Malaria Vaccines: Current Achievements and Path Forward
Abstract
1. Introduction
2. Status of Malaria Vaccines
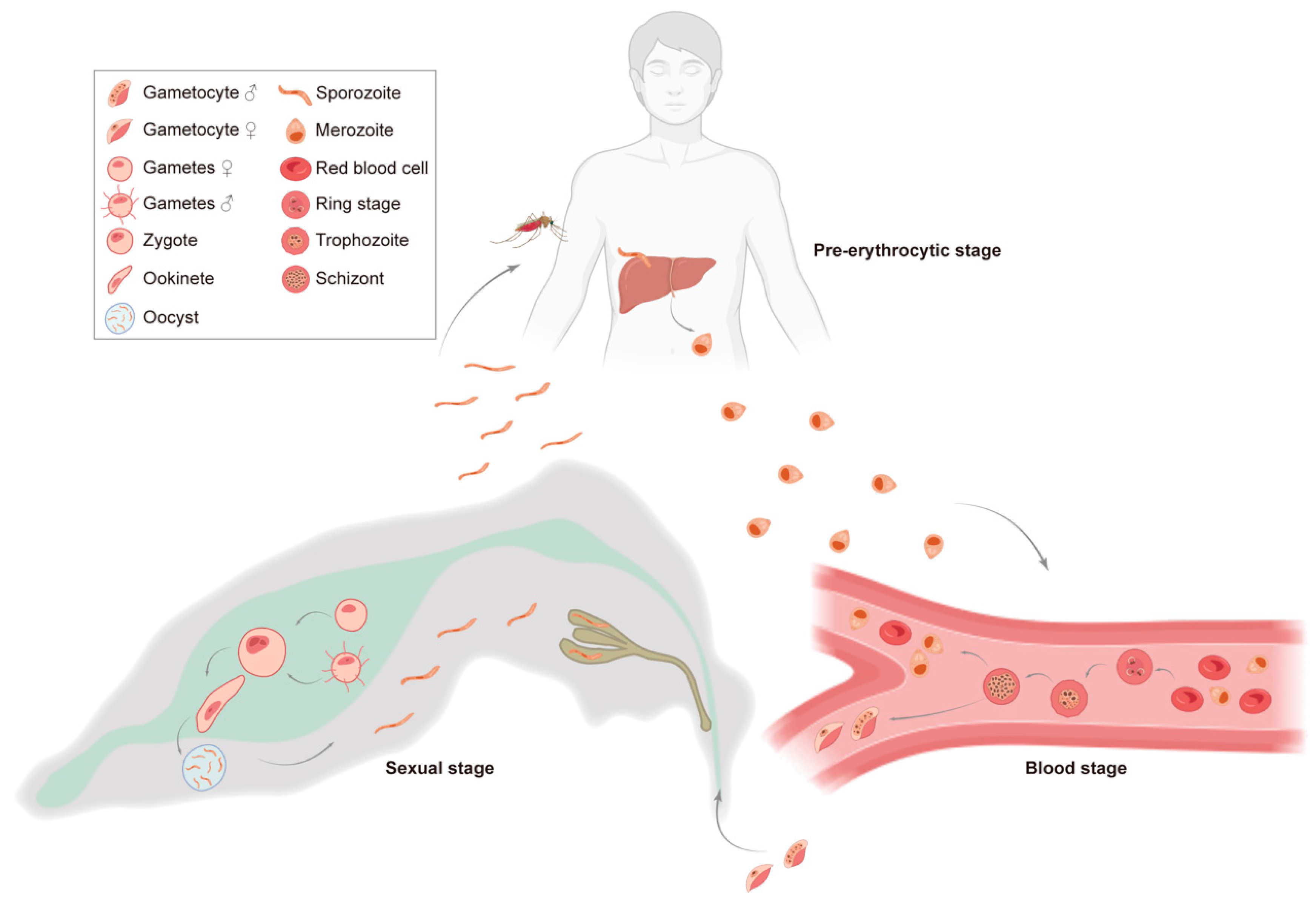
2.1. The Life Cycle of Plasmodium and Corresponding Malaria Vaccines
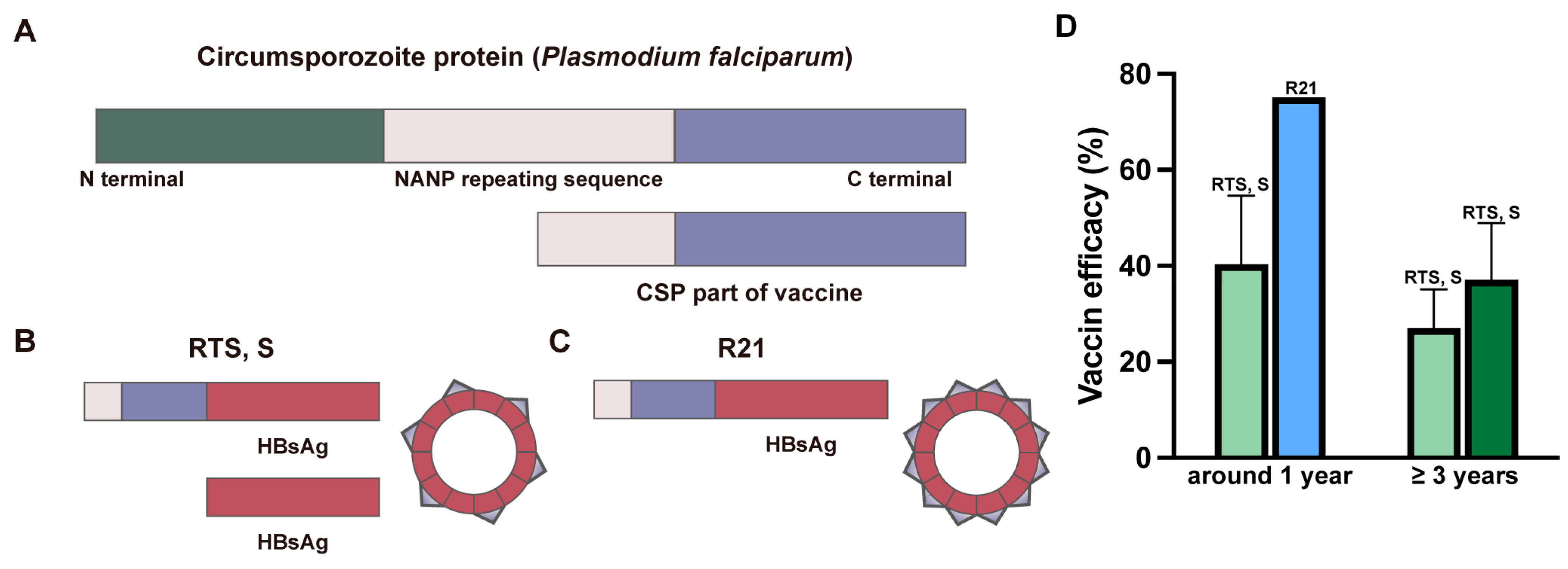
2.2. WHO-Recommended Malaria Vaccines
2.3. Other Vaccines in Development
2.3.1. Whole-Sporozoite Vaccine Candidates
2.3.2. Blood-Stage Vaccine Candidates
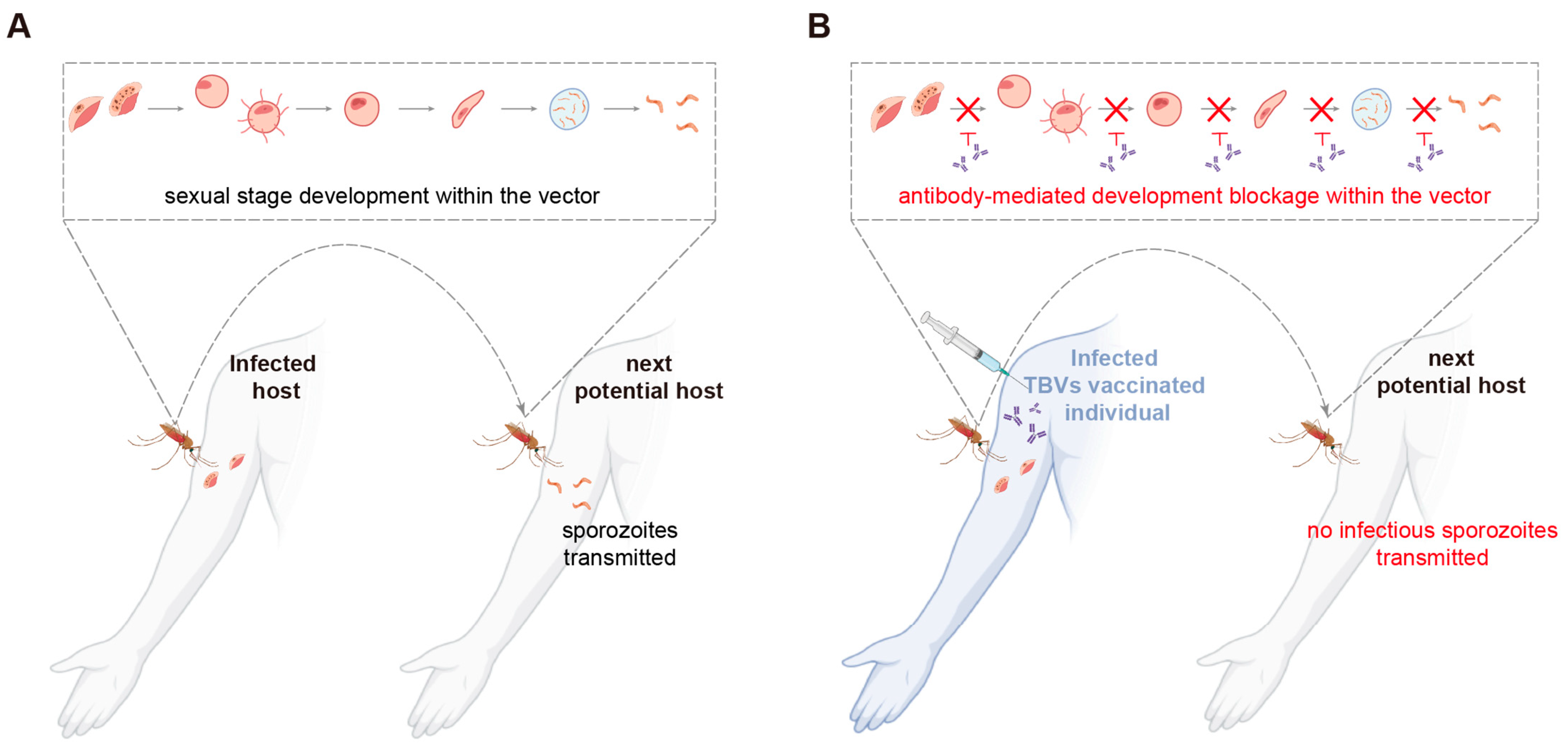
2.3.3. Sexual-Stage Vaccine Candidates
3. Potential Use of Advanced Technologies in Malaria Vaccines
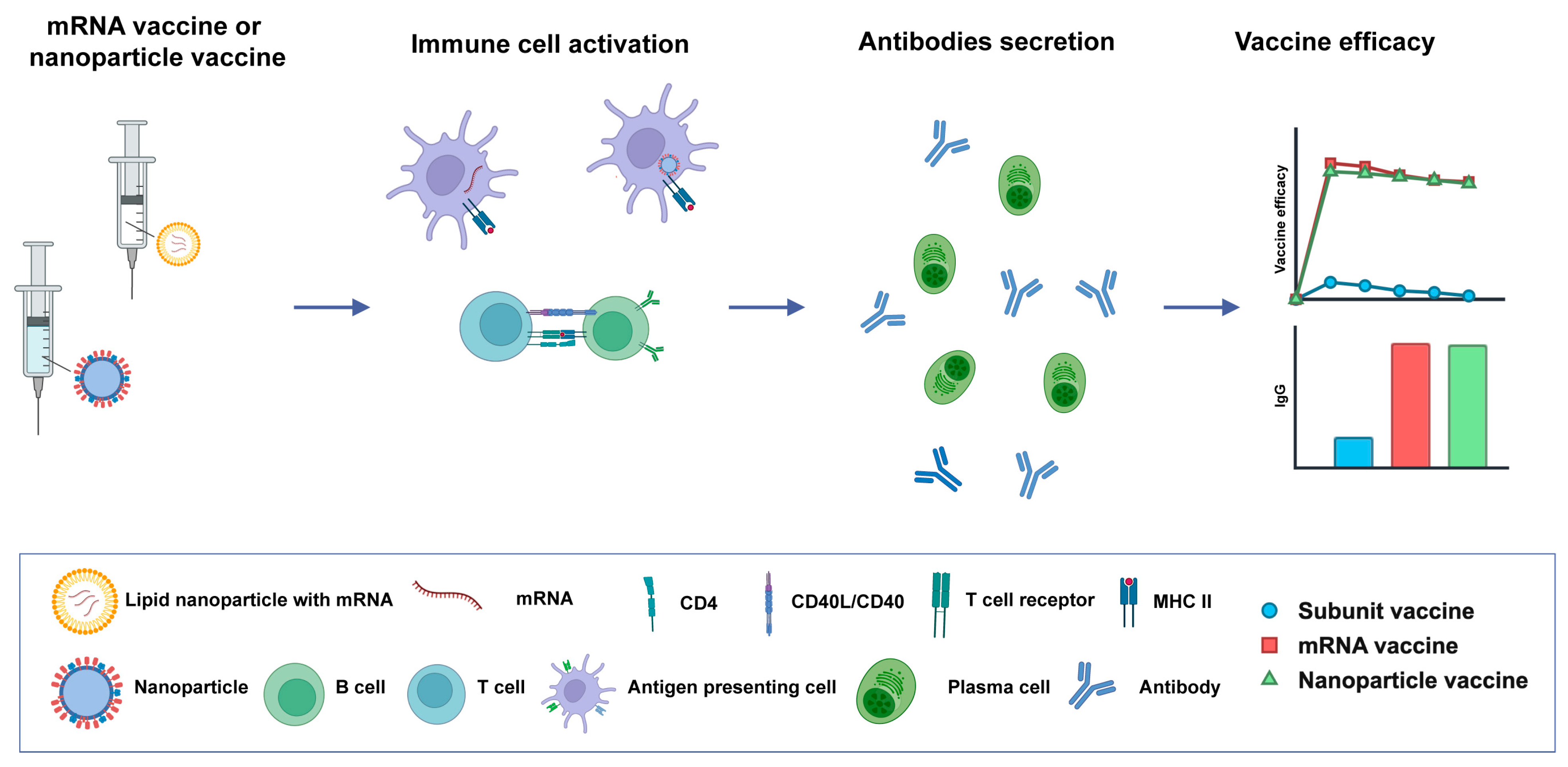
3.1. Novel Vaccine Platforms
3.2. Adjuvant Development and Improvements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Kim Sung, L.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Cox-Singh, J.; Singh, B. Knowlesi malaria: Newly emergent and of public health importance? Trends Parasitol. 2008, 24, 406–410. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Zhou, Y.; Zhang, W.-X.; Tembo, E.; Xie, M.-Z.; Zhang, S.-S.; Wang, X.-R.; Wei, T.-T.; Feng, X.; Zhang, Y.-L.; Du, J.; et al. Effectiveness of indoor residual spraying on malaria control: A systematic review and meta-analysis. Infect. Dis. Poverty 2022, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Musa, J.J.; Moore, S.J.; Moore, J.; Mbuba, E.; Mbeyela, E.; Kobe, D.; Swai, J.K.; Odufuwa, O.G. Long-lasting insecticidal nets retain bio-efficacy after 5 years of storage: Implications for malaria control programmes. Malar. J. 2020, 19, 110. [Google Scholar] [CrossRef]
- Gesase, S.; Gosling, R.D.; Hashim, R.; Ord, R.; Naidoo, I.; Madebe, R.; Mosha, J.F.; Joho, A.; Mandia, V.; Mrema, H.; et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS ONE 2009, 4, e4569. [Google Scholar] [CrossRef]
- Mixson-Hayden, T.; Jain, V.; McCollum, A.M.; Poe, A.; Nagpal, A.C.; Dash, A.P.; Stiles, J.K.; Udhayakumar, V.; Singh, N. Evidence of selective sweeps in genes conferring resistance to chloroquine and pyrimethamine in Plasmodium falciparum isolates in India. Antimicrob. Agents Chemother. 2010, 54, 997–1006. [Google Scholar] [CrossRef]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Haldar, K.; Bhattacharjee, S.; Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2018, 16, 156–170. [Google Scholar] [CrossRef]
- Bhattarai, A.; Ali, A.S.; Kachur, S.P.; Mårtensson, A.; Abbas, A.K.; Khatib, R.; Al-Mafazy, A.W.; Ramsan, M.; Rotllant, G.; Gerstenmaier, J.F.; et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007, 4, e309. [Google Scholar] [CrossRef]
- Valero, M.V.; Amador, L.R.; Galindo, C.; Figueroa, J.; Bello, M.S.; Murillo, L.A.; Mora, A.L.; Patarroyo, G.; Rocha, C.L.; Rojas, M.; et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet 1993, 341, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Alonso, P.L.; Smith, T.; Schellenberg, J.R.; Masanja, H.; Mwankusye, S.; Urassa, H.; Bastos de Azevedo, I.; Chongela, J.; Kobero, S.; Menendez, C.; et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 1994, 344, 1175–1181. [Google Scholar] [CrossRef]
- Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Gupta, R.P.; Singh, A.K.; Gautam, V.; Kumar, R. Advancements and Challenges in Developing Malaria Vaccines: Targeting Multiple Stages of the Parasite Life Cycle. ACS Infect. Dis. 2023, 9, 1795–1814. [Google Scholar] [CrossRef]
- Doolan, D.L.; Dobano, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria Vaccines: Preferred Product Characteristics and Clinical Development Considerations; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Quagliata, M.; Papini, A.M.; Rovero, P. Malaria vaccines. Expert Opin. Ther. Pat. 2023, 33, 169–178. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Good, M.F. Malaria Vaccines: Progress to Date. BioDrugs 2023, 37, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E.; Gorres, J.P.; Healy, S.A.; Fried, M. Malaria vaccines: A new era of prevention and control. Nat. Rev. Microbiol. 2024, 22, 756–772. [Google Scholar] [CrossRef]
- Prudêncio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Mikolajczak, S.A.; Wilson, E.M.; Grompe, M.; Kaushansky, A.; Camargo, N.; Bial, J.; Ploss, A.; Kappe, S.H. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Investig. 2012, 122, 3618–3628. [Google Scholar] [CrossRef]
- Baker, D.A. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 2010, 172, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.M.; Stewart, V.A. Immune mechanisms in malaria: New insights in vaccine development. Nat. Med. 2013, 19, 168–178. [Google Scholar] [CrossRef]
- Vijayan, A.; Chitnis, C.E. Development of Blood Stage Malaria Vaccines. Methods Mol. Biol. 2019, 2013, 199–218. [Google Scholar] [CrossRef]
- Coelho, C.H.; Rappuoli, R.; Hotez, P.J.; Duffy, P.E. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends Parasitol. 2019, 35, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, Immunogenicity, and Efficacy of a Recombinantly Produced Plasmodium falciparum Circumsporozoite Protein-Hepatitis B Surface Antigen Subunit Vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Alonso, P.L.; Sacarlal, J.; Aponte, J.J.; Leach, A.; Macete, E.; Milman, J.; Mandomando, I.; Spiessens, B.; Guinovart, C.; Espasa, M.; et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: Randomised controlled trial. Lancet 2004, 364, 1411–1420. [Google Scholar] [CrossRef]
- Bejon, P.; Lusingu, J.; Olotu, A.; Leach, A.; Lievens, M.; Vekemans, J.; Mshamu, S.; Lang, T.; Gould, J.; Dubois, M.C.; et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008, 359, 2521–2532. [Google Scholar] [CrossRef]
- The RTS, S Clinical Trials Partnership. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar] [CrossRef]
- The RTS, S Clinical Trials Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- World Health Organization = Organisation mondiale de la santé. Weekly Epidemiological Record, 2022, vol. 97, 09 [full issue]. Wkly. Epidemiol. Rec. Relev. Epidemiol. Hebd. 2022, 97, 61–80. [Google Scholar]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-M™ adjuvant: A critical component of vaccines for the 21(st) century. Hum. Vaccines Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.M.; Karlsson, K.H.; Lövgren-Bengtsson, K.; Magnusson, S.E.; Fuentes, A.; Stertman, L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS ONE 2012, 7, e41451. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Oneko, M.; Steinhardt, L.C.; Yego, R.; Wiegand, R.E.; Swanson, P.A.; Kc, N.; Akach, D.; Sang, T.; Gutman, J.R.; Nzuu, E.L.; et al. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: A double-blind, randomized, placebo-controlled phase 2 trial. Nat. Med. 2021, 27, 1636–1645. [Google Scholar] [CrossRef]
- Mordmüller, B.; Surat, G.; Lagler, H.; Chakravarty, S.; Ishizuka, A.S.; Lalremruata, A.; Gmeiner, M.; Campo, J.J.; Esen, M.; Ruben, A.J.; et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017, 542, 445–449. [Google Scholar] [CrossRef]
- Murphy, S.C.; Vaughan, A.M.; Kublin, J.G.; Fishbauger, M.; Seilie, A.M.; Cruz, K.P.; Mankowski, T.; Firat, M.; Magee, S.; Betz, W.; et al. A genetically engineered Plasmodium falciparum parasite vaccine provides protection from controlled human malaria infection. Sci. Transl. Med. 2022, 14, eabn9709. [Google Scholar] [CrossRef]
- Ogutu, B.R.; Apollo, O.J.; McKinney, D.; Okoth, W.; Siangla, J.; Dubovsky, F.; Tucker, K.; Waitumbi, J.N.; Diggs, C.; Wittes, J.; et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 2009, 4, e4708. [Google Scholar] [CrossRef]
- Spring, M.D.; Cummings, J.F.; Ockenhouse, C.F.; Dutta, S.; Reidler, R.; Angov, E.; Bergmann-Leitner, E.; Stewart, V.A.; Bittner, S.; Juompan, L.; et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE 2009, 4, e5254. [Google Scholar] [CrossRef]
- Silk, S.E.; Kalinga, W.F.; Salkeld, J.; Mtaka, I.M.; Ahmed, S.; Milando, F.; Diouf, A.; Bundi, C.K.; Balige, N.; Hassan, O.; et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet Infect. Dis. 2024, 24, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2021, 2, 701–719.e719. [Google Scholar] [CrossRef]
- Tamborrini, M.; Schäfer, A.; Hauser, J.; Zou, L.; Paris, D.H.; Pluschke, G. The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals. Malar. J. 2023, 22, 210. [Google Scholar] [CrossRef]
- Raj, D.K.; Das Mohapatra, A.; Jnawali, A.; Zuromski, J.; Jha, A.; Cham-Kpu, G.; Sherman, B.; Rudlaff, R.M.; Nixon, C.E.; Hilton, N.; et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature 2020, 582, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Chichester, J.A.; Green, B.J.; Jones, R.M.; Shoji, Y.; Miura, K.; Long, C.A.; Lee, C.K.; Ockenhouse, C.F.; Morin, M.J.; Streatfield, S.J.; et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine 2018, 36, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.R.; Ellis, R.D.; Hurd, J.; Hentrich, A.; Gabriel, E.; Hynes, N.A.; Rausch, K.M.; Zhu, D.; Muratova, O.; Herrera, R.; et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel®, a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naïve Adults. PLoS ONE 2016, 11, e0163144. [Google Scholar] [CrossRef]
- Sagara, I.; Healy, S.A.; Assadou, M.H.; Kone, M.; Swihart, B.J.; Kwan, J.L.; Fintzi, J.; Sissoko, K.; Kamate, B.; Samake, Y.; et al. Malaria transmission-blocking vaccines Pfs230D1-EPA and Pfs25-EPA in Alhydrogel in healthy Malian adults; a phase 1, randomised, controlled trial. Lancet Infect. Dis. 2023, 23, 1266–1279. [Google Scholar] [CrossRef]
- Alkema, M.; Smit, M.J.; Marin-Mogollon, C.; Totté, K.; Teelen, K.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Mordmüller, B.G.; Reimer, J.M.; Lövgren-Bengtsson, K.L.; et al. A Pfs48/45-based vaccine to block Plasmodium falciparum transmission: Phase 1, open-label, clinical trial. BMC Med. 2024, 22, 170. [Google Scholar] [CrossRef]
- Nussenzweig, R.S.; Vanderberg, J.; Most, H.; Orton, C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 1967, 216, 160–162. [Google Scholar] [CrossRef]
- Rieckmann, K.H.; Beaudoin, R.L.; Cassells, J.S.; Sell, K.W. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. World Health Organ. 1979, 57 (Suppl. 1), 261–265. [Google Scholar] [PubMed]
- Mordmüller, B.; Sulyok, Z.; Sulyok, M.; Molnar, Z.; Lalremruata, A.; Calle, C.L.; Bayon, P.G.; Esen, M.; Gmeiner, M.; Held, J.; et al. A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection. NPJ Vaccines 2022, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Bijker, E.M.; Bastiaens, G.J.; Teirlinck, A.C.; van Gemert, G.J.; Graumans, W.; van de Vegte-Bolmer, M.; Siebelink-Stoter, R.; Arens, T.; Teelen, K.; Nahrendorf, W.; et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 7862–7867. [Google Scholar] [CrossRef]
- Coulibaly, D.; Kone, A.K.; Traore, K.; Niangaly, A.; Kouriba, B.; Arama, C.; Zeguime, A.; Dolo, A.; Lyke, K.E.; Plowe, C.V.; et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: A randomized, controlled phase 1 trial. EClinicalMedicine 2022, 52, 101579. [Google Scholar] [CrossRef]
- Mueller, A.K.; Labaied, M.; Kappe, S.H.; Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 2005, 433, 164–167. [Google Scholar] [CrossRef]
- Spring, M.; Murphy, J.; Nielsen, R.; Dowler, M.; Bennett, J.W.; Zarling, S.; Williams, J.; de la Vega, P.; Ware, L.; Komisar, J.; et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 2013, 31, 4975–4983. [Google Scholar] [CrossRef]
- Cohen, S.; Mc, G.I.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Richards, J.S.; Gilson, P.R.; Chai, W.; Beeson, J.G. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 2010, 115, 4559–4568. [Google Scholar] [CrossRef]
- Combe, A.; Giovannini, D.; Carvalho, T.G.; Spath, S.; Boisson, B.; Loussert, C.; Thiberge, S.; Lacroix, C.; Gueirard, P.; Ménard, R. Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe 2009, 5, 386–396. [Google Scholar] [CrossRef]
- Blackman, M.J.; Scott-Finnigan, T.J.; Shai, S.; Holder, A.A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 1994, 180, 389–393. [Google Scholar] [CrossRef]
- Singh, S.; Miura, K.; Zhou, H.; Muratova, O.; Keegan, B.; Miles, A.; Martin, L.B.; Saul, A.J.; Miller, L.H.; Long, C.A. Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): Protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 2006, 74, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.H.; Thomas, A.W.; Margos, G.; Dluzewski, A.R.; Bannister, L.H. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 2004, 72, 154–158. [Google Scholar] [CrossRef]
- Srinivasan, P.; Beatty, W.L.; Diouf, A.; Herrera, R.; Ambroggio, X.; Moch, J.K.; Tyler, J.S.; Narum, D.L.; Pierce, S.K.; Boothroyd, J.C.; et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc. Natl. Acad. Sci. USA 2011, 108, 13275–13280. [Google Scholar] [CrossRef]
- Besteiro, S.; Michelin, A.; Poncet, J.; Dubremetz, J.F.; Lebrun, M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog. 2009, 5, e1000309. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Baldeviano, G.C.; Miura, K.; Diouf, A.; Ventocilla, J.A.; Leiva, K.P.; Lugo-Roman, L.; Lucas, C.; Orr-Gonzalez, S.; Zhu, D.; et al. A malaria vaccine protects Aotus monkeys against virulent Plasmodium falciparum infection. NPJ Vaccines 2017, 2, 14. [Google Scholar] [CrossRef]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef]
- Reddy, K.S.; Amlabu, E.; Pandey, A.K.; Mitra, P.; Chauhan, V.S.; Gaur, D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc. Natl. Acad. Sci. USA 2015, 112, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.C.; Yap, A.; Sisquella, X.; Thompson, J.K.; Lim, N.T.; Whitehead, L.W.; Chen, L.; Lampe, M.; Tham, W.H.; Wilson, D.; et al. Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes. Cell Host Microbe 2016, 20, 60–71. [Google Scholar] [CrossRef]
- Wong, W.; Huang, R.; Menant, S.; Hong, C.; Sandow, J.J.; Birkinshaw, R.W.; Healer, J.; Hodder, A.N.; Kanjee, U.; Tonkin, C.J.; et al. Structure of Plasmodium falciparum Rh5-CyRPA-Ripr invasion complex. Nature 2019, 565, 118–121. [Google Scholar] [CrossRef]
- Douglas, A.D.; Baldeviano, G.C.; Lucas, C.M.; Lugo-Roman, L.A.; Crosnier, C.; Bartholdson, S.J.; Diouf, A.; Miura, K.; Lambert, L.E.; Ventocilla, J.A.; et al. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe 2015, 17, 130–139. [Google Scholar] [CrossRef]
- Barr, P.J.; Green, K.M.; Gibson, H.L.; Bathurst, I.C.; Quakyi, I.A.; Kaslow, D.C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 1991, 174, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Burkhardt, M.; Nakuchima, S.; Herrera, R.; Muratova, O.; Gittis, A.G.; Kelnhofer, E.; Reiter, K.; Smelkinson, M.; Veltri, D.; et al. Structure and function of a malaria transmission blocking vaccine targeting Pfs230 and Pfs230-Pfs48/45 proteins. Commun. Biol. 2020, 3, 395. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.A.; Anderson, C.; Swihart, B.J.; Mwakingwe, A.; Gabriel, E.E.; Decederfelt, H.; Hobbs, C.V.; Rausch, K.M.; Zhu, D.; Muratova, O.; et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Investig. 2021, 131, e146221. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu, P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2023, 9, 9. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef]
- Bale, J.B.; Gonen, S.; Liu, Y.; Sheffler, W.; Ellis, D.; Thomas, C.; Cascio, D.; Yeates, T.O.; Gonen, T.; King, N.P.; et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science 2016, 353, 389–394. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Storni, T.; Lipowsky, G.; Schmid, M.; Pumpens, P.; Bachmann, M.F. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur. J. Immunol. 2002, 32, 3305–3314. [Google Scholar] [CrossRef]
- Tokatlian, T.; Kulp, D.W.; Mutafyan, A.A.; Jones, C.A.; Menis, S.; Georgeson, E.; Kubitz, M.; Zhang, M.H.; Melo, M.B.; Silva, M.; et al. Enhancing Humoral Responses Against HIV Envelope Trimers via Nanoparticle Delivery with Stabilized Synthetic Liposomes. Sci. Rep. 2018, 8, 16527. [Google Scholar] [CrossRef]
- Ingale, J.; Stano, A.; Guenaga, J.; Sharma, S.K.; Nemazee, D.; Zwick, M.B.; Wyatt, R.T. High-Density Array of Well-Ordered HIV-1 Spikes on Synthetic Liposomal Nanoparticles Efficiently Activate B Cells. Cell Rep. 2016, 15, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Yang, L.; Alirezaei, M.; Wilson, R.; Ota, T.; Doyle, E.D.; Cottrell, C.A.; Guenaga, J.; Tran, K.; Li, W.; et al. Fusion of the molecular adjuvant C3d to cleavage-independent native-like HIV-1 Env trimers improves the elicited antibody response. Front. Immunol. 2023, 14, 1180959. [Google Scholar] [CrossRef]
- Khairkhah, N.; Shahhosseini, F.; Agi, E.; Milani, A.; Bolhassani, A. Comparison of Adjuvant Effects of Montanide ISA-720 and Heat Shock Protein 27 in Increasing Immunostimulatory Properties of HIV-1 Nef-Vif Fusion Protein Construct. Protein Pept. Lett. 2023, 30, 401–410. [Google Scholar] [CrossRef]
- Shirota, H.; Sano, K.; Kikuchi, T.; Tamura, G.; Shirato, K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J. Immunol. 2000, 164, 5575–5582. [Google Scholar] [CrossRef]
- Kim, Y.H.; Girardi, M.; Duvic, M.; Kuzel, T.; Link, B.K.; Pinter-Brown, L.; Rook, A.H. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2010, 63, 975–983. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar] [CrossRef]
| Vaccine Immunogen | Adjuvant | Immunogen Type | Current Status | Outcome | Ref. | |
|---|---|---|---|---|---|---|
| Pre-erythrocytic stage | PfSPZ | / | Whole sporozoite (radiation attenuation) | Phase 2 | No significant protection in infants in endemic regions | [40] |
| PfSPZ-Cvac | / | Whole sporozoite (chemical attenuation) | Phase 1 | 100% homologous CHMI 1 efficacy in malaria-naïve healthy adults | [41] | |
| PfGAP3KO | / | Whole sporozoite (genetic attenuation) | Phase 1 | ~50% homologous CHMI 1 efficacy in malaria-naïve healthy adults | [42] | |
| Blood stage | MSP1 | AS02 | Subunit | Phase 2 | No significant protection in children in endemic regions | [43] |
| AMA1 | AS01B/AS02A | Subunit | Phase 1/2a | 0% homologous CHMI 1 efficacy in malaria-naïve healthy adults | [44] | |
| RH5.1 | Matrix-M | Subunit | Phase 1b | Up to 88% GIA 2 of total IgG at 2.5 mg/mL in children in endemic regions | [45] | |
| RH5.1 | AS01B | Subunit | Phase 1/2a | Up to 20% PMR 3 reduction during homologous CHMI 1 in malaria-naïve healthy vaccinated individuals | [46] | |
| PfCyRPA | GLA-SE | Subunit | Preclinical | ~80% GIA 2 of total IgG from vaccinated rabbits at 2.5 mg/mL | [47] | |
| PfGARP | / | mRNA | Preclinical | >94% GIA 2 by serum from vaccinated mice | [48] | |
| Sexual stage | Pfs25-VLP | Alhydrogel | Subunit | Phase 1 | No TRA 4 by neat serum from malaria-naïve healthy adult vaccinated individuals | [49] |
| Pfs25-EPA | Alhydrogel | Subunit | Phase 1 | 9 out of 11 malaria-naïve healthy adult vaccinated individuals displayed >50% TRA 4 after 4 doses at 47 ug | [50] | |
| Pfs230D1-EPA | Alhydrogel | Subunit | Phase 1 | 73.7% TRA 4 10 weeks post-dose 4 by serum from malaria-experienced Malian adults | [51] | |
| Pfs48/45 | Matrix-M | Subunit | Phase 1 | No TRA 4 by neat serum from malaria-naïve healthy adult vaccinated individuals | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, Q.; He, X.; Yang, B. Malaria Vaccines: Current Achievements and Path Forward. Vaccines 2025, 13, 542. https://doi.org/10.3390/vaccines13050542
Chen J, Wang Q, He X, Yang B. Malaria Vaccines: Current Achievements and Path Forward. Vaccines. 2025; 13(5):542. https://doi.org/10.3390/vaccines13050542
Chicago/Turabian StyleChen, Jiayan, Qi Wang, Xiaomeng He, and Bei Yang. 2025. "Malaria Vaccines: Current Achievements and Path Forward" Vaccines 13, no. 5: 542. https://doi.org/10.3390/vaccines13050542
APA StyleChen, J., Wang, Q., He, X., & Yang, B. (2025). Malaria Vaccines: Current Achievements and Path Forward. Vaccines, 13(5), 542. https://doi.org/10.3390/vaccines13050542





