Proteomic Profiling of Human Peripheral Blood Cell Targets of IgG Induced by SARS-CoV-2: Insights into Vaccine Safety
Abstract
1. Introduction
2. Methods
2.1. Sample Collection and Study Cohort
2.2. Human Proteome Microarray Analysis
2.3. Identification of IgG Targets and Bioinformatics Analysis
2.4. Statistical Adjustment for Unequal Sample Sizes
3. Results
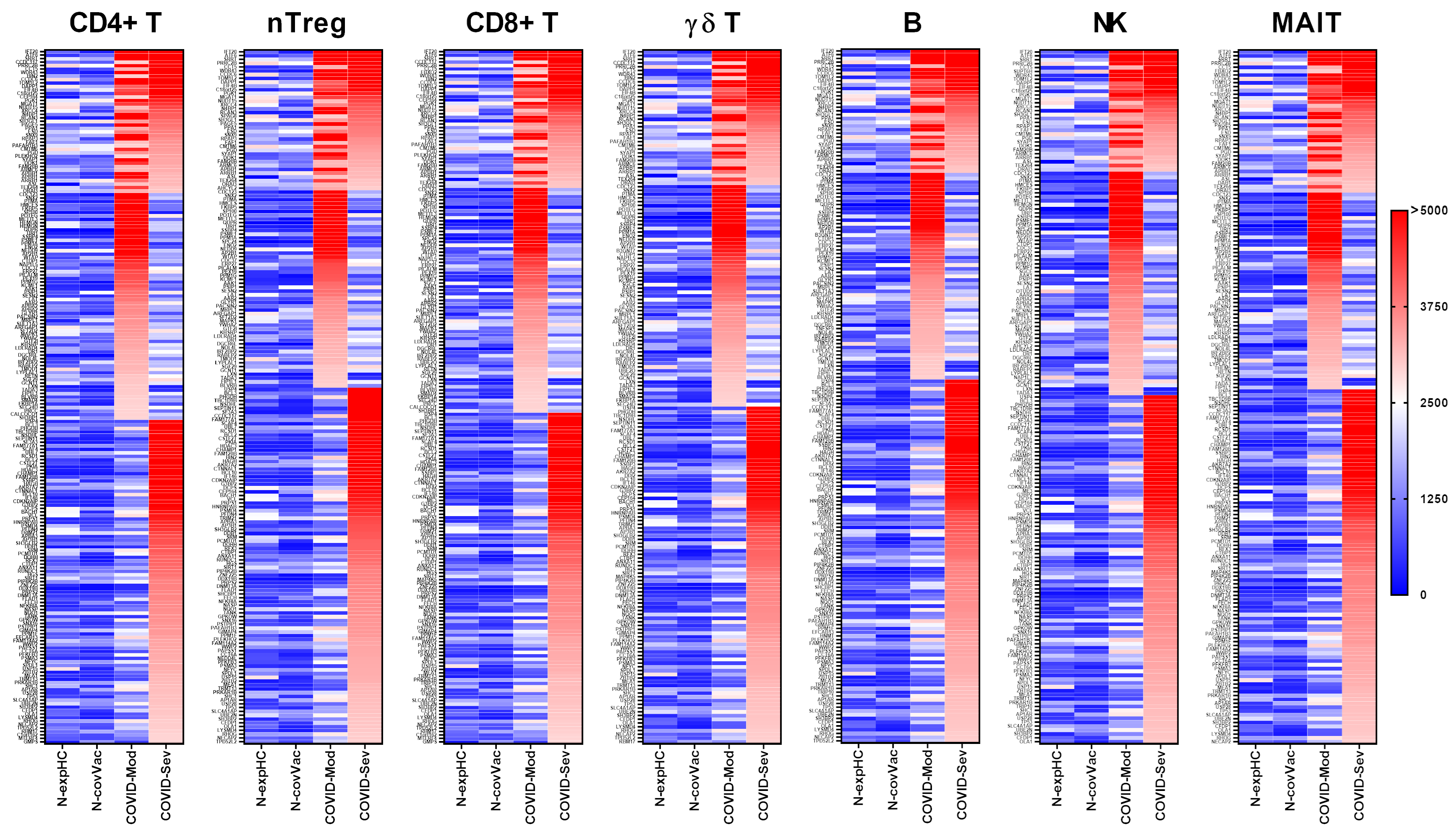
3.1. COVID-19 Patients Produce IgG Targeting Multiple Peripheral Lymphocyte Populations
3.1.1. CD4+ T Cells
3.1.2. nTreg Cells
3.1.3. CD8+ T Cells
3.1.4. γδ T Cells
3.1.5. B Cells
3.1.6. NK Cells
3.1.7. MAIT Cells
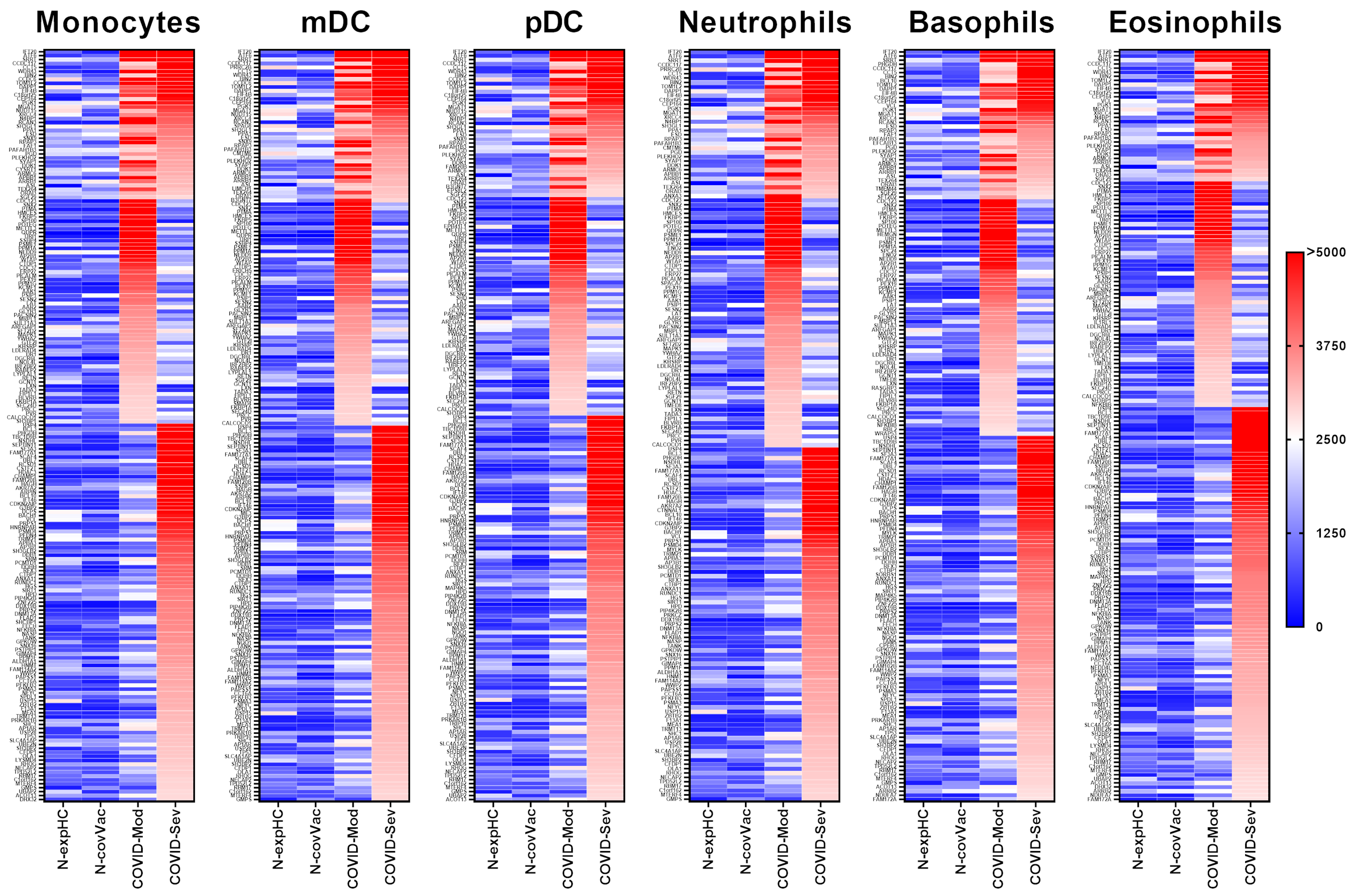
3.2. COVID-19 Patients Produce IgG Targeting Multiple Myeloid-Derived Innate Immune Cell Subsets
3.2.1. Monocytes
3.2.2. Myeloid Dendritic Cells (mDCs)
3.2.3. Plasmacytoid Dendritic Cells (pDCs)
3.2.4. Neutrophils
3.2.5. Basophils
3.2.6. Eosinophils
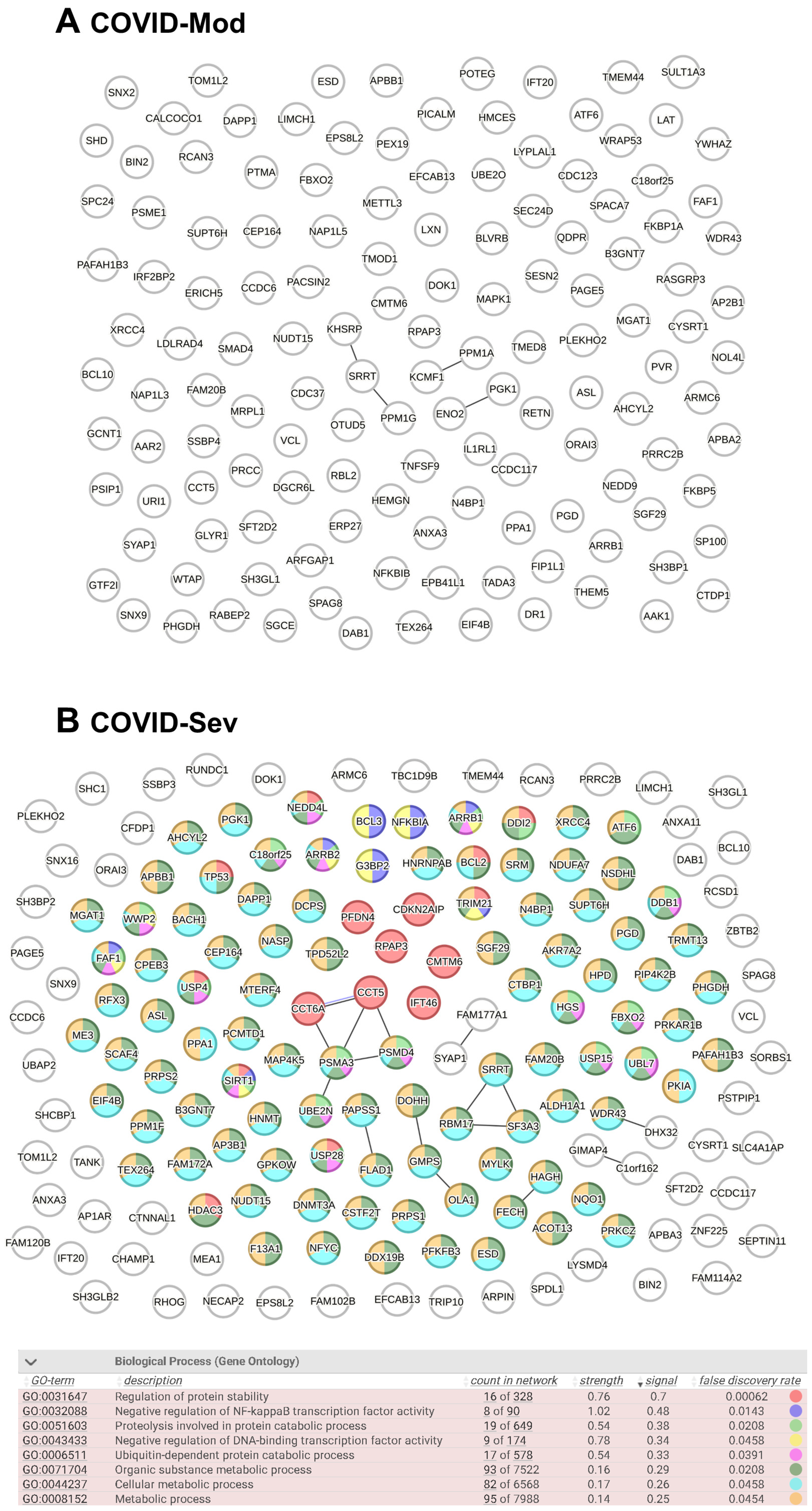
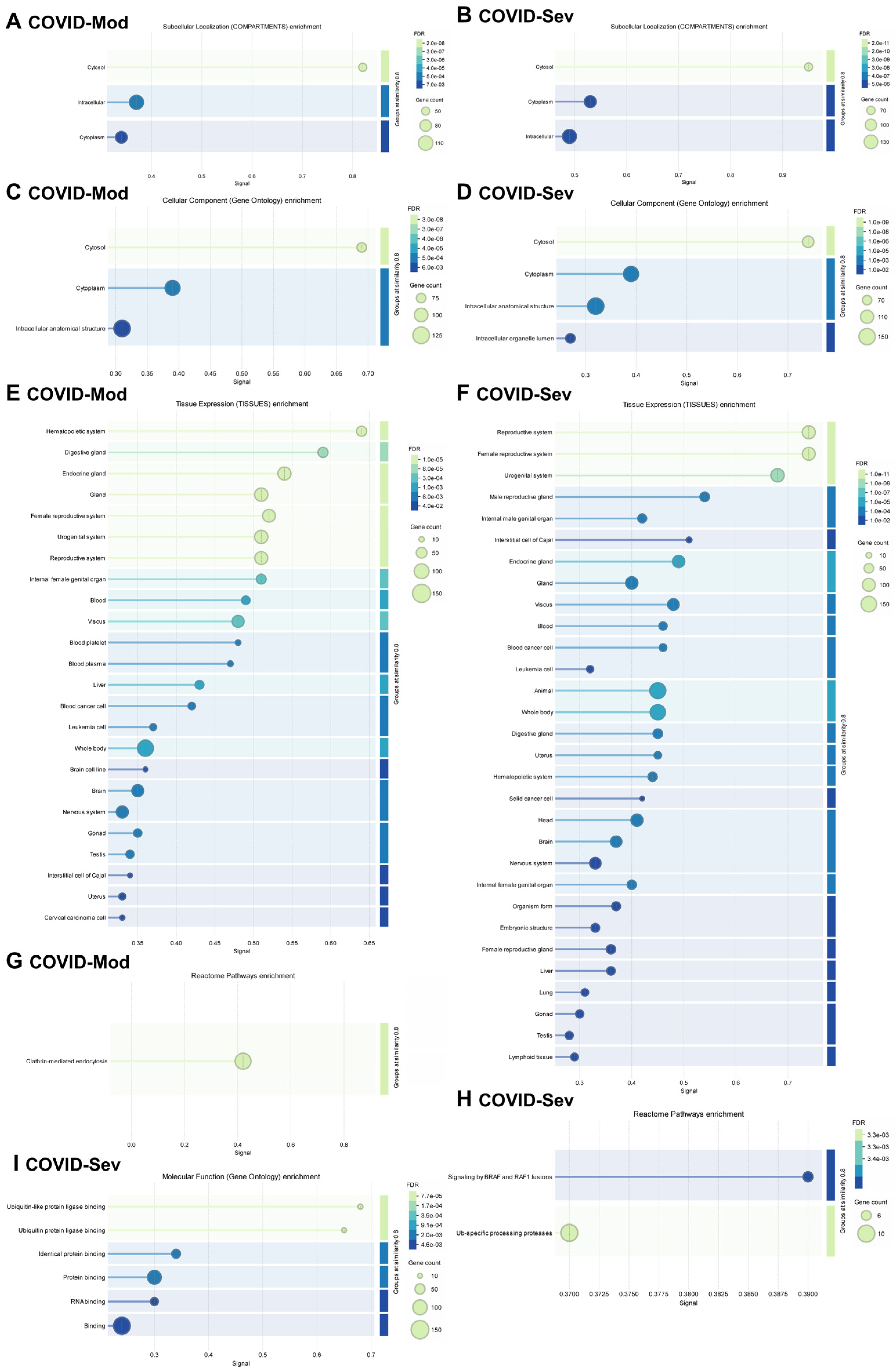
3.3. Evaluation of Known and Predicted Protein–Protein Interaction Networks (PPINs)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Rotondo, J.C.; Perna, B.; Guarino, M.; De Giorgio, R. Special Issue: Advances in SARS-CoV-2 Infection. Microorganisms 2023, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mi, Z.; Liu, Z.; Rong, P. SARS-CoV-2: Pathogenesis, therapeutics, variants, and vaccines. Front. Microbiol. 2024, 15, 1334152. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Lam, C.Y.; Tan, P.H.; Tsang, H.F.; Wong, S.C. Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 8155. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; De Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef]
- Kocivnik, N.; Velnar, T. A Review Pertaining to SARS-CoV-2 and Autoimmune Diseases: What Is the Connection? Life 2022, 12, 1918. [Google Scholar] [CrossRef]
- Guo, M.; Shang, S.; Li, M.; Cai, G.; Li, P.; Chen, X.; Li, Q. Understanding autoimmune response after SARS-CoV-2 infection and the pathogenesis/mechanisms of long COVID. Med. Rev. 2024, 4, 367–383. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Miller, J.S.; Zheng, S.G. An updated advance of autoantibodies in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102743. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. Knowledge Mapping of Exosomes in Autoimmune Diseases: A Bibliometric Analysis (2002–2021). Front. Immunol. 2022, 13, 939433. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Xia, X.; Wang, J. Knowledge mapping of COVID-19 and autoimmune diseases: A visual and bibliometric analysis. Clin. Exp. Med. 2023, 23, 3549–3564. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, X.; Pan, H.; Shi, H.; Shi, Y. Unveiling the intricacies of COVID-19: Autoimmunity, multi-organ manifestations and the role of autoantibodies. Scand. J. Immunol. 2024, 99, e13344. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Gervais, A.; Taniguchi, M.; Saare, L.; Särekannu, K.; Le Voyer, T.; Philippot, Q.; Rosain, J.; Bizien, L.; Asano, T.; et al. Higher COVID-19 pneumonia risk associated with anti-IFN-α than with anti-IFN-ω auto-Abs in children. J. Exp. Med. 2024, 221, 1353. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, V.; Muzslay, E.; Czipó, D.; Terkovics, L.; Takács, J.; Garai, R.; Kovács, F.; Luczay, A.; Körner, A.; Tóth-Heyn, P. Increasing prevalence of thyroid autoimmunity in childhood type 1 diabetes in the pre-COVID but not during the COVID era. Front. Endocrinol. 2024, 15, 1496155. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, A.; Mirhosseini, A.; Shariati, A.; Mohareri, M.; Valizadeh, N.; Mohammadi, F.S.; Shamsian, S.A.A.; Jafari Rad, M.; Rezaee, S.A. SARS-CoV-2 infection and increasing autoimmune disorders among ICU-hospitalized COVID-19 patients. Int. J. Rheum. Dis. 2023, 26, 2151–2156. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Zarębska-Michaluk, D.; Poniedziałek, B.; Jaroszewicz, J.; Flisiak, R.; Rzymski, P. Overview of autoantibodies in COVID-19 convalescents. J. Med. Virol. 2023, 95, e28864. [Google Scholar] [CrossRef]
- Notarte, K.I.; Carandang, T.H.D.C.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Manalo, G.N.; Ng, J.A.; Grecia, S.; Lippi, G.; Henry, B.M.; et al. Autoantibodies in COVID-19 survivors with post-COVID symptoms: A systematic review. Front. Immunol. 2024, 15, 1428645. [Google Scholar] [CrossRef]
- Delle Fave, R.F.; Polisini, G.; Giglioni, G.; Parlavecchio, A.; Dell’Atti, L.; Galosi, A.B. COVID-19 and male fertility: Taking stock of one year after the outbreak began. Arch. Ital. Urol. Androl. 2021, 93, 115–119. [Google Scholar] [CrossRef]
- Van Regemorter, E.; Zorzi, G.; Scohy, A.; Gruson, D.; Morelle, J. Impact of the COVID-19 pandemic on temporal trends of biological indicators of autoimmunity. J. Transl. Autoimmun. 2023, 7, 100222. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Achleitner, M.; Steenblock, C.; Dänhardt, J.; Jarzebska, N.; Kardashi, R.; Kanczkowski, W.; Straube, R.; Rodionov, R.N.; Bornstein, N.; Tselmin, S.; et al. Clinical improvement of Long-COVID is associated with reduction in autoantibodies, lipids, and inflammation following therapeutic apheresis. Mol. Psychiatry 2023, 28, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.R.; Fagundes, B.O.; do Nascimento, L.A.; Bergamasco, I.S.; Sgnotto, F.D.R.; Fernandes, I.G.; Fernandes, J.R.; Pinto, T.N.C.; da Borges, J.V.S.; Benard, G.; et al. Deciphering the IgG Idiotype Network Through Proteomic Analysis of Potential Targets in SARS-CoV-2-Induced Immune Responses. Immunology 2025, 175, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Kayalar, O.; Cetinkaya, P.D.; Eldem, V.; Argun Baris, S.; Kokturk, N.; Kuralay, S.C.; Rajabi, H.; Konyalilar, N.; Mortazavi, D.; Korkunc, S.K.; et al. Comparative Transcriptomic Analyses of Peripheral Blood Mononuclear Cells of COVID-19 Patients without Pneumonia and with Severe Pneumonia in the First Year of Follow-Up. Viruses 2024, 16, 1211. [Google Scholar] [CrossRef]
- Potts, M.; Fletcher-Etherington, A.; Nightingale, K.; Mescia, F.; Bergamaschi, L.; Calero-Nieto, F.J.; Antrobus, R.; Williamson, J.; Parsons, H.; Huttlin, E.L.; et al. Proteomic analysis of circulating immune cells identifies cellular phenotypes associated with COVID-19 severity. Cell Rep. 2023, 42, 112613. [Google Scholar] [CrossRef]
- Santinelli, L.; Lazzaro, A.; Sciarra, F.; Maddaloni, L.; Frasca, F.; Fracella, M.; Moretti, S.; Borsetti, A.; Bugani, G.; Alessandri, F.; et al. Cellular Immune Profiling of Lung and Blood Compartments in Patients with SARS-CoV-2 Infection. Pathogens 2023, 12, 442. [Google Scholar] [CrossRef]
- Cunha, F.R.M.; Fagundes, B.O.; Machado, N.R.; França, C.N.; Victor, J.R. IgG from individuals without atopy arising as mediators of a nonatopic profile in human peripheral CD4+ T cells. Ann. Allergy Asthma Immunol. 2024, 132, 770–772. [Google Scholar] [CrossRef]
- Fagundes, B.O.; de-Sousa, T.R.; Victor, J.R. Gamma-delta (γδ) T cell-derived cytokines (IL-4, IL-17, IFN-γ and IL-10) and their possible implications for atopic dermatitis development. Int. J. Dermatol. 2023, 62, 443–448. [Google Scholar] [CrossRef]
- Fagundes, B.O.; de Sousa, T.R.; Nascimento, A.; Fernandes, L.A.; Sgnotto, F.D.R.; Orfali, R.L.; Aoki, V.; Duarte, A.J.D.S.; Sanabani, S.S.; Victor, J.R. IgG from Adult Atopic Dermatitis (AD) Patients Induces Nonatopic Neonatal Thymic Gamma-Delta T Cells (γδT) to Acquire IL-22/IL-17 Secretion Profile with Skin-Homing Properties and Epigenetic Implications Mediated by miRNA. Int. J. Mol. Sci. 2022, 23, 6872. [Google Scholar] [CrossRef]
- de Sousa, T.R.; Fagundes, B.O.; Nascimento, A.; Fernandes, L.A.; Sgnotto, F.D.R.; Orfali, R.L.; Aoki, V.; Duarte, A.J.D.S.; Sanabani, S.S.; Victor, J.R. IgG from Adult Atopic Dermatitis (AD) Patients Induces Thymic IL-22 Production and CLA Expression on CD4+ T Cells: Possible Epigenetic Implications Mediated by miRNA. Int. J. Mol. Sci. 2022, 23, 6867. [Google Scholar] [CrossRef]
- Santos, L.S.; Sgnotto, F.D.R.; Inoue, A.H.S.; Padreca, A.F.; Menghini, R.P.; Duarte, A.J.D.S.; Victor, J.R. IgG from Non-atopic Individuals Induces In Vitro IFN-γ and IL-10 Production by Human Intra-thymic γδT Cells: A Comparison with Atopic IgG and IVIg. Arch. Immunol. Ther Exp. 2019, 67, 263–270. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; de Lima Lira, A.A.; da Ressureição Sgnotto, F.; Inoue, A.H.S.; Santos, L.S.; Nakamatsu, B.Y.; Duarte, A.J.D.S.; Leite-de-Moraes, M.; Victor, J.R. Maternal IgG impairs the maturation of offspring intrathymic IL-17-producing γδT cells: Implications for murine and human allergies. Clin. Exp. Allergy 2019, 49, 1000–1012. [Google Scholar] [CrossRef]
- Kwon, B.; Yang, S.J.; Cho, S.M.; Kim, M.E.; Nahm, D.H. Intramuscular administration of autologous total immunoglobulin G induces immunomodulatory effects on T cells in healthy human subjects: An open-labeled prospective single-arm trial. Medicine 2022, 101, e29486. [Google Scholar] [CrossRef] [PubMed]
- Nahm, D.H.; Ye, Y.M.; Shin, Y.S.; Park, H.S.; Kim, M.E.; Kwon, B.; Cho, S.M.; Han, J. Efficacy, Safety, and Immunomodulatory Effect of the Intramuscular Administration of Autologous Total Immunoglobulin G for Atopic Dermatitis: A Randomized Clinical Trial. Allergy Asthma Immunol. Res. 2020, 12, 949–963. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.E.; Shin, Y.S.; Ye, Y.M.; Park, H.S.; Nahm, D.H. Safety of Ultra-rush Schedule of Subcutaneous Allergen Immunotherapy with House Dust Mite Extract Conducted in an Outpatient Clinic in Patients with Atopic Dermatitis and Allergic Rhinitis. Allergy Asthma Immunol. Res. 2019, 11, 846–855. [Google Scholar] [CrossRef]
- Cho, S.M.; Kim, M.E.; Kwon, B.; Nahm, D.H. Immunomodulatory effects induced by intramuscular administration of autologous total immunoglobulin G in patients with atopic dermatitis. Int. Immunopharmacol. 2017, 52, 1–6. [Google Scholar] [CrossRef]
- Nahm, D.H.; Ahn, A.; Kim, M.E.; Cho, S.M.; Park, M.J. Autologous Immunoglobulin Therapy in Patients with Severe Recalcitrant Atopic Dermatitis: Long-Term Changes of Clinical Severity and Laboratory Parameters. Allergy Asthma Immunol. Res. 2016, 8, 375–382. [Google Scholar] [CrossRef]
- Victor, J.R. Do different IgG repertoires play a role in B- and T-cell functional modulation during ontogeny? The “hooks without bait” theory. Immunol. Cell Biol. 2020, 98, 540–548. [Google Scholar] [CrossRef]
- Victor, J.R.; Nahm, D.H. Mechanism underlying polyvalent IgG-induced regulatory T cell activation and its clinical application: Anti-idiotypic regulatory T cell theory for immune tolerance. Front. Immunol. 2023, 14, 1242860. [Google Scholar] [CrossRef]
- Pugh, S.; Fosdick, B.K.; Nehring, M.; Gallichotte, E.N.; VandeWoude, S.; Wilson, A. Estimating cutoff values for diagnostic tests to achieve target specificity using extreme value theory. BMC Med. Res. Methodol. 2024, 24, 30. [Google Scholar] [CrossRef]
- Klumpp-Thomas, C.; Kalish, H.; Drew, M.; Hunsberger, S.; Snead, K.; Fay, M.P.; Mehalko, J.; Shunmugavel, A.; Wall, V.; Frank, P.; et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat. Commun. 2021, 12, 113. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, abd4585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Rakanidis Machado, N.; Fagundes, B.O.; Fernandes, I.G.; Terra De Apoena Reche, D.; Sato, M.N.; Victor, J.R. IgG from patients with mild or severe COVID-19 reduces the frequency and modulates the function of peripheral mucosal-associated invariant T cells in PBMCs from healthy individuals. Biomed. Rep. 2023, 19, 95. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022, 20, 129. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- de Prost, N.; Bastard, P.; Arrestier, R.; Fourati, S.; Mahévas, M.; Burrel, S.; Dorgham, K.; Gorochov, G.; Tandjaoui-Lambiotte, Y.; Azzaoui, I.; et al. Plasma Exchange to Rescue Patients with Autoantibodies Against Type I Interferons and Life-Threatening COVID-19 Pneumonia. J. Clin. Immunol. 2021, 41, 536–544. [Google Scholar] [CrossRef]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef]
- Gomes, C.; Zuniga, M.; Crotty, K.A.; Qian, K.; Tovar, N.C.; Lin, L.H.; Argyropoulos, K.V.; Clancy, R.; Izmirly, P.; Buyon, J.; et al. Autoimmune anti-DNA and anti-phosphatidylserine antibodies predict development of severe COVID-19. Life Sci. Alliance 2021, 4, e202101180. [Google Scholar] [CrossRef]
- Troya, J.; Bastard, P.; Planas-Serra, L.; Ryan, P.; Ruiz, M.; de Carranza, M.; Torres, J.; Martínez, A.; Abel, L.; Casanova, J.L.; et al. Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J. Clin. Immunol. 2021, 41, 914–922. [Google Scholar] [CrossRef]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and Immunological Dysregulation: Can Autoantibodies be Useful? Clin. Transl. Sci. 2021, 14, 502–508. [Google Scholar] [CrossRef]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. The Role of Nuclear Factor Kappa B (NF-κB) in Development and Treatment of COVID-19: Review. Int. J. Mol. Sci. 2022, 23, 5283. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Stanley, K.E.; Thomas, E.; Leaver, M.; Wells, D. Coronavirus disease-19 and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020, 114, 33–43. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef]
- Ma, L.; Sahu, S.K.; Cano, M.; Kuppuswamy, V.; Bajwa, J.; McPhatter, J.; Pine, A.; Meizlish, M.L.; Goshua, G.; Chang, C.H.; et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 2021, 6, abh2259. [Google Scholar] [CrossRef]
- Seyoum Tola, F. The Role of Ubiquitin-Proteasome System in the Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus-2 Disease. Int. J. Inflamm. 2023, 2023, 6698069. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Buonsenso, D.; Wood, J.; Mohandas, S.; Warburton, D. Mechanistic Insights into Long Covid: Viral Persistence, Immune Dysregulation, and Multi-Organ Dysfunction. Compr. Physiol. 2025, 15, e70019. [Google Scholar] [CrossRef] [PubMed]
- Syal, R.; Kaur, J.; Siddiqui, M.; Amatul-Raheem, H.; Suarez, C.; Bojanki, N.L.S.V.; Kapadia, S.D.; Yennam, A.K.; Kunchala, K.; Metry, S.; et al. Long-Term Impacts of COVID-19 on Thyroid Health: Insights from Clinical Studies. Cureus 2024, 16, e71469. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ely, E.W. How long COVID could lift the fog on neurocognitive disorders. Nature 2024, 634, S11. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Wang, G.; Yuan, Z.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, abc8511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A.; Ghosh, R. COVID-19 polyradiculitis in 24 patients without SARS-CoV-2 in the cerebro-spinal fluid. J. Med. Virol. 2021, 93, 66–68. [Google Scholar] [CrossRef]
- Kanberg, N.; Ashton, N.J.; Andersson, L.M.; Yilmaz, A.; Lindh, M.; Nilsson, S.; Price, R.W.; Blennow, K.; Zetterberg, H.; Gisslén, M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020, 95, e1754–e1759. [Google Scholar] [CrossRef]
- Tang, K.T.; Hsu, B.C.; Chen, D.Y. Autoimmune and Rheumatic Manifestations Associated with COVID-19 in Adults: An Updated Systematic Review. Front. Immunol. 2021, 12, 645013. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Ramos, A.E.; Martin-Nares, E.; Hernández-Molina, G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021, 10, 3592. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.R.; do Nascimento, L.A.; Fagundes, B.O.; Borges, J.V.d.S.; Sgnotto, F.d.R.; Bergamasco, I.S.; Fernandes, J.R.; Pinto, T.N.C.; Pietrobon, A.J.; Benard, G.; et al. Proteomic Profiling of Human Peripheral Blood Cell Targets of IgG Induced by SARS-CoV-2: Insights into Vaccine Safety. Vaccines 2025, 13, 694. https://doi.org/10.3390/vaccines13070694
Machado NR, do Nascimento LA, Fagundes BO, Borges JVdS, Sgnotto FdR, Bergamasco IS, Fernandes JR, Pinto TNC, Pietrobon AJ, Benard G, et al. Proteomic Profiling of Human Peripheral Blood Cell Targets of IgG Induced by SARS-CoV-2: Insights into Vaccine Safety. Vaccines. 2025; 13(7):694. https://doi.org/10.3390/vaccines13070694
Chicago/Turabian StyleMachado, Nicolle Rakanidis, Lais Alves do Nascimento, Beatriz Oliveira Fagundes, João Vitor da Silva Borges, Fabio da Ressureição Sgnotto, Isabella Siuffi Bergamasco, Juliana Ruiz Fernandes, Thalyta Nery Carvalho Pinto, Anna Julia Pietrobon, Gil Benard, and et al. 2025. "Proteomic Profiling of Human Peripheral Blood Cell Targets of IgG Induced by SARS-CoV-2: Insights into Vaccine Safety" Vaccines 13, no. 7: 694. https://doi.org/10.3390/vaccines13070694
APA StyleMachado, N. R., do Nascimento, L. A., Fagundes, B. O., Borges, J. V. d. S., Sgnotto, F. d. R., Bergamasco, I. S., Fernandes, J. R., Pinto, T. N. C., Pietrobon, A. J., Benard, G., Sato, M. N., & Victor, J. R. (2025). Proteomic Profiling of Human Peripheral Blood Cell Targets of IgG Induced by SARS-CoV-2: Insights into Vaccine Safety. Vaccines, 13(7), 694. https://doi.org/10.3390/vaccines13070694





