Impact of Chitosan Nanoparticles-Coated Dendritic Cell-Based Vaccine as Cancer Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of CH-NPs
2.1.1. Preparation of Chitosan Nanoparticles
2.1.2. Characterization of Chitosan Nanoparticles
2.2. Cell Lines and Cell Culture
2.2.1. Cell Culture Preparation
2.2.2. Cell Culture Treatment
2.3. Generation of Mouse Bone Marrow Dendritic Cells
2.3.1. Ethical Approval
2.3.2. Experimental Protocol
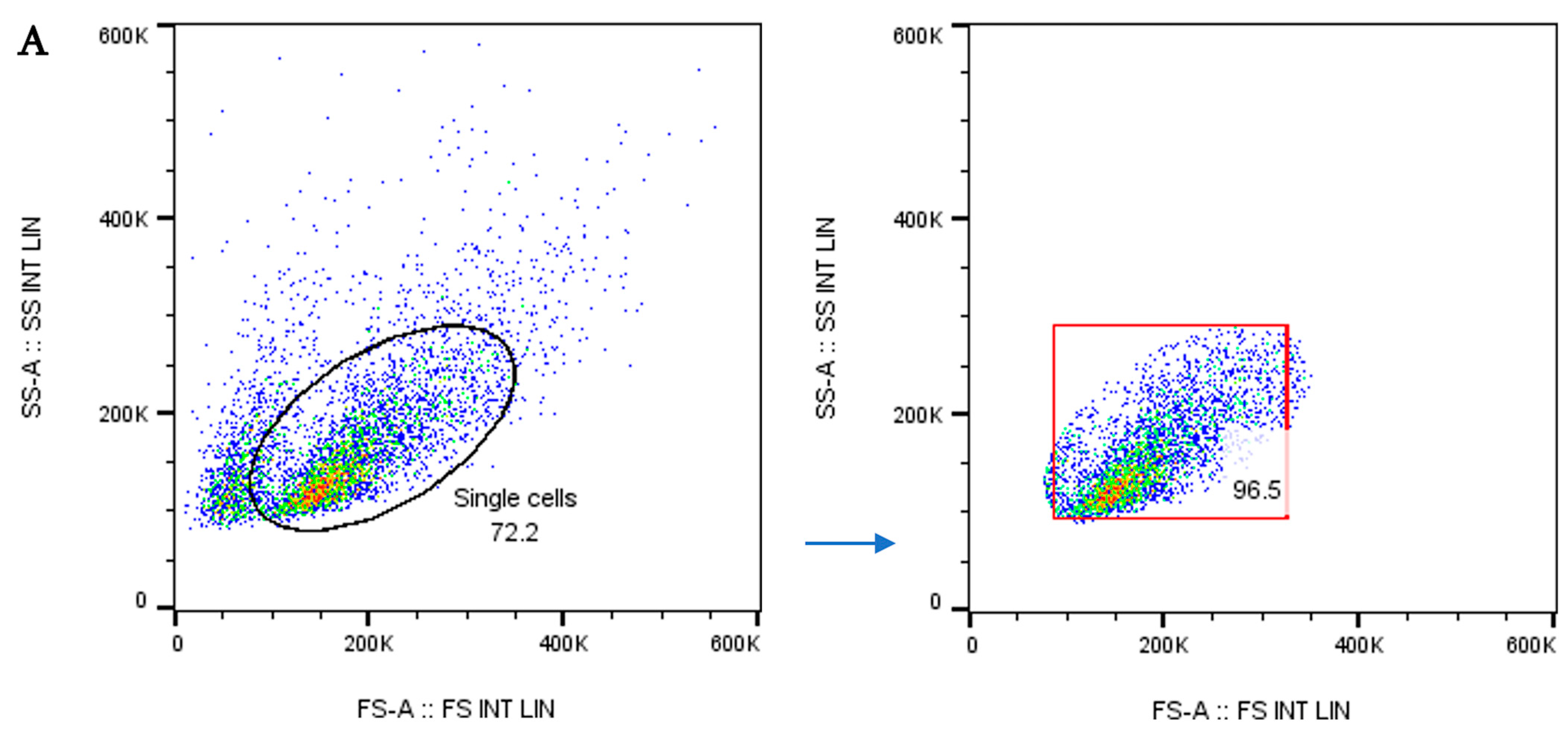
2.3.3. Flow Cytometry for Dendritic Cell Morphology and Phenotype
2.4. Adsorption of Chitosan Nanoparticles onto Dendritic Cells
2.5. In Vivo Assessment
2.5.1. Animal Model
2.5.2. Experimental Study Design
2.5.3. Assessing DC and T Cell Activation
2.5.4. Histological Studies
2.6. Statistical Analysis
3. Results
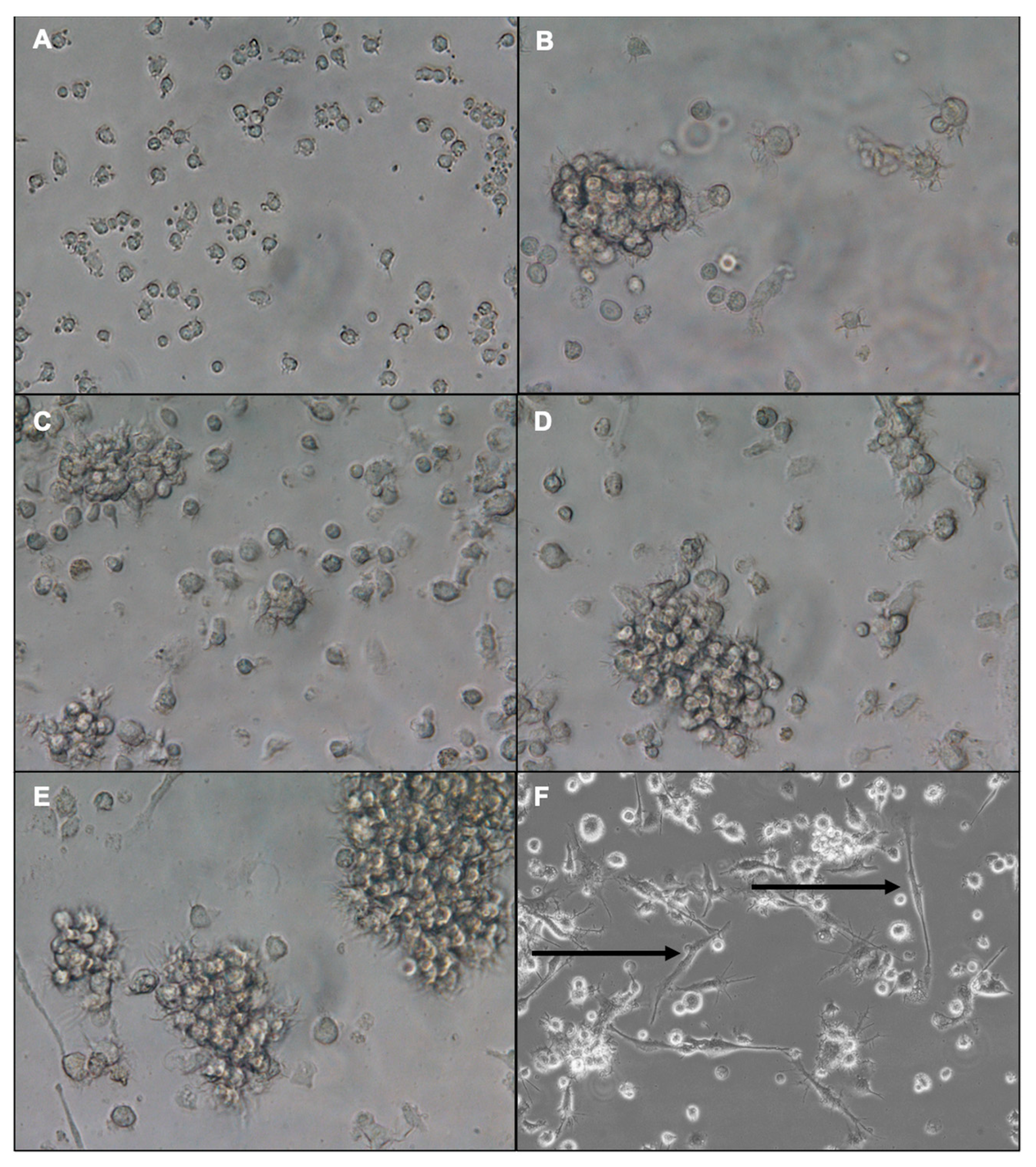
3.1. Morphology of Differentiated Bone Marrow-Derived Dendritic Cells
3.2. Dendritic Cell Phenotype by Flow Cytometry
3.3. Size, Zeta Potential, and Morphology of Chitosan Nanoparticles
3.4. Cytotoxicity Study Using MTT Assay
3.5. In Vivo Assessment
3.5.1. Tumor Induction and Vaccination Effect on Body Weight
3.5.2. Assessing Dendritic Cell Activation in Mice Blood
3.5.3. Assessing Dendritic Cell Activation in Mice Spleen
3.5.4. Assessing T Cell Activation
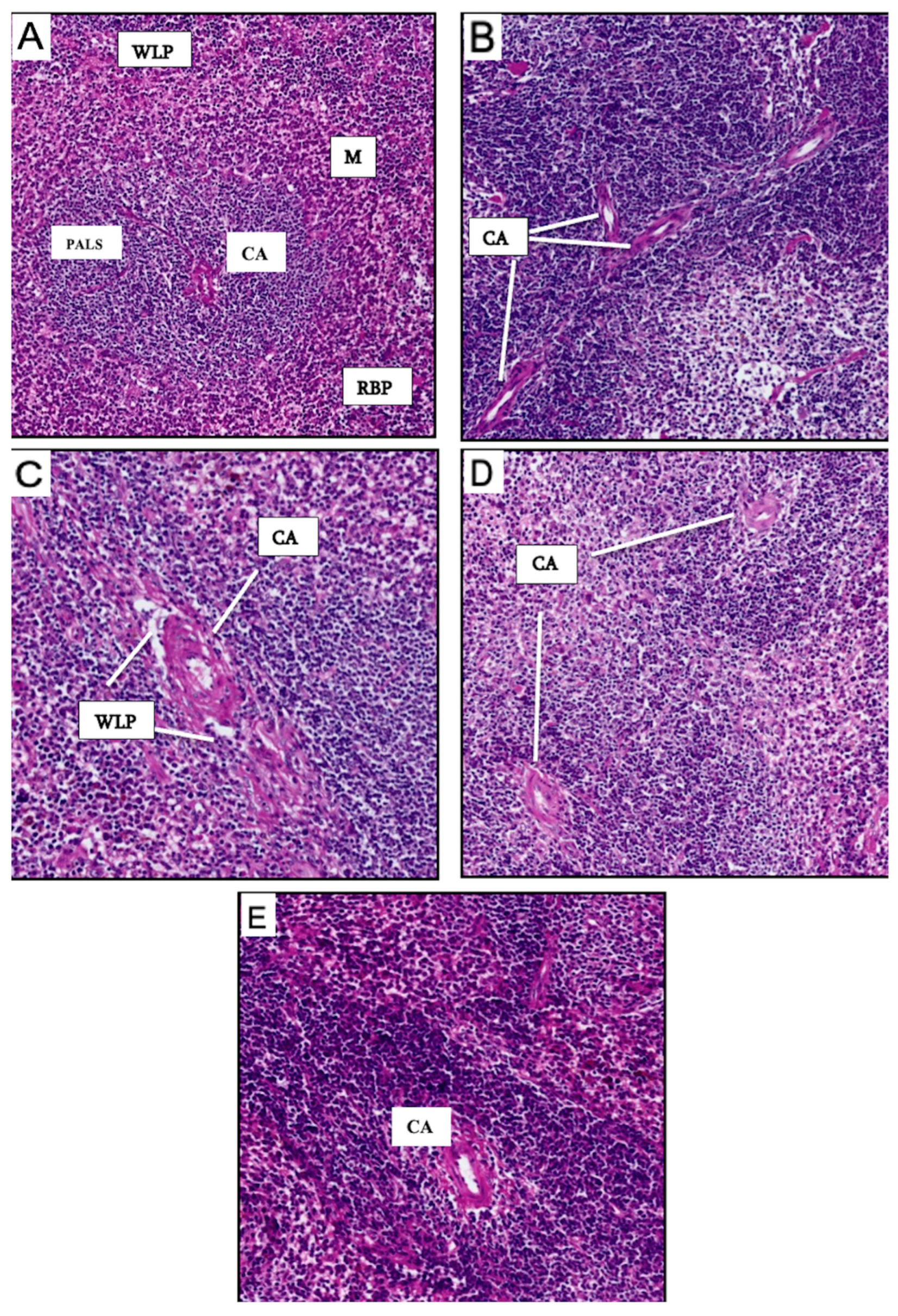
3.6. Histological Studies of Spleen and Lymph Nodes of Vaccinated Mice Groups
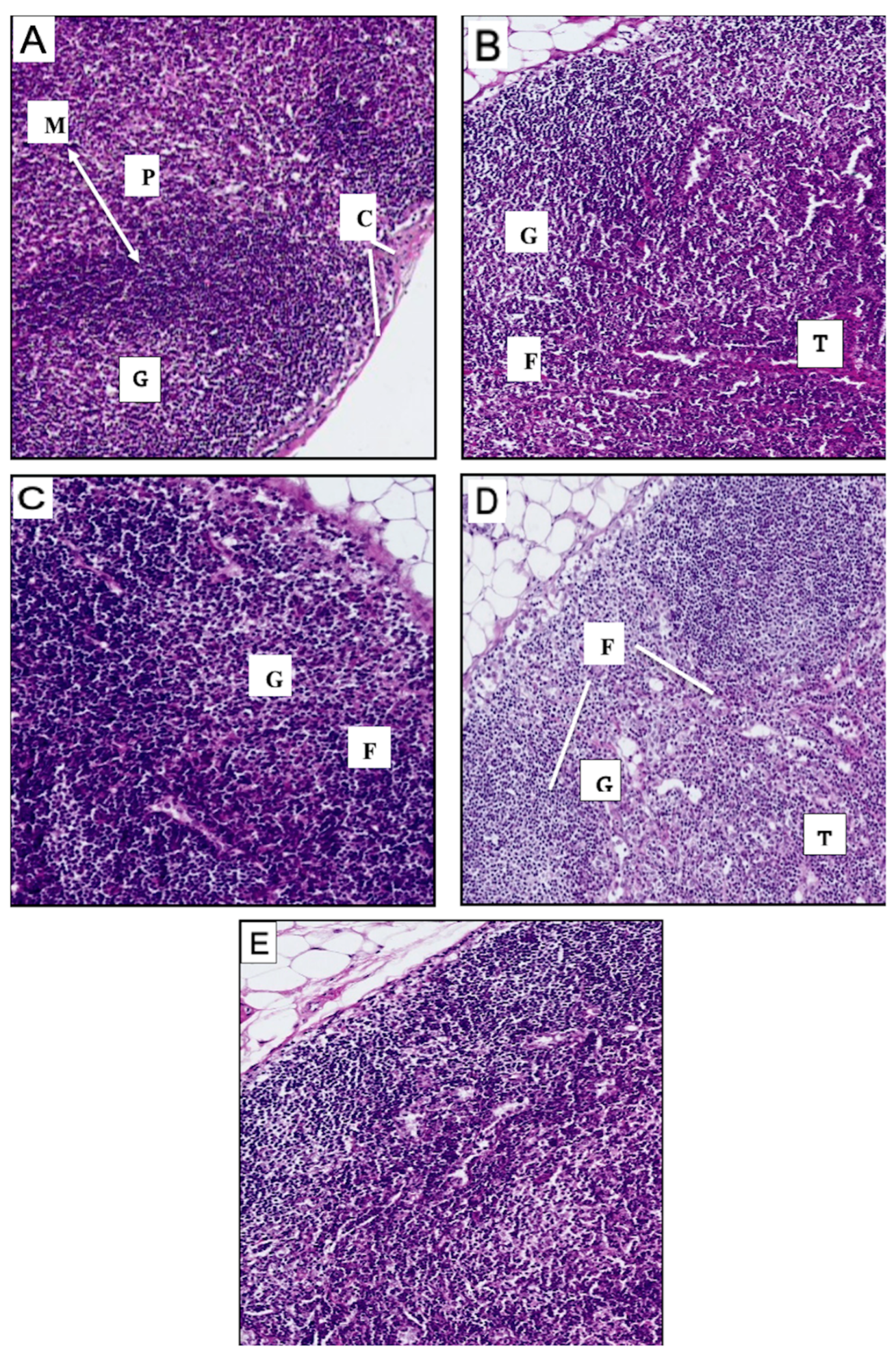
3.6.1. Histological Findings for Spleen
3.6.2. Histological Findings of the Lymph Nodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| APC | Antigen-presenting cell |
| BMDCs | Bone marrow dendritic cells |
| CA | Central artery |
| cDCs | Conventional Dendritic Cells |
| CH | Chitosan |
| CH-NP | Chitosan nanoparticles |
| cP | Centipoise |
| CSF-1 | Colony-stimulating factor 1 |
| CTL | Cytotoxic T lymphocyte |
| CTLA-4 | Cytotoxic T lymphocyte Associated Proteins-4 |
| DCs | Dendritic cells |
| DMSO | Dimethyl Sulfoxide |
| FACS | Fluorescence-activated cell sorting |
| FCS | Fetal Calf Serum |
| FT-IR | Fourier-transform infrared spectroscopy |
| IF- γ | Interferon-gamma |
| IL | Interleukin |
| mAbs | Monoclonal antibodies |
| MFI | Mean fluorescence intensity |
| MHC | Major histocompatibility complex |
| MoDCs | Monocyte-derived dendritic cells |
| MZ | Marginal zone |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NKs | Natural killer cells |
| NPs | Nanoparticles |
| PAMPs | Pathogen associated molecular patterns |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed death-ligand 1 |
| PLGA | Poly D, L-lactic-co-glycolic acid |
| RBP | Red blood pulp |
| SEM | Standard error of means |
| TAAs | Tumor-associated antigens |
| TAMs | Tumor-associated macrophages |
| TCE | Thymic Epithelial Cells |
| TCR | T cell receptor |
| TNF- α | Tumor necrosis factor-alpha |
| TPP | Tripolyphosphate |
| WLP | White lymph pulp |
References
- Akkın, S.; Varan, G.; Bilensoy, E. A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers. Molecules 2021, 26, 3382. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharm. Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Song, E.J.; Jeon, S.G.; Kim, K.A.; Kim, J.I.; Moon, M. Restricted CD4+ T cell receptor repertoire impairs cognitive function via alteration of Th2 cytokine levels. Neurogenesis 2017, 4, e1256856. [Google Scholar] [CrossRef][Green Version]
- Moreau, A.; Alliot-Licht, B.; Cuturi, M.C.; Blancho, G. Tolerogenic dendritic cell therapy in organ transplantation. Transpl. Int. 2017, 30, 754–764. [Google Scholar] [CrossRef]
- Thomson, A.W.; Metes, D.M.; Ezzelarab, M.B.; Raïch-Regué, D. Regulatory dendritic cells for human organ transplantation. Transplant. Rev. 2019, 33, 130–136. [Google Scholar] [CrossRef]
- Pittet, M.J.; Di Pilato, M.; Garris, C.; Mempel, T.R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef]
- Takahashi, A.; Kono, K.; Ichihara, F.; Sugai, H.; Fujii, H.; Matsumoto, Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol. Immunother. 2004, 53, 543–550. [Google Scholar] [CrossRef]
- Adamik, J.; Munson, P.V.; Maurer, D.M.; Hartmann, F.J.; Bendall, S.C.; Argüello, R.J.; Butterfield, L.H. Immuno-metabolic dendritic cell vaccine signatures associate with overall survival in vaccinated melanoma patients. Nat. Commun. 2023, 14, 7211. [Google Scholar] [CrossRef]
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 2004, 103, 2187–2195. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Phase I/II Pilot Study of Wilms’ Tumor 1 Peptide-Pulsed Dendritic Cell Vaccination Combined With Conventional Chemotherapy in Patients With Head and Neck Cancer. Ther. Apher. Dial. 2019, 23, 279–288. [Google Scholar] [CrossRef]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- El-Sissi, A.F.; Mohamed, F.H.; Danial, N.M.; Gaballah, A.Q.; Ali, K.A. Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 2020, 10, 88. [Google Scholar] [CrossRef]
- Hunsawong, T.; Sunintaboon, P.; Warit, S.; Thaisomboonsuk, B.; Jarman, R.G.; Yoon, I.K.; Ubol, S.; Fernandez, S. Immunogenic Properties of a BCG Adjuvanted Chitosan Nanoparticle-Based Dengue Vaccine in Human Dendritic Cells. PLoS Neglected Trop. Dis. 2015, 9, e0003958. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Kheshtchin, N.; Arab, S.; Hassannia, H.; Ajami, M.; Mirsanei, Z.; Habibi, S.; Masoumi, F.; et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J. Control. Release 2017, 246, 46–59. [Google Scholar] [CrossRef]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR study of montmorillonite-chitosan nanocomposite materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Metlay, J.P.; Crowley, M.T.; Witmer-Pack, M.; Steinman, R.M. Dendritic cells as antigen presenting cells in vivo. Int. Rev. Immunol. 1990, 6, 197–206. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [PubMed]
- Han, H.D.; Byeon, Y.; Jang, J.H.; Jeon, H.N.; Kim, G.H.; Kim, M.G.; Pack, C.G.; Kang, T.H.; Jung, I.D.; Lim, Y.T.; et al. In vivo stepwise immunomodulation using chitosan nanoparticles as a platform nanotechnology for cancer immunotherapy. Sci. Rep. 2016, 6, 38348. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Karimi, M.; Azadmanesh, K.; Naderi Manesh, H.; Hassan, Z.M.; Moazzeni, S.M. The effect of chitosan-tripolyphosphate nanoparticles on maturation and function of dendritic cells. Comp. Clin. Pathol. 2014, 23, 1421–1427. [Google Scholar] [CrossRef]
- Walter, F.; Winter, E.; Rahn, S.; Heidland, J.; Meier, S.; Struzek, A.M.; Lettau, M.; Philipp, L.M.; Beckinger, S.; Otto, L.; et al. Chitosan nanoparticles as antigen vehicles to induce effective tumor specific T cell responses. PLoS ONE 2020, 15, e0239369. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, S.; Lu, Z.; Chen, Z.; Chu, C.; Liu, A.; Xia, F.; Meng, S.; Guo, F.; Qiu, H.; et al. An optimized method for the induction and purification of mouse bone marrow dendritic cells. J. Immunol. Methods 2021, 495, 113073. [Google Scholar] [CrossRef]
- Jaksits, S.; Kriehuber, E.; Charbonnier, A.S.; Rappersberger, K.; Stingl, G.; Maurer, D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J. Immunol. 1999, 163, 4869–4877. [Google Scholar] [CrossRef]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rössner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Ali, S.; Audiger, C.; Autenrieth, S.E.; Berod, L.; Bigley, V.; Cyran, L.; Dalod, M.; Dörrie, J.; Dudziak, D.; et al. Guidelines for mouse and human DC generation. Eur. J. Immunol. 2023, 53, e2249816. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J. Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug-A Comprehensive Review. Pharmaceutics 2023, 15, 503. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Maroof, A.; Penny, M.; Kingston, R.; Murray, C.; Islam, S.; Bedford, P.A.; Knight, S.C. Interleukin-4 can induce interleukin-4 production in dendritic cells. Immunology 2006, 117, 271–279. [Google Scholar] [CrossRef]
- Gupta, U.; Hira, S.K.; Singh, R.; Paladhi, A.; Srivastava, P.; Pratim Manna, P. Essential role of TNF-α in gamma c cytokine aided crosstalk between dendritic cells and natural killer cells in experimental murine lymphoma. Int. Immunopharmacol. 2020, 78, 106031. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 2018, 3, 7. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Qiu, H.; Liu, J.; Qin, S.; Hou, Y.; Wang, C. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma Through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 317. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, D.; Zhou, X.; Xu, H.; Huang, Y.; Chu, C.; Shen, B. AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication. Pharmaceutics 2022, 14, 2147. [Google Scholar] [CrossRef]
- Fernández-Urrusuno, R.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Negi, J.S. Novel controlled ionic gelation strategy for chitosan nanoparticles preparation using TPP-β-CD inclusion complex. Eur. J. Pharm. Sci. 2018, 112, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ju, X.; Silveira, P.A.; Abadir, E.; Hsu, W.H.; Hart, D.N.J.; Clark, G.J. CD83: Activation Marker for Antigen Presenting Cells and Its Therapeutic Potential. Front. Immunol. 2019, 10, 1312. [Google Scholar] [CrossRef]
- Zhou, Z.; Wagar, N.; DeVos, J.R.; Rottinghaus, E.; Diallo, K.; Nguyen, D.B.; Bassey, O.; Ugbena, R.; Wadonda-Kabondo, N.; McConnell, M.S.; et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS ONE 2011, 6, e28184. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796. [Google Scholar] [CrossRef]
- von Rohrscheidt, J.; Petrozziello, E.; Nedjic, J.; Federle, C.; Krzyzak, L.; Ploegh, H.L.; Ishido, S.; Steinkasserer, A.; Klein, L. Thymic CD4 T cell selection requires attenuation of March8-mediated MHCII turnover in cortical epithelial cells through CD83. J. Exp. Med. 2016, 213, 1685–1694. [Google Scholar] [CrossRef]
- Tze, L.E.; Horikawa, K.; Domaschenz, H.; Howard, D.R.; Roots, C.M.; Rigby, R.J.; Way, D.A.; Ohmura-Hoshino, M.; Ishido, S.; Andoniou, C.E.; et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 2011, 208, 149–165. [Google Scholar] [CrossRef]
- Collin, M.; McGovern, N.; Haniffa, M. Human dendritic cell subsets. Immunology 2013, 140, 22–30. [Google Scholar] [CrossRef]
- McGovern, N.; Schlitzer, A.; Gunawan, M.; Jardine, L.; Shin, A.; Poyner, E.; Green, K.; Dickinson, R.; Wang, X.N.; Low, D.; et al. Human dermal CD14+ cells are a transient population of monocyte-derived macrophages. Immunity 2014, 41, 465–477. [Google Scholar] [CrossRef]
- Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the Epidemiological Features of Coronavirus Disease 2019 (COVID-19) Outbreak in the Republic of Korea from January 19 to March 2, 2020. J. Korean Med. Sci. 2020, 35, e112. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.; Germain, R.N. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu. Rev. Immunol. 2006, 24, 519–540. [Google Scholar] [CrossRef]




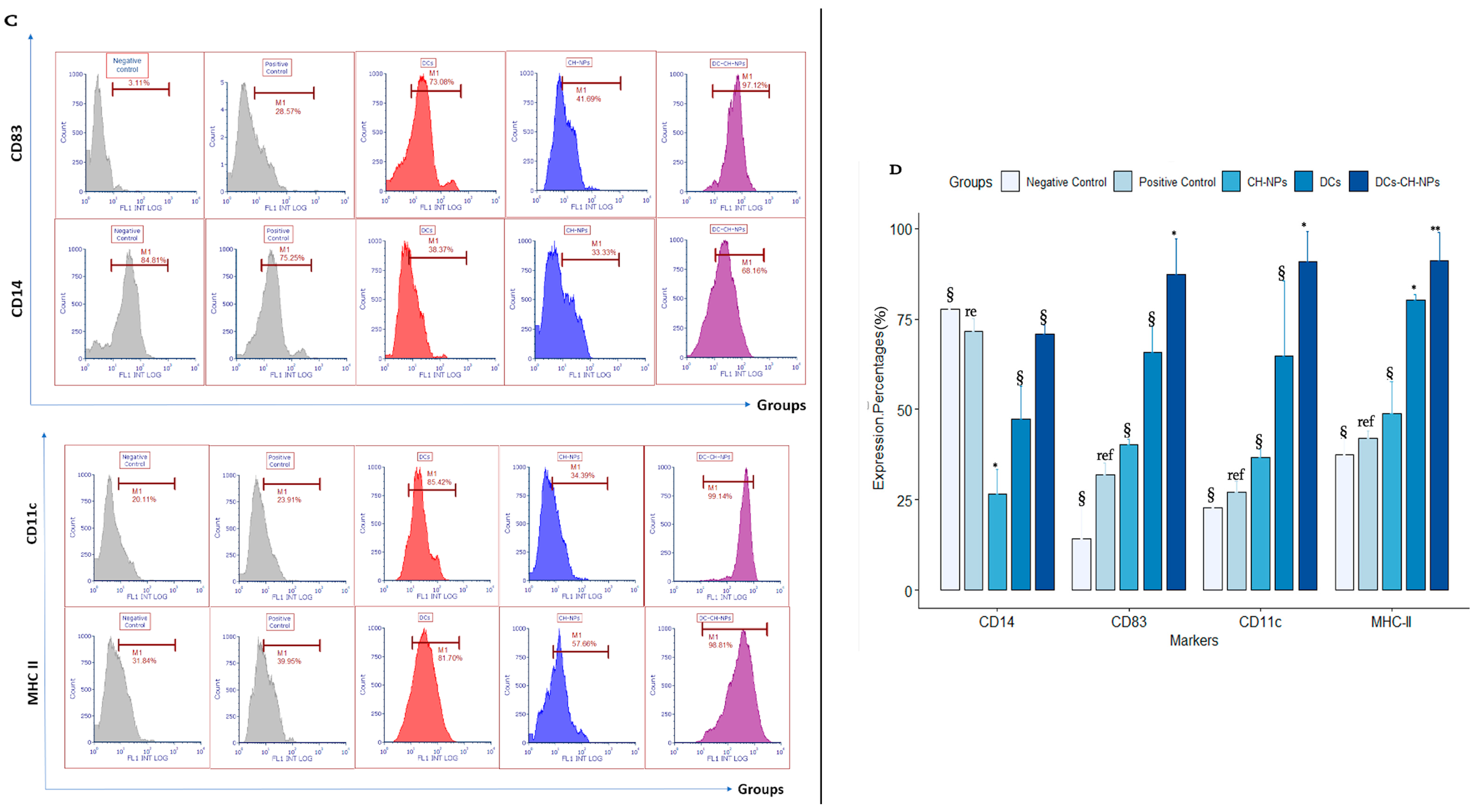

| Type of DCs | +LPS Treated DCs (MFI) | −LPS Treated DCs (MFI) | ap Value |
|---|---|---|---|
| Markers | |||
| CD14 | 181.9 ± 2.8 | 506.6 ± 4.7 | 0.001 *** |
| CD83 | 522.2 ± 3.6 | 125.9 ± 5.3 | 0.01 ** |
| CD80 | 235.3 ± 6.6 | 154.6 ± 3.2 | 0.001 *** |
| CD86 | 329.6 ± 4.7 | 222.9 ± 1.2 | 0.02 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrahimi, J.S.; Alotaibi, N.S.; Aldahlawi, A.M.; Basingab, F.S.; Zaher, K.A. Impact of Chitosan Nanoparticles-Coated Dendritic Cell-Based Vaccine as Cancer Immunotherapy. Vaccines 2025, 13, 474. https://doi.org/10.3390/vaccines13050474
Alrahimi JS, Alotaibi NS, Aldahlawi AM, Basingab FS, Zaher KA. Impact of Chitosan Nanoparticles-Coated Dendritic Cell-Based Vaccine as Cancer Immunotherapy. Vaccines. 2025; 13(5):474. https://doi.org/10.3390/vaccines13050474
Chicago/Turabian StyleAlrahimi, Jehan S., Najla S. Alotaibi, Alia M. Aldahlawi, Fatemah S. Basingab, and Kawther A. Zaher. 2025. "Impact of Chitosan Nanoparticles-Coated Dendritic Cell-Based Vaccine as Cancer Immunotherapy" Vaccines 13, no. 5: 474. https://doi.org/10.3390/vaccines13050474
APA StyleAlrahimi, J. S., Alotaibi, N. S., Aldahlawi, A. M., Basingab, F. S., & Zaher, K. A. (2025). Impact of Chitosan Nanoparticles-Coated Dendritic Cell-Based Vaccine as Cancer Immunotherapy. Vaccines, 13(5), 474. https://doi.org/10.3390/vaccines13050474





