Pneumococcal Vaccine Uptake in Adults Before and After Hospitalization for Pneumococcal Infections in Hong Kong, 2015 to 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Statistical Analysis
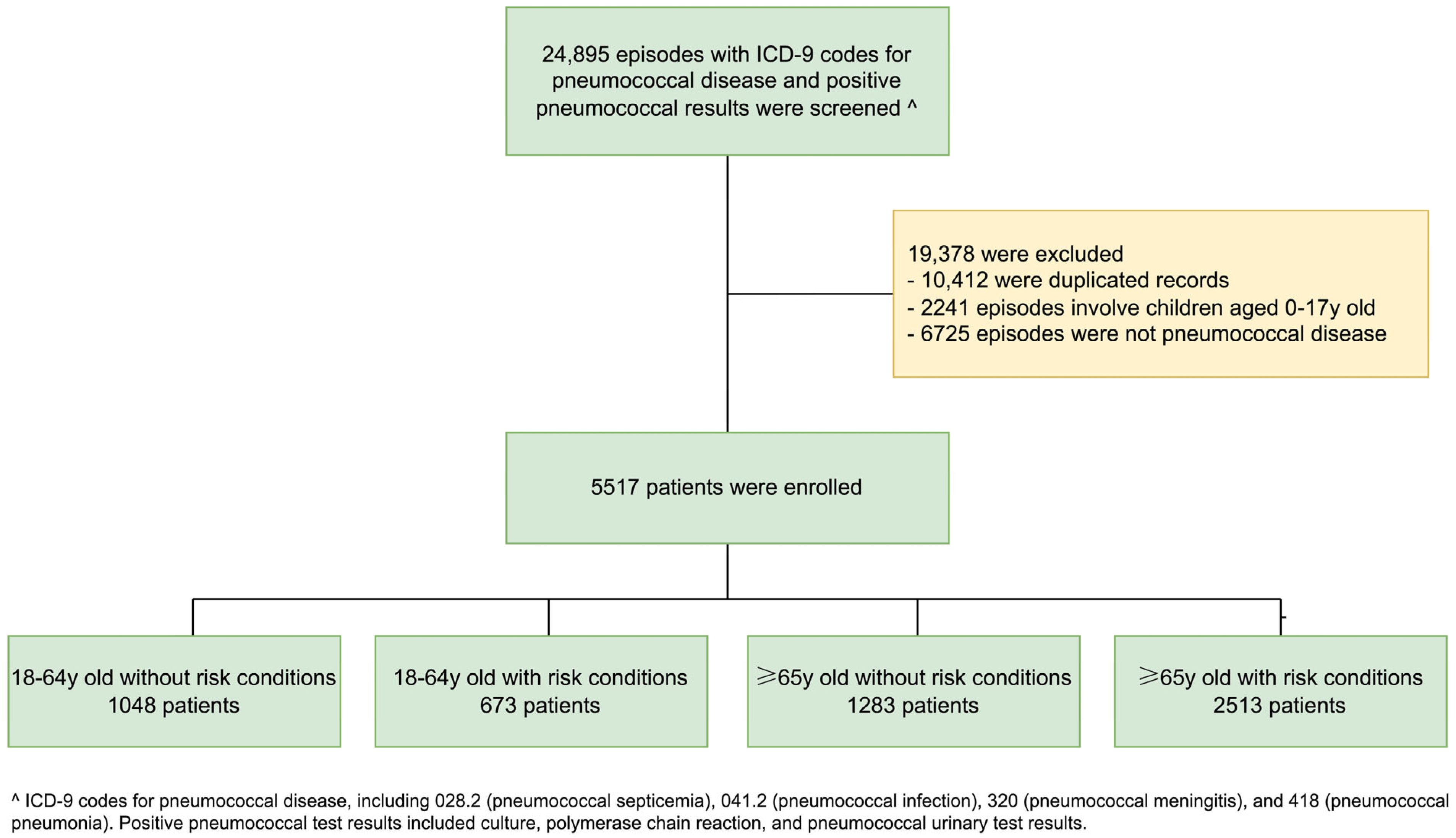
3. Results
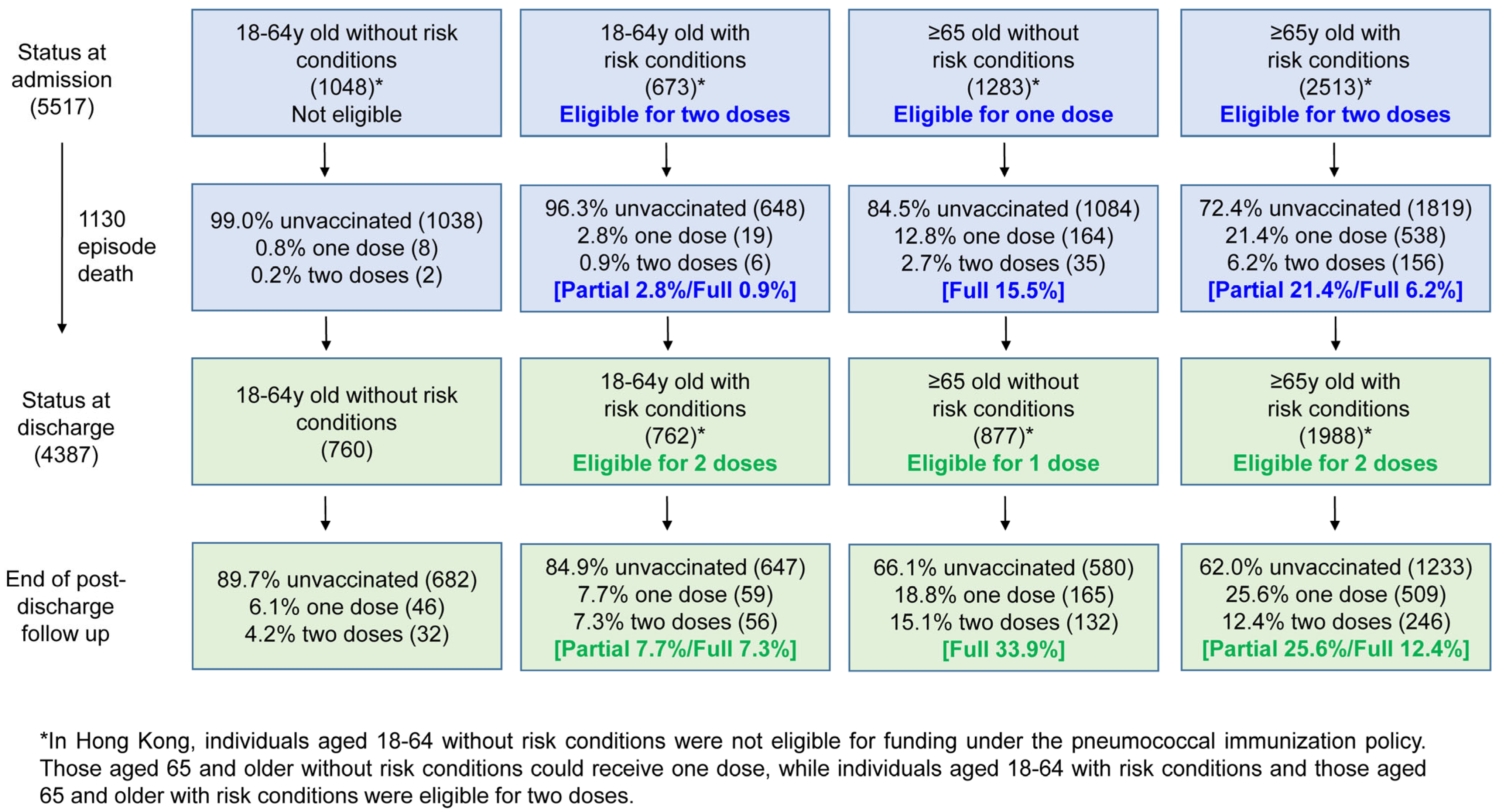
3.1. Uptake of Pneumococcal Vaccination Among the Study Population
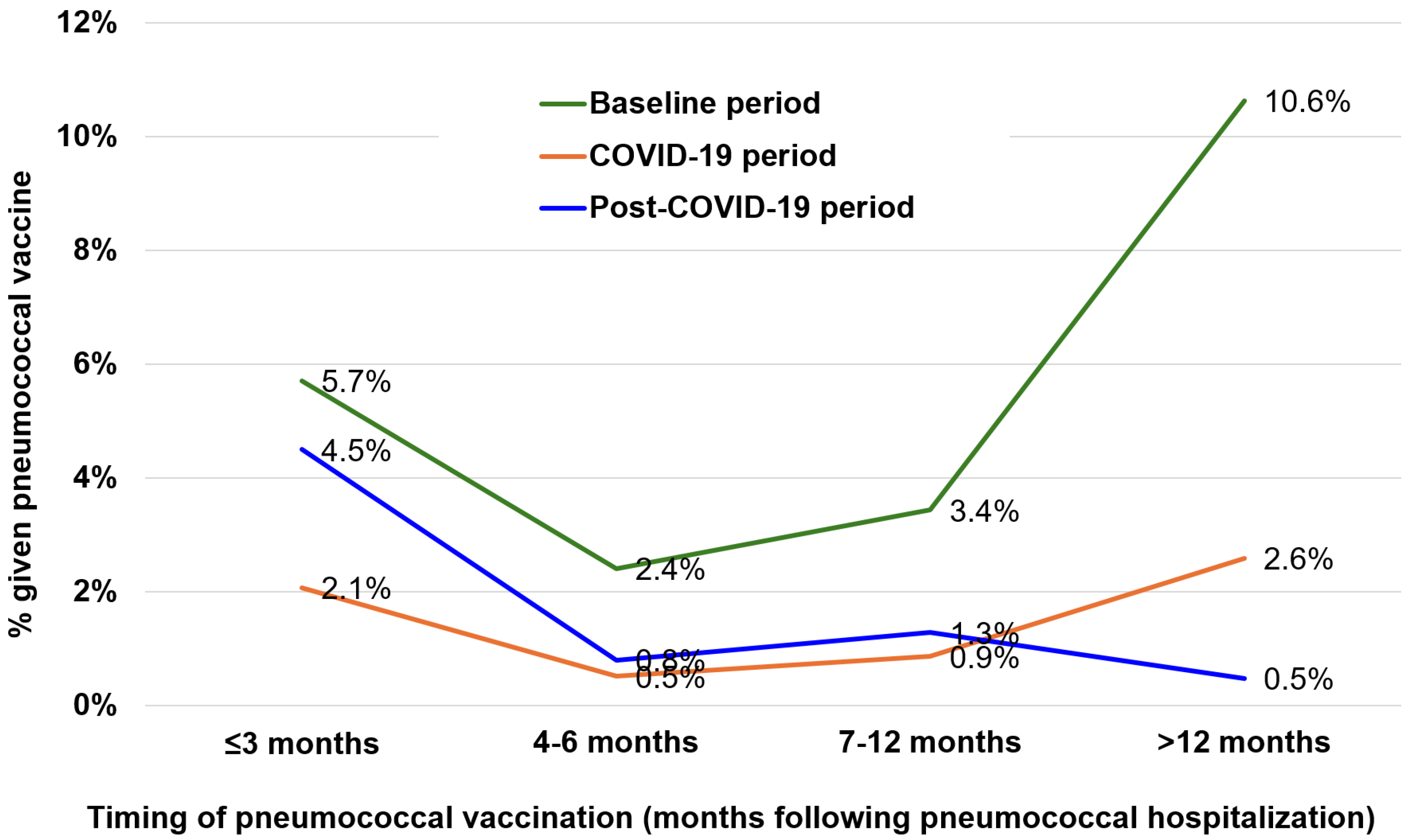
3.2. Missed Opportunities for Pneumococcal Vaccination
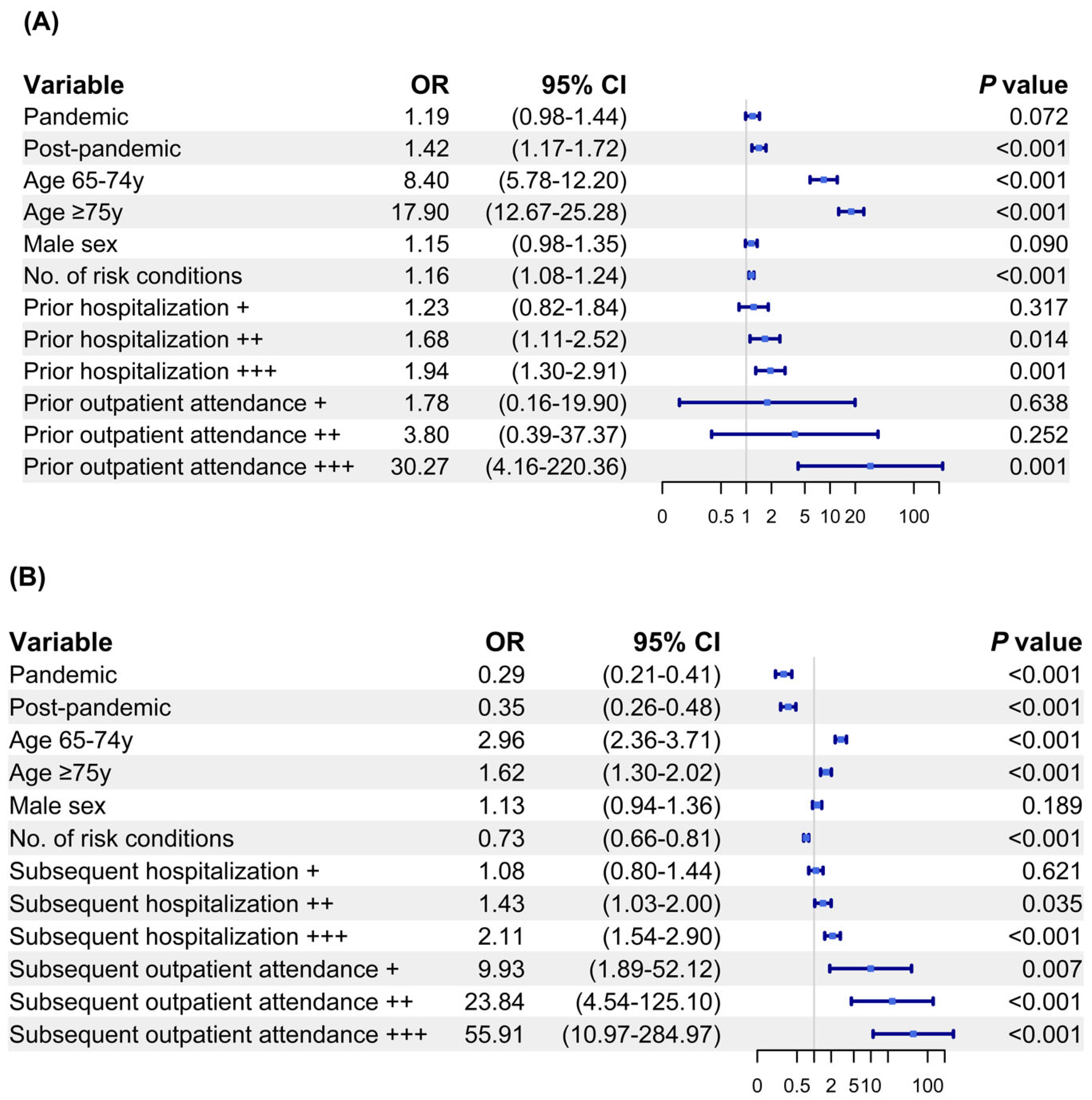
3.3. Factors Associated with Pneumococcal Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPD | invasive pneumococcal disease |
| 23vPPV | 23-valent polysaccharide pneumococcal vaccine |
| PCV | pneumococcal conjugate vaccine |
| COVID-19 | Coronavirus disease |
| CDARS | Clinical Data Analysis and Reporting System |
| ORs | odd ratios |
References
- Drijkoningen, J.J.; Rohde, G.G. Pneumococcal infection in adults: Burden of disease. Clin. Microbiol. Infect. 2014, 20 (Suppl. S5), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Ma, T.F.; Ip, M.S.; Ho, P.L. Invasive pneumococcal disease, pneumococcal pneumonia and all-cause pneumonia in Hong Kong during the COVID-19 pandemic compared with the preceding 5 years: A retrospective observational study. BMJ Open 2021, 11, e055575. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal Vaccination Strategies. An Update and Perspective. Ann. Am. Thorac. Soc. 2016, 13, 933–944. [Google Scholar] [CrossRef]
- Farrar, J.L.; Childs, L.; Ouattara, M.; Akhter, F.; Britton, A.; Pilishvili, T.; Kobayashi, M. Systematic Review and Meta-Analysis of the Efficacy and Effectiveness of Pneumococcal Vaccines in Adults. Pathogens 2023, 12, 732. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Lazarus, R.; Yu, L.M.; Bowman, J.; Bateman, E.A.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.C.; Mant, D.; et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J. Infect. Dis. 2012, 205, 1408–1416. [Google Scholar] [CrossRef]
- Jha, V.; Janoff, E.N. Complementary Role of CD4+ T Cells in Response to Pneumococcal Polysaccharide Vaccines in Humans. Vaccines 2019, 7, 18. [Google Scholar] [CrossRef]
- Jackson, L.A.; Gurtman, A.; van Cleeff, M.; Frenck, R.W.; Treanor, J.; Jansen, K.U.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013, 31, 3594–3602. [Google Scholar] [CrossRef]
- Fletcher, M.A.; Vojicic, J.; Daigle, D.; Taysi, B.; Haridy, H.; Abalos, M.G.; Del Carmen Morales, G. National recommendations for adult pneumococcal vaccination in countries of the WHO regions of Americas, Africa, Eastern Mediterranean, South East Asia, and Western Pacific. Vaccine 2024, 42, 126390. [Google Scholar] [CrossRef]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pilishvili, T.; Farrar, J.L.; Leidner, A.J.; Gierke, R.; Prasad, N.; Moro, P.; Campos-Outcalt, D.; Morgan, R.L.; Long, S.S.; et al. Pneumococcal Vaccine for Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Bertran, M.; Litt, D.J.; Ladhani, S.N.; Miller, E. Potential impact of replacing the 13-valent pneumococcal conjugate vaccine with 15-valent or 20-valent pneumococcal conjugate vaccine in the 1 + 1 infant schedule in England: A modelling study. Lancet Public Health 2024, 9, e654–e663. [Google Scholar] [CrossRef]
- Kobayashi, M.; Leidner, A.J.; Gierke, R.; Xing, W.; Accorsi, E.; Moro, P.; Kamboj, M.; Kuchel, G.A.; Schechter, R.; Loehr, J.; et al. Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal Wkly. Rep. 2025, 74, 1–8. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Adam, H.J.; Baxter, M.R.; Karlowsky, J.A.; Golden, A.R.; Martin, I.; Zhanel, G.G. Comparing serotype coverage of pneumococcal vaccines with PCV21 (V116), a new 21-valent conjugate pneumococcal vaccine, and the epidemiology of its eight unique Streptococcus pneumoniae serotypes (15A, 15C, 16F, 23A, 23B, 24F, 31 and 35B) causing invasive pneumococcal disease in adult patients in Canada: SAVE study, 2018–21. J. Antimicrob. Chemother. 2025, 80, 1377–1385. [Google Scholar] [CrossRef]
- Centre for Health Protection, Department of Health, HKSAR, Scientific Committee on Vaccine Preventable Diseases. Available online: https://www.chp.gov.hk/en/static/24008.html (accessed on 20 April 2025).
- Ho, P.L.; Chiu, S.S.; Law, P.Y.; Chan, E.L.; Lai, E.L.; Chow, K.H. Increase in the nasopharyngeal carriage of non-vaccine serogroup 15 Streptococcus pneumoniae after introduction of children pneumococcal conjugate vaccination in Hong Kong. Diagn. Microbiol. Infect. Dis. 2015, 81, 145–148. [Google Scholar] [CrossRef]
- Chan, K.C.; Subramanian, R.; Chong, P.; Nelson, E.A.; Lam, H.S.; Li, A.M.; Ip, M. Pneumococcal carriage in young children after introduction of PCV13 in Hong Kong. Vaccine 2016, 34, 3867–3874. [Google Scholar] [CrossRef]
- Centre for Health Protection. Statistics on Antimicrobial Resistance Control. Pneumococcal Vaccination. Available online: https://www.chp.gov.hk/en/statistics/data/10/100044/6870.html (accessed on 20 April 2025).
- Kyaw, M.H.; Greene, C.M.; Schaffner, W.; Ray, S.M.; Shapiro, M.; Barrett, N.L.; Gershman, K.; Craig, A.S.; Roberson, A.; Zell, E.R.; et al. Adults with invasive pneumococcal disease: Missed opportunities for vaccination. Am. J. Prev. Med. 2006, 31, 286–292. [Google Scholar] [CrossRef]
- Schulz, P.S.; Moore, S.E.; Smith, D.; Javed, J.; Wilde, A.M. Missed Pneumococcal Vaccination Opportunities in Adults with Invasive Pneumococcal Disease in a Community Health System. Open Forum Infect. Dis. 2022, 9, ofac075. [Google Scholar] [CrossRef]
- Malo, J.A.; Ware, R.S.; Lambert, S.B. Estimating the risk of recurrent invasive pneumococcal disease in Australia, 1991–2016. Vaccine 2021, 39, 5748–5756. [Google Scholar] [CrossRef]
- Einarsdóttir, H.M.; Erlendsdóttir, H.; Kristinsson, K.G.; Gottfredsson, M. Nationwide study of recurrent invasive pneumococcal infections in a population with a low prevalence of human immunodeficiency virus infection. Clin. Microbiol. Infect. 2005, 11, 744–749. [Google Scholar] [CrossRef]
- Hedlund, J.; Kalin, M.; Ortqvist, A. Recurrence of pneumonia in middle-aged and elderly adults after hospital-treated pneumonia: Aetiology and predisposing conditions. Scand. J. Infect. Dis. 1997, 29, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-P.F.; Ma, T.-F.; Fang, H.; Tsui, W.-K.; Ho, J.C.-M.; Ip, M.S.-M.; Ho, P.-L. Changes in the incidence, viral coinfection pattern and outcomes of pneumococcal hospitalizations during and after the COVID-19 pandemic. Pneumonia 2025, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Centre for Health Protection, Department of Health, HKSAR. Report on IPD. Available online: https://www.chp.gov.hk/en/resources/29/636.html (accessed on 20 April 2025).
- Bertran, M.; D’Aeth, J.C.; Abdullahi, F.; Eletu, S.; Andrews, N.J.; Ramsay, M.E.; Litt, D.J.; Ladhani, S.N. Invasive pneumococcal disease 3 years after introduction of a reduced 1 + 1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: A prospective national observational surveillance study. Lancet Infect. Dis. 2024, 24, 546–556. [Google Scholar] [CrossRef]
- Paguio, J.A.; Yao, J.S.; Dee, E.C. Silver lining of COVID-19: Heightened global interest in pneumococcal and influenza vaccines, an infodemiology study. Vaccine 2020, 38, 5430–5435. [Google Scholar] [CrossRef]
- Tavares Costa, M.; Ribeiro, A.S.; Oliveira Almeida, D.; Ochoa-Leite, C. Pneumococcal vaccination in a northern Portugal health centre during COVID-19 pandemic. Semergen 2022, 48, 293–294. [Google Scholar] [CrossRef]
- Matta, M.G.; Pulido, L.; Herrera-Paz, J.J.; Picco, J.M.; Wolff, S.; Tse, G.; Garcia-Zamora, S. Influenza and pneumococcal vaccine prescription for adults during COVID-19 first wave in three regions of Argentina. Vaccine 2023, 41, 1541–1544. [Google Scholar] [CrossRef]
- Atkinson, K.M.; Ntacyabukura, B.; Hawken, S.; Laflamme, L.; Wilson, K. Effects of the COVID-19 pandemic on self-reported 12-month pneumococcal vaccination series completion rates in Canada. Hum. Vaccines Immunother. 2022, 18, 2158005. [Google Scholar] [CrossRef]
- Chan, K.F.; Ma, T.F.; Sridhar, S.; Lui, M.M.; Ho, J.C.; Lam, D.C.; Ip, M.S.; Ho, P.L. Changes in the incidence, clinical features and outcomes of tuberculosis during COVID-19 pandemic. J. Infect. Public Health 2024, 17, 102511. [Google Scholar] [CrossRef]
- Guo, R.; Wat, D.; Lam, S.H.M.; Bucci, T.; Tsang, C.T.; Cai, A.P.; Chan, Y.H.; Ren, Q.W.; Huang, J.Y.; Zhang, J.N.; et al. Cardiovascular benefits and safety profile of macrolide maintenance therapy in patients with bronchiectasis. Eur. Respir. J. 2025, 65, 2401574. [Google Scholar] [CrossRef]
- Hospital Authority Statistical Report. Available online: https://www3.ha.org.hk/Data/HAStatistics/StatisticalReport (accessed on 20 April 2025).
- Agresti, A. Categorical Data Analysis, 3rd ed.; Wiley: Somerset, UK, 2012; Volume 792. [Google Scholar]
- Casella, G.; Berger, R.L. Statistical Inference, 2nd ed.; Duxbury: Pacific Grove, CA, USA, 2002. [Google Scholar]
- Gelman, A.; Jakulin, A.; Pittau, M.G.; Su, Y.-S. A weakly informative default prior distribution for logistic and other regression models. Ann. Appl. Stat. 2008, 2, 1360–1383. [Google Scholar] [CrossRef]
- Chan, P.S.; Poon, J.; Han, S.C.; Ye, D.; Yu, F.Y.; Fang, Y.; Wong, M.C.S.; Mo, P.K.H.; Wang, Z. Changes in the Pneumococcal Vaccination Uptake and Its Determinants before, during, and after the COVID-19 Pandemic among Community-Living Older Adults in Hong Kong, China: Repeated Random Telephone Surveys. Vaccines 2024, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- McFadden, K.; Heywood, A.; Dyda, A.; Kaufman, J.; Seale, H. Minimising missed opportunities to promote and deliver immunization services to middle and older age adults: Can hospital-based programs be a solution? Vaccine 2021, 39, 3467–3472. [Google Scholar] [CrossRef] [PubMed]
- Dexter, P.R.; Perkins, S.M.; Maharry, K.S.; Jones, K.; McDonald, C.J. Inpatient computer-based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: A randomized trial. JAMA 2004, 292, 2366–2371. [Google Scholar] [CrossRef]
- Tam, C.F.; Cheung, K.S.; Lam, S.; Wong, A.; Yung, A.; Sze, M.; Fang, J.; Tse, H.F.; Siu, C.W. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheter. Cardiovasc. Interv. 2021, 97, E194–E197. [Google Scholar] [CrossRef]
- Hayes, B.H.; Haberling, D.L.; Kennedy, J.L.; Varma, J.K.; Fry, A.M.; Vora, N.M. Burden of Pneumonia-Associated Hospitalizations: United States, 2001–2014. Chest 2018, 153, 427–437. [Google Scholar] [CrossRef]
- Li, X.; Blais, J.E.; Wong, I.C.K.; Tam, A.W.Y.; Cowling, B.J.; Hung, I.F.N.; Chan, E.W.Y. Population-based estimates of the burden of pneumonia hospitalizations in Hong Kong, 2011–2015. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 553–561. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, H.; Wannaadisai, W.; Vietri, J.; Chaiyakunapruk, N. Systematic literature review of cost-effectiveness analyses of adult 15- and 20-valent pneumococcal vaccines. Vaccine 2025, 46, 126656. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.K.M.; Hung, I.F.N.; Lam, B.; Lin, A.W.C.; So, T.M.K.; Wong, A.T.Y.; Wong, M.C.S. The role of a single-shot higher-valency pneumococcal vaccine in overcoming challenges regarding invasive pneumococcal disease in Hong Kong. Hong Kong Med. J. 2023, 29, 11–14. [Google Scholar] [CrossRef]
| Age Group | Outcome | n | Previous Hospitalization | Previous Outpatient Attendance | Subsequent Hospitalization | Subsequent Outpatient Attendance | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (%) | No | Total (%) | No | Total (%) | No | Total (%) | No | |||
| 18–64 y old without risk conditions | Survived | 983 | 576 (58.6) | 3 (1–6) | 794 (80.8) | 35 (12–81) | 659 (67.0) | 3 (1–7) | 894 (90.9) | 19 (5–47) |
| Died | 65 | 47 (72.3) | 7 (3–17) | 56 (86.2) | 25 (7–66) | - | - | - | - | |
| Subtotal | 1048 | 623 (59.4) | 3 (1–6) | 850 (81.1) | 26 (7–70) | - | - | - | - | |
| 18–64 y old with risk conditions | Survived | 539 | 444 (82.4) | 8 (3–17) | 489 (90.7) | 71 (30–142) | 453 (84.0) | 5 (2–13) | 517 (95.9) | 34 (11–73) |
| Died | 134 | 109 (81.3) | 6 (3–13) | 112 (83.6) | 69 (21–110) | - | - | - | - | |
| Subtotal | 673 | 553 (82.2) | 8 (3–16) | 601 (89.3) | 70 (28–134) | - | - | - | - | |
| ≥65 y old without risk conditions | Survived | 1027 | 799 (77.8) | 4 (2–9) | 948 (92.3) | 68 (28–118) | 890 (86.7) | 4 (2–9) | 966 (94.1) | 32 (19–61) |
| Died | 256 | 199 (77.7) | 5 (2–9) | 231 (90.2) | 65 (29–117) | - | - | - | - | |
| Subtotal | 1283 | 998 (77.8) | 4 (2–9) | 1179 (91.9) | 67 (28–118) | - | - | - | - | |
| ≥65 y old with risk conditions | Survived | 1838 | 1749 (95.2) | 10 (5–18) | 1809 (98.4) | 108 (67–165) | 1714 (93.3) | 5 (2–10) | 1720 (93.6) | 23 (9–52) |
| Died | 675 | 645 (95.6) | 9 (5–17) | 666 (98.7) | 109 (60–168) | - | - | - | - | |
| Subtotal | 2513 | 2394 (95.3) | 10 (5–18) | 2475 (98.5) | 108 (65–166) | - | - | - | - | |
| All patients | Survived | 4387 | 3568 (81.3) | 7 (3–15) | 4040 (92.1) | 79 (32–138) | 3716 (84.7) | 4 (2–10) | 4097 (93.4) | 25 (9–56) |
| Died | 1130 | 1000 (88.5) | 8 (4–15) | 1065 (94.2) | 96 (44–152) | - | - | - | - | |
| Total | 5517 | 4568 (82.8) | 7 (3–15) | 5105 (92.5) | 82 (33–141) | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.-P.F.; Ma, T.-F.; Ho, J.C.-M.; Hung, I.F.-N.; Ip, M.S.-M.; Ho, P.-L. Pneumococcal Vaccine Uptake in Adults Before and After Hospitalization for Pneumococcal Infections in Hong Kong, 2015 to 2024. Vaccines 2025, 13, 541. https://doi.org/10.3390/vaccines13050541
Chan K-PF, Ma T-F, Ho JC-M, Hung IF-N, Ip MS-M, Ho P-L. Pneumococcal Vaccine Uptake in Adults Before and After Hospitalization for Pneumococcal Infections in Hong Kong, 2015 to 2024. Vaccines. 2025; 13(5):541. https://doi.org/10.3390/vaccines13050541
Chicago/Turabian StyleChan, King-Pui Florence, Ting-Fung Ma, James Chung-Man Ho, Ivan Fan-Ngai Hung, Mary Sau-Man Ip, and Pak-Leung Ho. 2025. "Pneumococcal Vaccine Uptake in Adults Before and After Hospitalization for Pneumococcal Infections in Hong Kong, 2015 to 2024" Vaccines 13, no. 5: 541. https://doi.org/10.3390/vaccines13050541
APA StyleChan, K.-P. F., Ma, T.-F., Ho, J. C.-M., Hung, I. F.-N., Ip, M. S.-M., & Ho, P.-L. (2025). Pneumococcal Vaccine Uptake in Adults Before and After Hospitalization for Pneumococcal Infections in Hong Kong, 2015 to 2024. Vaccines, 13(5), 541. https://doi.org/10.3390/vaccines13050541








