Folder7/Folder1/Folder107/MeningococcalDiseaseInfo.pdf?rev=df6a3de5f6c241de93381c0d30ec6b38#:~:text=Begin%20follow%2Dup%20case%20investigation,24%20hours%20of%20case%20notification.&text=Enter%20the%20meningococcal%20disease%20case,Meningococcal%20Disease%20case%20report%20form (accessed on 24 November 2023).
- Minnesota Department of Health. Meningococcal Disease and Vaccine: What College Students Need to Know. Available online: https://www.health.state.mn.us/diseases/meningococcal/collegefact.html (accessed on 21 July 2025).
- Minnesota Department of Health. Meningococcal Information for College Students. Available online: https://www.health.state.mn.us/diseases/meningococcal/college.html (accessed on 10 November 2023).
- Mississippi Board of Trustees of State Institutions of Higher Learning. Policies and Bylaws. Available online: http://www.ihl.state.ms.us/board/downloads/policiesandbylaws.pdf (accessed on 21 July 2025).
- Mississippi State Department of Health. Immunizations. Available online: https://msdh.ms.gov/msdhsite/_static/14,0,71.html (accessed on 10 November 2023).
- Centers For Disease Control and Prevention. Vaccine Information for Adults. Available online: https://www.cdc.gov/vaccines-adults/index.html (accessed on 21 July 2025).
- Missouri Revisor of Statutes. Title XI Education and Libraries: Chapter 174. Available online: https://revisor.mo.gov/main/OneSection.aspx?section=174.335&bid=33759 (accessed on 10 November 2023).
- Missouri Department of Health and Senior Services. Meningococcal Disease: What You Need to Know. Available online: https://health.mo.gov/living/wellness/immunizations/pdf/716.pdf (accessed on 21 July 2025).
- Missouri Revised Statutes. Mo. Rev. Stat. § 167.638. Available online: https://law.justia.com/codes/missouri/title-xi/chapter-167/section-167-638/ (accessed on 21 July 2025).
- Montana Department of Public Health and Human Services. Documentation of Immunization Status of Persons Commencing Attendance in a Postsecondary School. Available online: https://rules.mt.gov/gateway/RuleNo.asp?RN=37.114.712 (accessed on 10 November 2023).
- Montana DPHHS. Adolescent Vaccines. Available online: https://dphhs.mt.gov/publichealth/immunization/AdolescentVaccines#what (accessed on 10 November 2023).
- Nebraska Department of Health and Human Services. DHHS Urges Catch Up on Child Immunizations and All Nebraskans to Get Flu Vaccine Once Its Available. Available online: https://dhhs.ne.gov/Pages/DHHS-Urges-Catch-Up-on-Child-Immunizations-and-All-Nebraskans-to-get-Flu-Vaccine-Once-its-Available.aspx (accessed on 10 November 2023).
- Nebraska Legislature. Nebraska Revised Statute 85-902. Available online: https://nebraskalegislature.gov/laws/statutes.php?statute=85-902 (accessed on 10 November 2023).
- Nevada Administration Code. Chapter 441a—Infectious Diseases; Toxic Agents General Provisions: NAC 441A.755. Available online: https://www.leg.state.nv.us/nac/nac-441a.html#NAC441ASec755 (accessed on 21 July 2025).
- New Hampshire DHHS. Meningococcal Disease. Available online: https://www.dhhs.nh.gov/programs-services/disease-prevention/infectious-disease-control/meningococcal-disease (accessed on 10 November 2023).
- National Vaccine Information Center. New Hampshire State Vaccine Requirements. Available online: https://www.nvic.org/law-policy-state/vaccine-laws-new-hampshire (accessed on 10 November 2023).
- New Jersey Legislature. Chapter 332: An Act Concerning Immunizations for Meningococcal Disease and Amending P.L.2003, c.284. Available online: https://pub.njleg.gov/bills/2018/PL19/332_.PDF (accessed on 10 November 2023).
- New Jersey Legislature. P.L. 2003, Chapter 284: An Act Requiring [Meningitis] Meningococcal Vaccinations for Students at Certain Institutions of Higher Education, Amending P.L.2000, c.25 and Supplementing Title 18A of the New Jersey Statutes. Available online: https://pub.njleg.gov/bills/2002/AL03/284_.PDF (accessed on 10 November 2023).
- New Jersey Department of Health. 2022 College Immunization Status Report. Available online: https://www.nj.gov/health/cd/documents/imm_requirements/college_status_report.pdf (accessed on 10 November 2023).
- NJ Health. Implementation of Meningococcal Vaccine Requirements: Guidance for Institutions of Higher Education. Available online: https://www.nj.gov/health/cd/documents/topics/meningo/meningo_requirements_highered.pdf (accessed on 10 November 2023).
- New Jersey Administrative Code. N.J. Admin. Code § 8:57-6.10. Available online: https://www.law.cornell.edu/regulations/new-jersey/N-J-A-C-8-57-6-10 (accessed on 21 July 2025).
- New Mexico Department of Health. Immunization Schedules. Available online: https://www.nmhealth.org/about/phd/idb/imp/imsc/ (accessed on 10 November 2023).
- Centers for Disease Control and Prevention. Adult Immunization Schedule by Age. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/adult-age.html (accessed on 21 July 2025).
- New York State Department of Health. Section IX: Appendix A—Article 21, Title 6, Section 2167: Immunization Against Meningococcal Meningitis. Available online: https://www.health.ny.gov/prevention/immunization/handbook/section_9_appendices/appendix_a/public_health_law/article_21/title_6/section_2167.htm (accessed on 10 November 2023).
- New York State Department of Health. Information for College/University Student Health Services. Available online: https://www.health.ny.gov/prevention/immunization/meningococcal/info_college_student_health_services.htm (accessed on 10 November 2023).
- North Carolina Department of Health and Human Services. Vaccine Preventable Diseases: Meningococcal Disease. Available online: https://epi.dph.ncdhhs.gov/cd/diseases/vpd.html (accessed on 21 July 2025).
- North Carolina Department of Health and Human Services. Immunization Requirements for Colleges and Universities. Available online: https://immunization.dph.ncdhhs.gov/schools/collegesuniversities.htm (accessed on 21 July 2025).
- North Dakota Public Health Division. Meningococcal Disease Fact Sheet. Available online: https://www.hhs.nd.gov/sites/www/files/documents/DOH%20Legacy/Immunization/Meningococcal%20Disease.pdf (accessed on 10 November 2023).
- North Dakota State Board of Higher Education. Policy Manual: 506 Immunizations and Tuberculosis Screening. Available online: https://ndusbpos.sharepoint.com/:w:/s/NDUSPoliciesandProcedures/Ee0NekYhiutLkVYQWfXFEfUBqFtQAWWQKLThkGnzt0fjBw?rtime=TmVgCavI3Ug (accessed on 21 July 2025).
- Oklahoma Senate. Oklahoma Statutes: Title 70. Schools. Available online: https://oksenate.gov/sites/default/files/2019-12/os70.pdf (accessed on 10 November 2023).
- Oklahoma State Department of Health. Vaccines by Age Group. Available online: https://oklahoma.gov/health/services/personal-health/immunizations/imm-vaccines-by-age-group.html (accessed on 10 November 2023).
- Oregon Health Authority. Immunization Requirements for Colleges. Available online: https://www.oregon.gov/oha/PH/PREVENTIONWELLNESS/VACCINESIMMUNIZATION/GETTINGIMMUNIZED/Pages/college.aspx (accessed on 14 November 2023).
- Oregon Health Authority. Vax to School: Check Your Immunization Record. Available online: https://sharedsystems.dhsoha.state.or.us/DHSForms/Served/le4682.pdf (accessed on 14 November 2023).
- Pennsylvania General Assembly. College and University Student Vaccination Act. Available online: https://www.legis.state.pa.us/cfdocs/legis/li/uconsCheck.cfm?yr=2002&sessInd=0&act=83# (accessed on 10 November 2023).
- Pennsylvania Department of Health. PreTeen and Teen Immunizations. Available online: https://www.health.pa.gov/topics/programs/immunizations/Pages/Adolescent.aspx (accessed on 10 November 2023).
- Immunize.org. Vaccinations for Preteens and Teens. Available online: https://immunize.org/wp-content/uploads/catg.d/p4020.pdf (accessed on 10 November 2023).
- Rhode Island Code of Regulations. Immunization and Communicable Disease Testing in Preschool, School, Colleges or Universities (216-RICR-30-05-3). Available online: https://rules.sos.ri.gov/regulations/part/216-30-05-3 (accessed on 10 November 2023).
- Rhode Island Department of Health. Immunization. Available online: https://health.ri.gov/immunization/ (accessed on 10 November 2023).
- South Carolina Department of Health. Frequently Asked Questions: Vaccinations. Available online: https://dph.sc.gov/public/vaccinations/frequently-asked-questions-vaccinations (accessed on 21 July 2025).
- South Carolina Department of Health. Preteen & Teen Vaccines. Available online: https://dph.sc.gov/health-wellness/child-teen-health/vaccine-requirements-info/preteen-teen-vaccines (accessed on 21 July 2025).
- University of South Carolina Upstate. Immunization Requirements. Available online: https://uscupstate.edu/student-experience/student-services-resources/health-services/immunization-requirements/ (accessed on 21 July 2025).
- South Dakota Department of Health. Learn About Vaccine Protection. Available online: https://doh.sd.gov/media/jy5leqak/sdbor_collegevaccinationsbrochure.pdf (accessed on 10 November 2023).
- State of Tennessee. Tenn. Code Ann. § 49-7-124. Available online: https://advance.lexis.com/documentpage/?pdmfid=1000516&crid=6deef5e8-4348-46fd-859e-9e237037165a&nodeid=ABXAAHAABAAZ&nodepath=%2fROOT%2fABX%2fABXAAH%2fABXAAHAAB%2fABXAAHAABAAZ&level=4&haschildren=&populated=false&title=49-7-124.+Jacob+Nunley+Act+%E2%80%94+Requirement+of+proving+immunization+against+meningococcal+disease+%E2%80%94+Exemptions.&config=025054JABlOTJjNmIyNi0wYjI0LTRjZGEtYWE5ZC0zNGFhOWNhMjFlNDgKAFBvZENhdGFsb2cDFQ14bX2GfyBTaI9WcPX5&pddocfullpath=%2fshared%2fdocument%2fstatutes-legislation%2furn%3acontentItem%3a4X55-GPX0-R03K-P4T6-00008-00&ecomp=L38_kkk&prid=5bf99721-194e-46f4-91a1-57e008134de3 (accessed on 10 November 2023).
- Tennessee Department of Health. College Immunization Requirements. Available online: https://www.tn.gov/health/cedep/immunization-program/ip/immunization-requirements/college-immunization-requirements.html (accessed on 10 November 2023).
- Tennessee Immunization Program. Diseases Covered by Tennessee Child Care and School Immunization Requirements. Available online: https://www.tn.gov/content/dam/tn/health/documents/immunizationrequirements/tenniis/TN_Immunization_Summary_Table.pdf (accessed on 10 November 2023).
- Texas Education Code. Chapter 51. Provisions Generally Applicable to Higher Education. Available online: https://statutes.capitol.texas.gov/Docs/ED/htm/ED.51.htm#51.9192 (accessed on 10 November 2023).
- Texas Health and Human Services. Meningococcal Invasive Disease; Prevention and Vaccination. Available online: https://www.dshs.texas.gov/vaccine-preventable-diseases/meningococcal-invasive-disease (accessed on 21 July 2025).
- Centers for Disease Control and Prevention. The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-14-meningococcal-disease.html#cdc_report_pub_study_section_8-vaccination-schedule-and-use (accessed on 21 July 2025).
- Utah Department of Health & Human Services. Stay Up to Date on Your Immunizations and Health Screenings. Available online: https://dhhs.utah.gov/health-screenings/ (accessed on 15 November 2023).
- Vermont Department of Health. College Entry Immunization Requirements. Available online: https://www.healthvermont.gov/sites/default/files/documents/pdf/ID_IZ_K12_iz_college_letter.pdf (accessed on 21 July 2025).
- Vermont Department of Health. Immunizations for Adults. Available online: https://www.healthvermont.gov/disease-control/immunization/immunizations-adults (accessed on 15 November 2023).
- Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: For Ages 19 Years or Older. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/adult/adult-combined-schedule.pdf (accessed on 21 July 2025).
- Commonwealth of Virginia. Code of Virginia. Available online: https://law.lis.virginia.gov/vacode/title23.1/chapter8/section23.1-800/ (accessed on 15 November 2023).
- Virginia Department of Health. Immunization Practices (ACIP) & VISS. Available online: https://www.vdh.virginia.gov/immunization/immunization-manual/ (accessed on 15 November 2023).
- Washington State Department of Health. For College Students and Administrators. Available online: https://doh.wa.gov/you-and-your-family/immunization/college-students (accessed on 15 November 2023).
- Washington State Department of Health. Immunizations—For Adults. Available online: https://doh.wa.gov/you-and-your-family/immunization/adult (accessed on 15 November 2023).
- Wisconsin State Legislature. 2003 Wisconsin Act 61. Available online: https://docs.legis.wisconsin.gov/2003/related/acts/61 (accessed on 15 November 2023).
- Wisconsin Department of Health Services. College Students and Meningococcal Disease General Information. Available online: https://www.dhs.wisconsin.gov/immunization/student-mening.htm (accessed on 15 November 2023).
- Wyoming Department of Health. Imunization Unit: Adult Vaccines. Available online: https://health.wyo.gov/publichealth/immunization/adult-vaccines/ (accessed on 15 November 2023).
- Texas A&M University. At a Glance. Available online: https://www.tamu.edu/about/at-a-glance.html (accessed on 14 December 2023).
- University of Central Florida. UCF Facts 2022-23. Available online: https://www.ucf.edu/document/ucf-facts-and-stats/ (accessed on 14 December 2023).
- University of Florida. Facts: Enrollment & Demographics. Available online: https://ir.aa.ufl.edu/facts/enrollment/ (accessed on 14 December 2023).
- The Ohio State University. Statistical Summary 2022-2023. Available online: https://facts.osu.edu/sites/default/files/documents/2023/11/2022-23-Statistical-Summary_0.pdf (accessed on 14 December 2023).
- Arizona State University. Facts and Figures. Available online: https://www.asu.edu/about/facts-and-figures (accessed on 21 July 2025).
- University of Illinois Urbana-Champaign. Illinois Welcomes Second-Largest Freshman Class. Available online: https://news.illinois.edu/view/6367/1784595294 (accessed on 14 December 2023).
- University of Minnesota. Institutional Analysis: Institutional Data and Research (IDR). Available online: https://idr.umn.edu/reports-by-topic-enrollment/enrollments?utm_medium=browser&utm_id=oir_redirect&utm_source=0lYd6 (accessed on 14 December 2023).
- The University of Texas at Austin. UT Austin Enrolls Largest-Ever Student Body, Sets All-Time Highs for Graduation Rates. Available online: https://news.utexas.edu/2022/09/22/ut-austin-enrolls-largest-ever-student-body-sets-all-time-highs-for-graduation-rates/ (accessed on 14 December 2023).
- Kobzowicz, A.; Kramer, S.; Baker, W.; Lukens, K.; Hartwell, M. Meningitis: Immunizations on Pennsylvania College and University Campuses; General Assembly of the Commonwealth of Pennsylvania: Harrisburg, PA, USA, 2020; p. 139. [Google Scholar]
- American College Health Association. ACHA Guidelines: Immunization Recommendations for College Students. Available online: https://www.acha.org/resource/immunization-recommendations-for-college-students/ (accessed on 21 July 2025).
- MacNeil, J.R.; Rubin, L.; Folaranmi, T.; Ortega-Sanchez, I.R.; Patel, M.; Martin, S.W. Use of serogroup B meningococcal vaccines in adolescents and young adults: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Alderfer, J.; Isturiz, R.E.; Srivastava, A. Lessons from mass vaccination response to meningococcal B outbreaks at US universities. Postgrad. Med. 2020, 132, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Candrilli, S.D.; Kurosky, S. The Response to and Cost of Meningococcal Disease Outbreaks in University Campus Settings: A Case Study in Oregon, United States; RTI Press: Research Triangle Park, NC, USA, 2019. [Google Scholar]
- Soeters, H.M.; McNamara, L.A.; Blain, A.E.; Whaley, M.; MacNeil, J.R.; Hariri, S.; Mbaeyi, S.A. Serogroup B Meningococcal Disease University Outbreak Group. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013-2018. Emerg. Infect. Dis. 2019, 25, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Alderfer, J.; Srivastava, A. US States’ Policies for Meningococcal Vaccination vs Disease Epidemiology. In Proceedings of the IDWeek 2019, Washington, DC, USA, 2–6 October 2019. [Google Scholar]
- Oliver, S.E.; Patton, M.E.; Hoban, M.; Leino, V.; Mbaeyi, S.A.; Hariri, S.; MacNeil, J.R. Evaluation of meningococcal vaccination policies among colleges and universities—United States, 2017. J. Am. Coll. Health 2021, 69, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, L.M.; Yakely, A.E.; Hansen, C.E. Up-to-date coverage with meningococcal vaccine among adolescents age 17 years: Patterns and correlates in the United States, 2017. Vaccine 2019, 37, 5934–5938. [Google Scholar] [CrossRef] [PubMed]
- Kurosky, S.K.; Esterberg, E.; Irwin, D.E.; Trantham, L.; Packnett, E.; Novy, P.; Whelan, J.; Hogea, C. Meningococcal Vaccination Among Adolescents in the United States: A Tale of Two Age Platforms. J. Adolesc. Health 2019, 65, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2015. Available online: https://stacks.cdc.gov/view/cdc/49451 (accessed on 9 November 2022).
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2016. Available online: https://stacks.cdc.gov/view/cdc/49452 (accessed on 3 March 2025).
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2017. Available online: https://stacks.cdc.gov/view/cdc/75419 (accessed on 9 November 2022).
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2018. Available online: https://stacks.cdc.gov/view/cdc/111348 (accessed on 3 March 2025).
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; Stokley, S.; Singleton, J.A. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| State | MenACWY Required | Students Given Information About MenACWY Vaccine; Students May Decline Vaccination After Review | MenACWY Recommended | MenB Required | MenB Recommended | Exemptions |
|---|---|---|---|---|---|---|
| Alabama [23,30] | NO | NO; N/A | YES * | NO | NO | N/A |
| Alaska [23,31,32] | NO | YES; YES | YES | NO | NO | N/A |
| Arizona [23,33,34] | NO | NO; N/A | YES | NO | YES | N/A |
| Arkansas [23,35,36,37] | NO | NO; N/A | YES | NO | NO | N/A |
| California [23,38] | NO | YES; YES | YES | NO | YES | N/A |
| Colorado [26,39,40,41] | YES for students residing in student housing | YES; YES | N/A | NO | NO | N/A |
| Connecticut [26,42,43] | YES for students residing in on-campus housing | YES; NO (compulsory for students in on-campus housing) | N/A | NO | YES | Medical and religious |
| Delaware [23,26,44] | YES required within 5 years before enrollment | YES; NO (compulsory for students who wish to enroll in post-secondary schools) | N/A | NO | NO | Students may submit a written request to the postsecondary educational institution for exemption |
| Florida [26,45,46] | YES for students residing in on-campus housing | YES; YES | N/A | NO | NO | N/A |
| Georgia [23,26,47,48] | YES for students residing in campus housing or sorority/fraternity houses | YES; YES | N/A | NO | YES | N/A |
| Hawaii [26,49,50,51] | YES for first-year students residing in campus housing | NO; N/A | N/A | NO | YES * | Medical and religious; religious exemptions to specific immunizing agents will not be granted |
| Idaho [23,52,53,54] | NO | YES; YES | NO | NO | NO | N/A |
| Illinois [23,26,55] | YES for incoming students aged <22 years | NO; N/A | N/A | NO | NO | Medical and religious |
| Indiana [26,56,57,58] | YES for students aged ≤23 years | NO; N/A | N/A | YES for students aged ≤23 years | N/A | Medical and religious |
| Iowa [23,59,60] | NO | YES; YES | NO | NO | NO | N/A |
| Kansas [26,41,61] | YES for students residing in student housing | YES; YES | N/A | NO | NO | N/A |
| Kentucky [23,62] | NO | NO; N/A | YES | NO | NO | N/A |
| Louisiana [26,41,63,64] | YES for all on-campus students | YES; YES | N/A | NO | YES | Medical, religious, and personal; requires review of information by individual seeking an exemption for religious or personal reasons |
| Maine [23,65,66,67] | NO | NO; N/A | YES for freshman residing in a dormitory | NO | YES * | N/A |
| Maryland [26,68,69] | YES for students residing in on-campus student housing | YES; YES | N/A | NO | YES * | N/A |
| Massachusetts [26,70,71] | YES for full-time students aged <22 years residing in dormitory or with living arrangements licensed/approved by the school | YES; YES | N/A | NO | NO | Medical, religious, and personal; requires review of information by individual seeking an exemption for religious or personal reasons |
| Michigan [23,72] | NO | NO; N/A | YES for freshman residing in a dormitory | NO | YES * | N/A |
| Minnesota [23,73,74] | NO | YES; YES | YES for freshman residing in a dormitory | NO | YES * | N/A |
| Mississippi [23,75,76,77] | NO | NO; N/A | NO | NO | YES * | N/A |
| Missouri [26,41,78,79,80] | YES for students residing in campus housing or sorority/fraternity houses | YES; NO (compulsory for students residing in campus housing or sorority/fraternity houses) | N/A | NO | YES * | Medical and religious |
| Montana [23,81,82] | NO | NO; N/A | YES * | NO | NO | N/A |
| Nebraska [23,83,84] | NO | YES; YES | YES for college freshman, in particular those residing in a dormitory and for students who did not receive dose 2 at age 16 years | NO | NO | N/A |
| Nevada [26,85] | YES for all on-campus freshman aged <23 years | NO; N/A | N/A | NO | NO | Medical and religious; students enrolled in distance education only |
| New Hampshire [23,86,87] | NO | NO; N/A | NO | NO | NO | N/A |
| New Jersey [26,88,89,90,91,92] | YES | YES; NO | N/A | NO † | YES | Medical and religious |
| New Mexico [23,93,94] | NO | NO; N/A | YES for freshman residing in student housing if not previously vaccinated at age 16 years or older | NO | YES * | N/A |
| New York [26,95,96] | YES ‡ for students enrolled for ≥6 h per semester or ≥4 h per quarter | YES; YES ‡ | N/A | YES ‡ | N/A | N/A |
| North Carolina [23,97,98] | NO | NO; N/A | YES for freshman residing in a dormitory | NO | NO | N/A |
| North Dakota [26,99,100] | YES for students aged <22 years | NO; N/A | N/A | NO | YES * | Medical and religious; exemptions also provided to students enrolled only in distance learning, continuing education, noncredit, or off-campus courses and for students attending camps, workshops, courses, or programs contracted to a third party |
| Ohio [24,25] | NO (but students residing in on-campus student housing must disclose meningitis vaccination status) | NO; N/A § | YES | NO | YES | N/A |
| Oklahoma [26,41,94,101,102] | YES for incoming students residing in on-campus housing | YES; YES | N/A | NO | YES * | N/A |
| Oregon [23,103,104] | NO | YES; YES ¶ | YES | NO | YES | N/A |
| Pennsylvania [26,41,105,106,107] | YES for students residing in a dormitory or housing unit | YES; YES | N/A | NO | YES * | Medical and religious; exemptions also provide for “other reasons” if the institution provides detailed information on the risks associated with meningococcal disease and the availability and effectiveness of any vaccine |
| Rhode Island [26,77,108,109] | YES for full-time previously unvaccinated students aged <22 years residing in dormitory or with living arrangements approved by the school | NO; N/A | N/A | NO | YES * | Medical and religious (214-30-05-3.6.3) |
| South Carolina [23,110,111,112] | NO | YES; YES | YES * | NO | YES * | N/A |
| South Dakota [23,113] | NO | NO; N/A | YES | NO | YES | N/A |
| Tennessee [26,114,115,116] | YES for incoming students aged <22 years residing in on-campus housing | NO; N/A | N/A | NO | NO | Medical and religious |
| Texas [26,117,118,119] | YES for incoming on-campus students aged <22 years | YES; YES | N/A | NO | YES * | Medical, religious, and personal belief |
| Utah [23,94,120] | NO | NO; N/A | YES for freshman residing in a dormitory who had not been vaccinated at age ≥16 years | NO | YES * | N/A |
| Vermont [26,121,122,123] | YES for freshman aged <22 years residing in a dormitory who received dose 1 before age 16 years | NO; N/A | N/A | NO | YES * | Medical and religious |
| Virginia [26,94,124,125] | YES for incoming full-time students | YES; YES | N/A | NO | YES * | Medical and religious; also, any student may submit a written waiver after reviewing provided information |
| Washington [23,94,126,127] | NO | YES; YES ** | YES for freshman residing in a dormitory who had not been vaccinated at age ≥16 years | NO | YES * | N/A |
| Washington, DC [26,27] | YES for students residing in school housing and athletes | NO; N/A | N/A | NO | NO | Medical and religious |
| West Virginia [28] | YES | NO; N/A | N/A | NO | YES for students aged 16–23 years who are not at increased risk of disease or for any student at risk due to anatomical or functional asplenia, persistent complement deficiency, complement inhibitor use, or a meningococcal B outbreak | Medical; students attending in an all-virtual capacity may also qualify |
| Wisconsin [23,94,128,129] | NO | YES; YES †† | YES for freshman residing in a dormitory who had not been vaccinated at age ≥16 years | NO | YES * | N/A |
| Wyoming [23,94,130] | NO | NO; N/A | YES for freshman residing in a dormitory who had not been vaccinated at age ≥16 years | NO | YES * | N/A |
| State | Colleges with MenB Required | Colleges with MenB Recommended | College Cases/ Outbreaks of MenB ‡ |
|---|---|---|---|
| Alabama | NO | NO | NO |
| Alaska | NO | NO | NO |
| Arizona | NO | YES | NO |
| Arkansas | NO | YES | NO |
| California | YES | YES | YES |
| Colorado | NO | YES | NO |
| Connecticut | NO | YES | YES |
| Delaware | YES | YES | NO |
| Florida | YES | YES | NO |
| Georgia | YES | YES | NO |
| Hawaii | NO | NO | NO |
| Idaho | YES | NO | NO |
| Illinois | NO | YES | YES |
| Indiana | YES (MenB mandated at state level) | YES | NO |
| Iowa | YES | YES | NO |
| Kansas | NO | NO | NO |
| Kentucky | NO | YES | NO |
| Louisiana | NO | NO | NO |
| Maine | NO | NO | NO |
| Maryland | NO | YES | NO |
| Massachusetts | NO | YES | YES |
| Michigan | YES | YES | YES |
| Minnesota | NO | YES | NO |
| Mississippi | YES | NO | NO |
| Missouri | NO | YES | YES |
| Montana | NO | NO | NO |
| Nebraska | NO | NO | NO |
| Nevada | NO | NO | NO |
| New Hampshire | YES | YES | NO |
| New Jersey | YES | YES | YES |
| New Mexico | NO | NO | NO |
| New York | YES (MenB mandated at state level *) | YES | YES |
| North Carolina | NO | NO | NO |
| North Dakota | NO | NO | NO |
| Ohio | YES | YES | YES |
| Oklahoma | NO | NO | NO |
| Oregon | NO | YES | YES |
| Pennsylvania | YES | YES | YES |
| Rhode Island | NO | YES | YES |
| South Carolina | YES | YES | NO † |
| South Dakota | NO | YES | NO |
| Tennessee | NO | YES | NO |
| Texas | NO | YES | NO |
| Utah | NO | YES | NO |
| Vermont | NO | YES | NO |
| Virginia | YES | YES | NO |
| Washington | NO | YES | NO |
| Washington, DC | NO | NO | YES |
| West Virginia | NO | NO | NO |
| Wisconsin | NO | YES | YES |
| Wyoming | NO | NO | NO |
| State | Outbreaks of MenB Disease | Single Cases of MenB Disease | State-Level MenB Vaccine Requirement/ Recommendation for College Students |
|---|---|---|---|
| California | 2014: University of California, Santa Barbara (5 cases) 2016: Santa Clara University (3 cases) 2018: San Diego State University (3 cases) | 2014: Palomar College 2014: San Diego State University (resulted in death) 2015: University of California, Davis 2017: California Polytechnic State University 2017: Santa Barbara City College | Recommended |
| Connecticut | 2018: Central Connecticut State University | Recommended | |
| Illinois | 2017: University of Illinois at Urbana, Champaign | None | |
| Massachusetts | 2017: University of Massachusetts Amherst and Five College Consortium (3 cases) | 2018: Smith College | None |
| Michigan | 2013: Kalamazoo College (resulted in death) | Recommended † | |
| Missouri | 2015: Missouri University | Recommended † | |
| New Jersey | 2013–2014: Princeton University (9 cases/1 death) 2016: Rutgers University (2 cases) | Recommended | |
| New York | 2008: Cornell University (2 cases) 2019: Columbia University School of International and Public Affairs (2 cases) | 2008: State University College, Oswego (resulted in death) 2018: Colgate University 2018: Syracuse University | Required ‡ |
| Ohio | 2008–2010: Ohio University (10 cases/1 death) | Recommended | |
| Oregon | 2015: University of Oregon (7 cases/1 death) 2016: Oregon State University (5 cases) | Recommended | |
| Pennsylvania | 2009: University of Pennsylvania (3 cases) 2011: Lehigh University (2 cases) 2017: Bucknell University (2 cases) | 2014: Drexel University § (resulted in death) 2017: Kutztown University 2018: Pennsylvania State University | Recommended † |
| Rhode Island | 2015: Providence College (2 cases) | Recommended † | |
| Washington, DC | 2014: Georgetown University (resulted in death) | None | |
| Wisconsin | 2016: University of Wisconsin, Madison (3 cases) | 2013: University of Wisconsin, Madison (resulted in death) | Recommended † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presa, J.; Jodar, E.; Ochapa, M.; Mullenix, T.A.; Barrett, S.E.; Cane, A. A Narrative Review of College Meningococcal Vaccination Mandates Across the United States. Vaccines 2025, 13, 784. https://doi.org/10.3390/vaccines13080784
Presa J, Jodar E, Ochapa M, Mullenix TA, Barrett SE, Cane A. A Narrative Review of College Meningococcal Vaccination Mandates Across the United States. Vaccines. 2025; 13(8):784. https://doi.org/10.3390/vaccines13080784
Chicago/Turabian StylePresa, Jessica, Eva Jodar, Monica Ochapa, Tim A. Mullenix, Sharon E. Barrett, and Alejandro Cane. 2025. "A Narrative Review of College Meningococcal Vaccination Mandates Across the United States" Vaccines 13, no. 8: 784. https://doi.org/10.3390/vaccines13080784
APA StylePresa, J., Jodar, E., Ochapa, M., Mullenix, T. A., Barrett, S. E., & Cane, A. (2025). A Narrative Review of College Meningococcal Vaccination Mandates Across the United States. Vaccines, 13(8), 784. https://doi.org/10.3390/vaccines13080784






