Abstract
Background/Objectives: The clinical impact of replacing the 23-valent pneumococcal polysaccharide vaccine (PPSV23) for the vaccination of older (≥60 years) and at-risk German adults with either the 20-valent (PCV20) or 21-valent (V116) pneumococcal conjugate vaccine (PCV) was evaluated. Methods: An age- and serotype-specific transmission model was adapted to Germany to evaluate the impact of V116 versus PCV20 vaccination on pneumococcal disease (PD) incidence, including invasive pneumococcal disease (IPD) and inpatient and outpatient non-bacteremic pneumococcal pneumonia, over 10 years. A reference strategy (PPSV23 vaccination at a constant 30% vaccine coverage rate (VCR)) was compared against eight strategies varying by PCV (PCV20 vs. V116), VCR (30% vs. 60%), with or without the PCV revaccination of previously PPSV23-vaccinated adults (0% vs. 50% revaccination). Results: Vaccination with PCV20 and V116 initially decreased PD incidence, but incidence returned to pre-vaccine levels after five and eight years, respectively. Increasing the VCR to 60% prevented this resurgence. At a 10-year time horizon, V116 with 30% VCR reduced IPD cases by 9%, inpatient NBPP cases by 10%, and outpatient NBPP cases by 7% compared to the reference strategy. PCV20 with 30% VCR reduced these cases by 6%, 5%, and 4%, respectively. Increasing the VCR to 60% and revaccinating 50% of previously PPSV23-vaccinated adults further reduced IPD cases by 14% and 13% for V116, and by 9% and 9% for PCV20. Conclusions: Increasing the vaccination coverage rate to 60% and strategically revaccinating previously PPSV23-vaccinated adults significantly enhanced the effectiveness of pneumococcal vaccines, with V116 showing greater overall reductions in disease incidence compared to PCV20 or PPSV23.
1. Introduction
Pneumococcal diseases, caused by the bacterium Streptococcus pneumoniae, remain a significant public health concern in Germany, particularly affecting young children, the elderly, and individuals with underlying health conditions [1]. Approximately 100 different S. pneumoniae serotypes have been identified, and several vaccine formulations, including pneumococcal conjugate vaccines (PCVs) and pneumococcal polysaccharide vaccines (PPSVs), are available which target varying subsets of these serotypes [1,2].
The history of pneumococcal vaccines in Germany dates back to the early 20th century. Initial efforts involved whole-cell vaccines, which were eventually replaced by polysaccharide vaccines. The first pneumococcal polysaccharide vaccine (PPSV14) was licensed in 1977, covering 14 serotypes. This was followed by the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in 1983, which expanded coverage to 23 serotypes. However, these vaccines were less effective in young children and immunocompromised individuals [2,3].
The Standing Committee on Vaccination (STIKO) at the Robert Koch Institute recommended the introduction of PCV7 into the pediatric national immunization program (NIP) of Germany in July 2006 [4]. STIKO has since continuously updated its recommendations to include PCV10, PCV13, and PCV15 for the routine immunization of infants and young children. In 2025, PCV13 and PCV15 are recommended for routine PCV vaccination below 2 years of age in a 2 + 1 schedule for full-term infants and a 3 + 1 schedule for preterm infants. The pediatric vaccine coverage rate (VCR) in Germany for children up to 2 years of age who have completed the vaccination schedule was 74% as of 2023 [5]. For adults aged 60 years and older, STIKO has historically recommended the 23-valent pneumococcal polysaccharide vaccine (PPSV23) since 1998. Since September 2023, only PCV20 is recommended for adult vaccination [6]. Adult VCR remains low, with only 23.3% of adults aged 60–74 years having received the recommended pneumococcal vaccination as of 2022 [5].
The incidence of pneumococcal diseases (PD) in adults in Germany has shown notable trends over the years. From 2003 to 2006, prior to the inclusion of PCV7 in the NIP, the incidence of invasive pneumococcal disease (IPD) in adults aged 60 and older was 1.64 cases per 100,000 population. This incidence increased to 10.08 cases per 100,000 population in subsequent years [7]. From 2016 to 2019, the overall incidence rate of all-cause pneumonia (ACP) among individuals aged 16 years and older in Germany was 1345 per 100,000 person–years, while the incidence rate for IPD was 8.25 per 100,000 person–years [1]. These rates highlight the significant burden of PD among adults, particularly in older age groups and those with underlying health conditions [1]. Non-bacteremic pneumococcal pneumonia (NBPP), a form of pneumonia caused by S. pneumoniae without the presence of bacteria in the bloodstream, significantly contributes to the burden of pneumococcal diseases, and is another major health concern in Germany [8]. It is a common cause of community-acquired pneumonia (CAP) and affects both children and adults, with higher incidence rates in the elderly and individuals with comorbidities [9]. Non-bacteremic pneumococcal pneumonia (NBPP) is associated with significant morbidity and mortality, particularly among high-risk groups, and leads to substantial healthcare utilization, including both outpatient and inpatient services [10].
The incidence of IPD in German children declined from 2006 to 2019 following the introduction of PCV7 in the NIP [7]. Vaccine-type PD also declined in adults over this period; however, the overall burden of PD in this population increased, particularly in older adults [7]. From 2020 to 2021, IPD incidence declined with the implementation of non-pharmaceutical interventions employed during the SARS-CoV-2 pandemic; however, since that time, IPD incidence in Germany has returned to or exceeded pre-pandemic levels [11].
Pneumococcal vaccination recommendations continue to be evaluated in Germany to address the growing burden of NBPP and IPD, especially among older adults. A 20-valent pneumococcal conjugate vaccine (PCV20) was approved in the European Union (EU) in 2022, and has been recommended as the only adult vaccine since September 2023 in Germany; in addition, an adult-focused 21-valent PCV (V116) received a positive opinion from the EU’s Committee for Medicinal Products for Human Use (CHMP) in January 2025, and has been approved by the EU commission in late March 2025 [12]. This analysis evaluated the impact of PCV20 and V116 as a replacement for PPSV23 on the epidemiological burden of pneumococcal disease among older adults in Germany, by comparing these two scenarios as well as assessing the implications of increasing vaccination uptake rates among this population, in combination with the re-vaccination of individuals previously vaccinated with PPSV23.
2. Methods
We adapted a previously published age-stratified S-I-S ordinary differential equation dynamic transmission model (DTM) of pneumococcal carriage transmission to the German setting, by updating demographic and epidemiological inputs [10,13] (see Supplemental Figure S1). The DTM was stratified by age into six age strata: <2 years, 2–5 years, 5–18 years, 18–50 years, 50–60 years, and ≥60 years. Individuals in different age strata interacted based on mixing rates defined by an age-stratified contact matrix derived from published data for Germany [14]. The DTM was further stratified by grouping pneumococcal serotypes into 11 serotype classes (STCs), based on inclusion in various current, past, and future vaccines, including PCV7, PCV13, PCV15, PCV20, PCV21 (V116), and PPSV23 (Table 1). The classification is based on whether a serotype is targeted by an individual vaccine so that the assessment of each vaccine can be carried out by grouping some of the classes. For example, PPSV23 includes STCs 1–4 and STCs 6–9, while V116 includes STCs 3–10 with STC 10 representing the unique serotypes not in previous vaccines. Corresponding parameters were estimated according to the age and serotype grouping.

Table 1.
Grouping of pneumococcal serotypes into serotype classes, and visualization of inclusion of classes in vaccines included in the current and historical German NIP. ST = serotype; STC = serotype class.
Concurrent cocolonization with serotypes from up to three STCs was possible, based on vaccination status and inter-serotype competition [10,13]. Progression to pneumococcal disease (i.e., IPD, inpatient NBPP, and outpatient NBPP) was estimated by multiplying the number of colonization events by the disease-specific case-to-carrier ratio. The model estimated pneumococcal disease burden in Germany under varying future vaccination strategies over a 10-year time-horizon.
We estimated age- and serotype-specific transmission and competition coefficients (for competition between colonized and invading serotypes), disease-specific case-to-carrier ratios, and vaccine efficacies against carriage acquisition through model calibration to German historical time series data for the incidence of IPD and NBPP, and to regional (UK) pre-vaccine pneumococcal carriage data [15,16,17,18]. The calibration process involved multiple steps. First, the model was calibrated to pre-vaccine pneumococcal carriage and IPD data, followed by another calibration from the onset of the first vaccine introduction to the currently recommended vaccine (while ensuring that all vaccine policies through the years were fully captured). Once calibration to IPD incidence data was achieved, a secondary calibration was conducted to estimate the case-to-carrier ratios of NBPP (inpatient and outpatient) by fitting the model to NBPP data, respectively. Calibrated parameters and model fitting results are shown in Supplement Tables S3–S8 and Figures S2–S4. The model calibration was performed by minimizing the weighted sum of squared errors over annual age- and serotype-specific data utilizing the NMinimize function in Mathematica 14.1.
The target VCR for pediatric vaccination was assumed to remain constant at 85% over the entire time horizon, with an even split of PCV13 and PCV15 among children under two years old under the 2 + 1 vaccination schedule. Vaccine effectiveness (VE) against IPD and NBPP for pediatric vaccination varied by serotype and was based on published data [19,20,21,22,23]. For adult vaccination, VE against IPD for serotypes included in PCV13 was assumed to extend to covered serotypes in the V116 and PCV20 vaccines. VE estimates for covered serotypes were set to 75%, except for serotype 3, which was estimated at 26% [19,24]. For pediatric vaccination, VE against IPD for PPSV23 varied by serotype, based on published data [25,26]. Age-group specific VEs against IPD and NBPP are shown in Supplement Tables S9–S13. The average duration of protection for all PCV vaccines was assumed to be 10 years; the average duration of protection for PPSV23 was assumed to be 7.5 years [27,28]. Germany-specific input parameters are summarized in Table 2.

Table 2.
Germany-specific input parameter values for the demographic and transmission model components of the pneumococcal dynamic transmission model.
We evaluated the impact of three vaccines (PPSV23, V116, and PCV20) on the adult population aged 18+ years in Germany, while maintaining the status quo pediatric vaccination program across all vaccination scenarios and over the entire time horizon. For the reference scenario (scenario 0), we assumed that PPSV23 would continue at the current VCR level (30%). For all other scenarios, we assumed that PPSV23 would be replaced by either PCV20 or V116 for all adult vaccination moving forward. We varied adult vaccination along three dimensions (Table 3):

Table 3.
Adult vaccination scenarios evaluated over a 10-year time horizon.
- Vaccine formulation—replacement of PPSV23 (scenario 0) with either PCV20 (scenarios 1–4) or V116 (scenarios 5–8);
- Vaccine uptake—continuing at 30% uptake (scenarios 1, 2, 5, and 6), or increasing to 60% uptake (scenarios 3, 4, 7, and 8);
- Revaccination of adults previously vaccinated with PPSV23—no revaccination (scenarios 1, 3, 5, and 7), or revaccination at 50% uptake with the same vaccine formulation as for routine vaccination (scenarios 2, 4, 6, and 8).
3. Results
3.1. Invasive Pneumococcal Disease
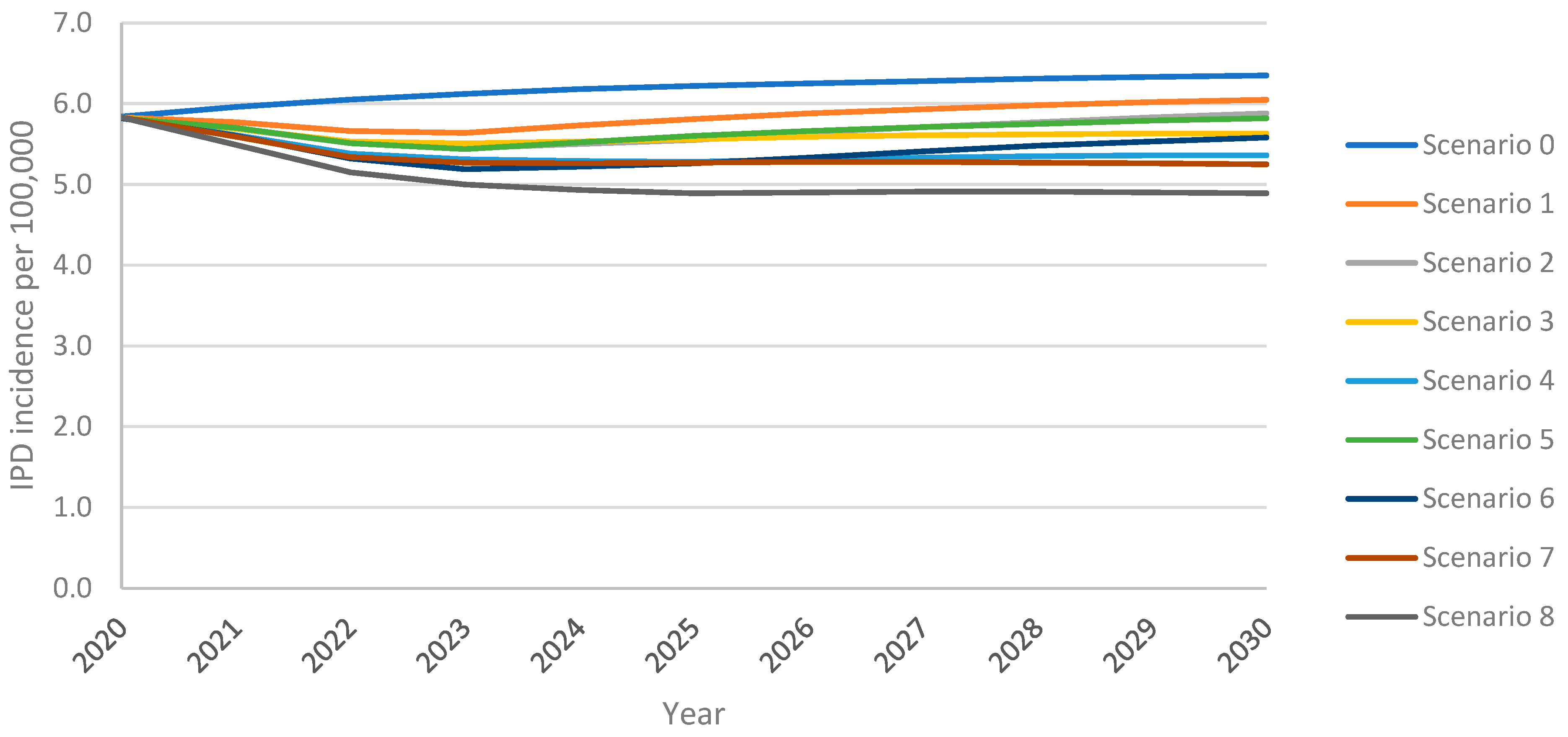
The model projected an increase in IPD incidence in adults 18+ years over the 10-year time horizon under all vaccination scenarios, with the greatest increase resulting from the continuation of PPSV23 at the uptake of 30% (scenario 0) (Figure 1). Following an initial decline in incidence from the 2020 incidence of 5.83 IPD cases per 100,000 population, PCV20 with 30% uptake (scenario 1) led to a subsequent rebound in IPD which exceeded the 2020 level within five years; with V116 with 30% uptake (scenario 5), this return in IPD was delayed by an additional four years (Figure 1). Increasing uptake (scenarios 3 and 7) eliminated the return in IPD. Replacing PPSV23 with PCV20 (scenario 1) or V116 (scenario 5) at 30% uptake led to 4% or 8% lower overall IPD incidence at the 10-year time horizon, respectively, when compared with maintaining PPSV23 at 30% uptake (i.e., reference strategy) (Supplement Figure S1). IPD incidence was 4–36% lower for all vaccination strategies for all age groups when compared with the reference strategy, with the greatest reduction resulting from scenario 8 (vaccination with V116 with 60% uptake and 50% revaccination) (Supplement Figure S5).
Figure 1.
Overall IPD incidence among adults 18+ years over the 10-year time horizon under varying vaccination scenarios.
Scenarios implementing V116 led to greater reductions in IPD incidence than equivalent scenarios implementing PCV20. At both 30% and 60% uptake, and with or without revaccination, the introduction of V116 resulted in fewer IPD cases among adults 18+ years than the introduction of PCV20, when compared with the reference strategy (scenario 0; PPSV23, 30% uptake, no revaccination) (Table 4). Vaccination with V116 led to 3.8–8.8% lower overall IPD annual incidence among adults aged 18+ years and 6.2–16.1% lower for adults aged 60+ years, at the 10-year time horizon when compared with equivalent scenarios utilizing PCV20, resulting in 5.3–11.3% fewer cumulative cases over the 10-year time horizon for adults 60+ years (Supplement Tables S14 and S15). Because V116 averted more cases than PCV20 in adults aged 60+, the proportion of cumulative IPD cases associated with 60+ was lower in V116 strategies than in PCV20 strategies (Table 5).

Table 4.
Impact of adult vaccination scenarios on incremental cumulative clinical outcomes in adults 18+ years over 10-year time horizon.

Table 5.
Impact of adult vaccination scenarios on cumulative IPD cases over 10-year time horizon (total cases).
As expected, for V116 scenarios, there were more cases of IPD caused by serotypes covered by PCV20 but not by V116 than there were under the equivalent PCV20 scenario. Conversely, for PCV20 scenarios, there were more cases of IPD caused by serotypes covered by V116 but not by PCV20 than there were under the equivalent V116 scenarios (Supplement Table S16). Nevertheless, V116 scenarios resulted in fewer cumulative IPD cases overall and for all adults 18+ years.
Increasing uptake to 60% reduced overall IPD incidence among adults 18+ years by 7.0–12.4% and incidence in adults 60+ years by 11.5–21.8%, leading to a 5.4–9.9% reduction in cumulative cases in this age group when compared to equivalent scenarios with 30% uptake (Supplement Tables S14 and S15). The revaccination of individuals previously vaccinated with PPSV23 led to 5.4–9.9% fewer cumulative IPD cases in adults 60+ over the time horizon. The relative performance of V116 versus PCV20 was not impacted by either increasing VCR and/or revaccination, i.e., overall IPD cases were consistently lower for V116 scenarios than PCV20 (Supplement Table S15).
3.2. Non-Bacteremic Pneumococcal Pneumonia—Inpatient Cases
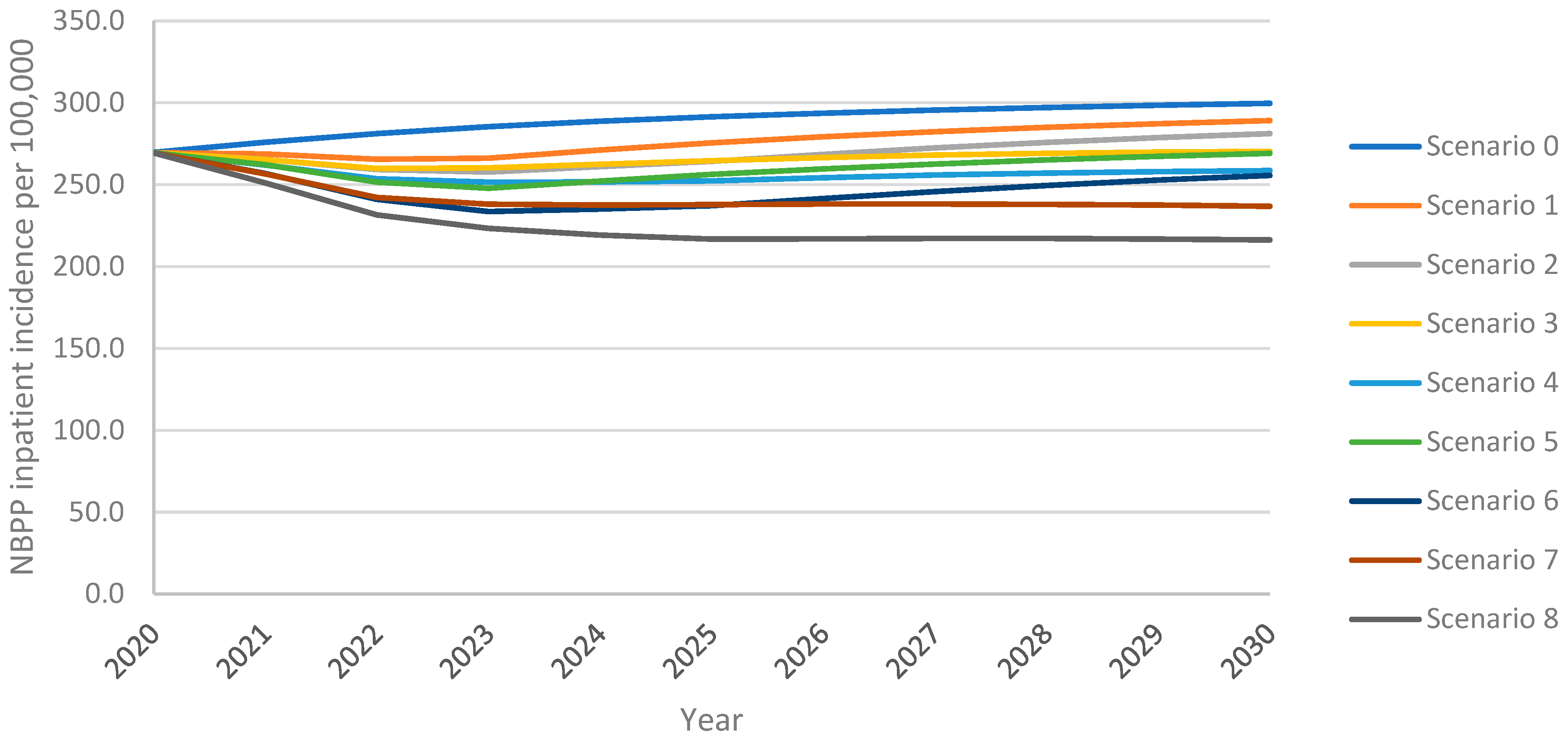
As observed with IPD incidence, the model projected an increase in inpatient NBPP incidence among adults aged 18+ years over the 10-year time horizon under all vaccination scenarios, with the greatest increase from the continued use of PPSV23 at 30% VCR (scenario 0) (Figure 2). As with IPD, inpatient NBPP incidence declined initially from the 2020 level of 269.4 NBPP inpatient cases per 100,0000 population, followed by an increase above 2020 levels within four years for PCV20 at 30% uptake (scenario 1); for V116 at 30% uptake (scenario 5) inpatient NBPP incidence had not yet returned to 2020 levels by the end of the 10-year time horizon. Increasing uptake with PCV20 or V116 (scenarios 3 and 7, respectively) prevented a rebound in incidence. Replacing PPSV23 with PCV20 with 30% uptake (scenario 1) led to 4% lower overall inpatient NBPP incidence at the 10-year time horizon, with 5% lower incidence in adults 60+ years, when compared with maintaining the reference strategy. In contrast, replacement with V116 (scenario 5) led to 10% lower overall inpatient NBPP incidence, 6% and 5% lower in 18–50- and 50–60-year-olds, respectively, and 14% lower in adults 60+. Inpatient NBPP incidence was 4–40% lower than the reference strategy for all vaccination strategies for all age groups, with the lowest incidence occurring in scenario 8 (vaccination with V116 with 60% uptake and 50% revaccination) (Supplement Figure S6).
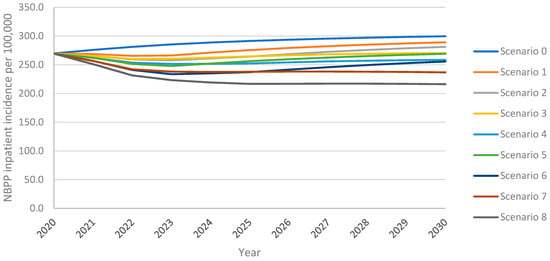
Figure 2.
Overall inpatient NBPP incidence in adults aged 18+ years over the 10-year time horizon under varying vaccination scenarios.
As with IPD, scenarios that implemented V116 led to lower inpatient NBPP incidence than equivalent scenarios implementing PCV20. As with cumulative adult IPD cases, at both 30% and 60% uptake, both with and without revaccination, the implementation of V116 prevented a greater number of inpatient NBPP cases among adults than the implementation of PCV20 when compared with the reference strategy (scenario 0) (Table 4). Vaccination with V116 led to 6.9–16.3% lower overall inpatient NBPP incidence, 10.1–25.3% lower for adults aged 60+ years, at the 10-year time horizon when compared with equivalent scenarios utilizing PCV20, resulting in 8.9–18.4% fewer cumulative inpatient NBPP cases over the time horizon for adults aged 60+ years (Supplement Tables S17 and S18). As seen with IPD, because V116 averted more inpatient NBPP cases than PCV20 in adults aged 60+, the proportion of cumulative inpatient NBPP cases associated with 60+ was lower in V116 strategies than in PCV20 strategies (Table 6).

Table 6.
Impact of adult vaccination scenarios on cumulative inpatient NBPP cases over 10-year time horizon (total cases).
As expected, and as observed with IPD cases, for V116 scenarios, there were more inpatient NBPP cases caused by serotypes covered by PCV20 but not V116, than there were under the equivalent PCV20 scenarios. Conversely, for PCV20 scenarios, there were more inpatient NBPP cases caused by serotypes covered by V116 but not PCV20, than there were under the equivalent V116 scenarios (Supplement Table S19). Nevertheless, V116 scenarios resulted in fewer cumulative inpatient NBPP cases (independent of serotype) overall and for all adults aged 18+ years.
Increasing VCR from 30% to 60% led to 6.5–15.4% lower overall inpatient NBPP incidence 9.4–23.9% lower in adults aged 60+ years, leading to 5.5–12.9% fewer cumulative cases in this age group (Supplement Tables S17 and S18). The revaccination of individuals previously vaccinated with PPSV23 led to 4.3–10.7% fewer cumulative inpatient NBPP cases in adults aged 60+ over the time horizon. As seen with IPD cases, the relative performance of V116 versus PCV20 was not impacted by either increasing VCR and/or revaccination, i.e., overall inpatient NBPP cases were consistently lower for V116 scenarios than PCV20. (Supplement Table S18).
3.3. Non-Bacteremic Pneumococcal Pneumonia—Outpatient Cases
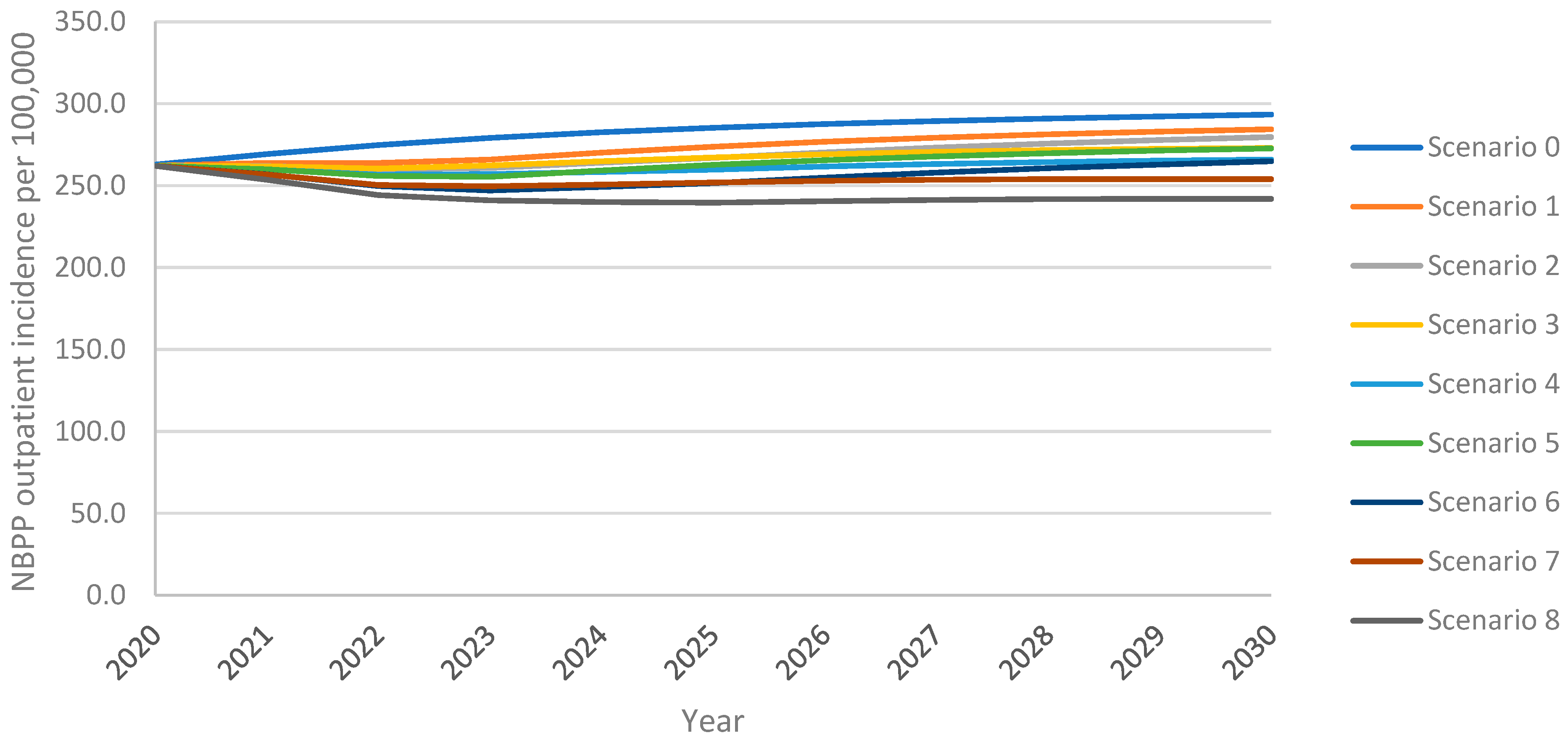
As above, the model projected an increase in overall outpatient NBPP incidence in adults aged 18+ years over the 10-year time horizon under all vaccination scenarios, with the greatest increase resulting from the continuation of PPSV23 at 30% VCR (scenario 0) (Figure 3). For PCV20 (scenario 1), outpatient NBPP incidence remained above the 2020 level 262.3 NBPP outpatient cases per 100,000 population for the entire time horizon; for V116 (scenario 5), incidence dropped initially, then increased, surpassing 2020 levels five years after introduction. Increasing uptake to 60% for PCV20 (scenario 3) delayed this rebound to 2023, whereas increasing uptake for V116 (scenario 7) prevented the rebound for the entirety of the time horizon. Replacing PPSV23 with PCV20 (scenario 1) led to a 3% lower overall outpatient NBPP incidence at the 10-year time horizon, 5% lower in adults aged 60+ years, when compared with the reference strategy. In contrast, replacement with V116 (scenario 5) led to 7% lower overall outpatient NBPP incidence, 6% and 5% lower in 18–50- and 50–60-year-olds, respectively, and 14% lower in adults aged 60+ years (Supplement Figure S7).
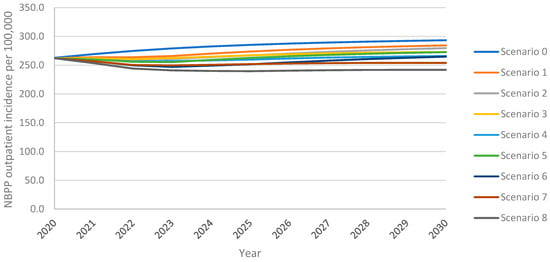
Figure 3.
Overall outpatient NBPP incidence in adults aged 18+ years over the 10-year time horizon under varying vaccination scenarios.
As with cumulative adult IPD cases and cumulative adult inpatient NBPP cases, at both 30% and 60% VCR, and both with and without revaccination, the implementation of V116 prevented a greater number of outpatient NBPP cases among adults over the 10-year time horizon than did the implementation of PCV20, when compared with the reference strategy (scenario 0) (Table 4).
Vaccination with V116 led to 4.1–9.0% lower overall outpatient NBPP incidence, 10.1–25.3% lower for adults aged 60+ years, at the 10-year time horizon when compared with equivalent scenarios utilizing PCV20, resulting in 8.9–18.4% fewer cumulative outpatient NBPP cases over the time horizon for adults aged 60+ years (Supplement Tables S20 and S21). As seen with IPD and inpatient NBPP, because V116 averted more outpatient NBPP cases than PCV20 in adults aged 60+ years, the proportion of cumulative outpatient NBPP cases associated with adults aged 60+ years was lower in V116 strategies than in PCV20 strategies (Table 7).

Table 7.
Impact of adult vaccination scenarios on cumulative outpatient NBPP cases over 10-year time horizon (total cases).
As expected, and as observed for both IPD and inpatient NBPP cases, for V116 scenarios, there were more outpatient cases of NBPP caused by serotypes covered by PCV20, but not V116, than there were under the equivalent PCV20 scenarios. Conversely, for PCV20 scenarios, there were more outpatient cases of NBPP caused by serotypes covered by V116 but not PCV20, than there were under the equivalent V116 scenarios (Supplement Table S22). Nevertheless, V116 scenarios resulted in fewer cumulative outpatient NBPP cases (independent of serotype) overall and for all adults aged 18+ years.
Increasing the uptake from 30% to 60% reduced the overall outpatient NBPP incidence in adults aged 18+ years by 4.0–8.6% and the incidence in adults aged 60+ years by 9.4–23.9%, leading to a 5.5–12.9% reduction in cumulative cases in this age group (Supplement Tables S20 and S21). The revaccination of individuals previously vaccinated with PPSV23 led to 4.3–10.7% fewer cumulative outpatient NBPP cases in adults aged 60+ years over the time horizon. As seen with IPD and inpatient NBPP cases, the relative performance of V116 versus PCV20 was not impacted by either increasing VCR and/or revaccination, i.e., overall outpatient NBPP cases were consistently lower for V116 scenarios than PCV20, with greater reductions resulting from vaccination with V116 (Supplementary Table S21).
4. Discussion
At present, the uptake of pneumococcal vaccine among adults aged 60+ years is low, with approximately 30% of older adults having received PPSV23 vaccination. With the availability of multiple adult pneumococcal vaccines in Germany, there is an opportunity to assess the adult vaccination program by evaluating different vaccines, coverage rates, and revaccination. In this analysis, we adapted a dynamic model for the transmission of S. pneumoniae carriage to the German setting and evaluated eight potential adult vaccination strategies varying vaccine type, uptake rate, and revaccination rate [10,13].
Pneumococcal disease due to IPD and NBPP was projected to increase in all adults aged 18+ years in Germany over a 10-year time horizon under the continuation of PPSV23. When PPSV23 was replaced by either V116 or PCV20, IPD and NBPP inpatient and outpatient incidence in this age group were projected to increase at a significantly slower rate than under the reference strategy of continuation of PPSV23. IPD and NBPP incidence among adults 60+ years were projected to be lower at the 10-year time horizon with the implementation of V116 versus PCV20.
Increasing VCR from 30% to 60% was projected to lead to a noticeable reduction in pneumococcal disease over the time horizon. Scenarios with 60% uptake (scenarios 3 and 7) were associated with fewer cumulative IPD and NBPP cases in the overall German population compared with 30% uptake scenarios, and noticeably fewer cases in the target population of adults 60+ years. These clinical improvements were greater when V116 was implemented, rather than PCV20.
The greatest projected overall impact on clinical outcomes resulted from the simultaneous implementation of increasing uptake from 30% to 60% and revaccinating previously PPSV23-vaccinated individuals (scenarios 4 and 8). The combination of increased uptake and revaccination was projected to lead to the lowest cumulative IPD and inpatient and outpatient NBPP cases over all age groups by the end of the 10-year time horizon. Scenario 8, which included V116 adult vaccination and V116 revaccination of PPSV23-vaccinated individuals, led to the greatest improvements in clinical outcomes across all scenarios.
The model projected that increasing adult VCRs and using V116 versus PCV20 would noticeably reduce the incidence of IPD and NBPP in Germany, and that revaccinating individuals previously vaccinated with PPSV23 would further reduce pneumococcal disease burden.
This analysis was subject to several limitations. First, the DTM assumed a static population which did not account for changes in the population age distribution over time. Given Germany’s aging population, this assumption may lead to an underestimation of the impacts of varying vaccination strategies among older adults [33]. In addition, due to the grouping of serotypes into STCs, the DTM is not capable of disaggregating single serotypes for discussion and prevents a detailed analysis of serotypes which may lead to higher disease in specific subpopulations. The incorporation of this capability into future versions of the DTM is planned. Further, non-pharmaceutical interventions implemented in Germany during the SARS-CoV-2 pandemic, which had a noticeable influence on pneumococcal disease, were not accounted for in this model, as the most recent year in the calibration dataset was 2019. Nevertheless, published data show that pneumococcal incidence rapidly returned to pre-pandemic levels following the cessation of these interventions [11].
Another limitation of this analysis lies in the use of UK-specific pneumococcal carriage data as a proxy for Germany-specific pre-PCV carriage data. Due to the unavailability of Germany-specific pneumococcal carriage data, reliance on UK data was necessary to complete the model calibration and derive meaningful insights from the model projections. However, differences in demographic, epidemiological, environmental, and healthcare factors between Germany and the UK could influence pneumococcal carriage patterns, rendering the proxy an imperfect representation of the German population. These factors may limit the generalizability of the findings to Germany-specific contexts and should be taken into consideration when interpreting the results. Future studies would benefit from concerted efforts to collect and analyze Germany-specific pneumococcal carriage data to improve accuracy and contextual relevance. Despite these limitations, this study serves as an important stepping-stone in addressing the broader research question and highlights areas requiring further investigation.
Finally, the historical IPD and NBPP incidence data utilized in model calibration were collected over a long time period, and included variations in reporting rate over time, which in turn may impact model outcomes.
5. Conclusions
Though the uptake of pneumococcal vaccine among adults 60+ years in Germany is currently low, the vaccination of older adults with V116 and implementing strategies to increase vaccine uptake and revaccinate previously vaccinated individuals was predicted to lead to substantial reductions in pneumococcal disease burden over a 10-year time horizon. Policymakers should consider taking advantage of the opportunity to reevaluate the adult vaccination program in alignment with these strategies to reduce the disease burden of S. pneumoniae in Germany.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines13050475/s1, Supplementary Materials: Model Equations, Model Calibration, Vaccine Efficacy, Additional Model Results. Reference [34] is cited in the Supplementary Materials.
Author Contributions
O.S., T.M.M., K.M.B., R.J.O. and M.d.L. led the study concept and design. O.S., T.M.M., K.M.B., R.J.O., M.d.L., S.M.-S., T.R. and C.S. supported model inputs, data analysis, and model adaptations. O.S., T.M.M., K.M.B., R.J.O. and G.M. supported model calibration. All authors contributed to the interpretation of results and in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Ltd., Inc., Rahway, NJ, USA. The funder provided support in the form of salaries or consulting fees for authors.
Institutional Review Board Statement
This study did not involve human participants, and thus ethical approval does not apply.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. This is a modeling study and, therefore, no primary data were collected in this study. All inputs were from published literature and included only anonymized data.
Acknowledgments
We would like to thank Colleen Burgess for her assistance in the preparation of this manuscript, and John Lang for his detailed review.
Conflicts of Interest
O.S., T.M.M., K.M.B. and R.J.O. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Ltd., Inc., Rahway, NJ, USA. M.d.L., S.M.-S., T.R. and C.S. are employees of MSD subsidiaries of Merck & Co., Ltd., Inc., Rahway, NJ, USA. G.M. is an employee of Wolfram Research Inc. under contract to Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Ltd., Inc., Rahway, NJ, USA. O.S., M.d.L., S.M.-S., T.R., C.S., T.M.M., K.M.B. and R.J.O. may hold stock or stock options in Merck & Co., Ltd., Inc., Rahway, NJ, USA.
References
- Deb, A.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Haeckl, D.; Mihm, S.; Johnson, K.D.; Weiss, T. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol. Infect. 2022, 150, e204. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- US Centers for Disease Control and Prevention. Pneumococcal Disease. Available online: https://www.cdc.gov/pneumococcal/vaccines/types.html (accessed on 4 January 2025).
- Htar, M.T.T.; Christopoulou, D.; Schmitt, H.J. Pneumococcal serotype evolution in Western Europe. BMC Infect. Dis. 2015, 15, 419. [Google Scholar] [CrossRef]
- Rieck, T.; Steffen, A.; Feig, M.; Rau, C. Impfquoten in Deutschland–aktuelle Ergebnisse aus dem RKI-Impfquotenmonitoring. Epidemiol. Bull. 2024, 50, 3–10. [Google Scholar] [CrossRef]
- Schlaberg, J.; Vygen-Bonnet, S.; Falman, A.; Wilhelm, J.; Hummers, E.; von Kries, R.; Ledig, T.; Bogdan, C. Aktualisierung der Empfehlungen der STIKO zur Standardimpfung von Personen ≥ 60 Jahre Sowie zur Indikationsimpfung von Risikogruppen Gegen Pneumokokken und Die Dazugehörige Wissenschaftliche Begründung. 2023. Available online: https://edoc.rki.de/handle/176904/11307.2 (accessed on 17 March 2025).
- van der Linden, M.; Imöhl, M.; Perniciaro, S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS ONE 2019, 14, e0220453. [Google Scholar] [CrossRef]
- Kuchenbecker, U.; Chase, D.; Reichert, A.; Schiffner-Rohe, J.; Atwood, M. Estimating the cost-effectiveness of a sequential pneumococcal vaccination program for adults in Germany. PLoS ONE 2018, 13, e0197905. [Google Scholar] [CrossRef]
- Perniciaro, S.; van der Linden, M. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: A retrospective cohort study. Lancet Reg. Health–Eur. 2021, 7, 100126. [Google Scholar] [CrossRef]
- Malik, T.M.; Bakker, K.M.; Oidtman, R.J.; Sharomi, O.; Meleleo, G.; Nachbar, R.B.; Elbasha, E.H. A dynamic transmission model for assessing the impact of pneumococcal vaccination in the United States. PLoS ONE 2025, 20, e0305892. [Google Scholar] [CrossRef]
- Perniciaro, S.; van der Linden, M.; Weinberger, D.M. Reemergence of invasive pneumococcal disease in Germany during the spring and summer of 2021. Clin. Infect. Dis. 2022, 75, 1149–1153. [Google Scholar] [CrossRef]
- Kellaher, C. Merck Gets European Commission Approval of Capvaxive Vaccine. Available online: https://www.morningstar.com/news/dow-jones/202503263462/merck-gets-european-commission-approval-of-capvaxive-vaccine (accessed on 26 March 2025).
- Oidtman, R.J.; Meleleo, G.J.; Sharomi, O.; Matthews, I.; Ntais, D.; Nachbar, R.; Malik, T.; Bakker, K.M. Modeling the epidemiological impact of different adult pneumococcal vaccination strategies in the United Kingdom. Infect. Dis. Ther. 2025, 14, 587–602. [Google Scholar] [CrossRef]
- Prem, K.; Cook, A.R.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef] [PubMed]
- Benfield, T.; Skovgaard, M.; Schønheyder, H.C.; Knudsen, J.D.; Bangsborg, J.; Østergaard, C.; Slotved, H.-C.; Konradsen, H.B.; Thomsen, R.W.; Lambertsen, L. Serotype distribution in non-bacteremic pneumococcal pneumonia: Association with disease severity and implications for pneumococcal conjugate vaccines. PLoS ONE 2013, 8, e72743. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Ewig, S.; Rohde, G.; Schuette, H.; Rupp, J.; Welte, T.; Suttorp, N.; Forstner, C.; Group, C.S. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine 2016, 34, 2342–2348. [Google Scholar] [CrossRef]
- van der Linden, M. Internal data on an ongoing IPD-surveillance in Germany, data on file. 2024. [Google Scholar]
- Cleary, D.W.; Jones, J.; Gladstone, R.A.; Osman, K.L.; Devine, V.T.; Jefferies, J.M.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Changes in serotype prevalence of Streptococcus pneumoniae in Southampton, UK between 2006 and 2018. Sci. Rep. 2022, 12, 13332. [Google Scholar] [CrossRef]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir. Med. 2016, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Givon-Lavi, N.; Dagan, R. Effectiveness of pneumococcal conjugate vaccines against community-acquired alveolar pneumonia attributable to vaccine-serotype Streptococcus pneumoniae among children. Clin. Infect. Dis. 2021, 73, e1423–e1433. [Google Scholar] [CrossRef] [PubMed]
- Ryman, J.; Weaver, J.; Yee, K.L.; Sachs, J.R. Predicting effectiveness of the V114 vaccine against invasive pneumococcal disease in children. Expert Rev. Vaccines 2022, 21, 1515–1521. [Google Scholar] [CrossRef]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; George, R.C.; Slack, M.P.; Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012, 30, 6802–6808. [Google Scholar] [CrossRef]
- Suzuki, M.; Dhoubhadel, B.G.; Ishifuji, T.; Yasunami, M.; Yaegashi, M.; Asoh, N.; Ishida, M.; Hamaguchi, S.; Aoshima, M.; Ariyoshi, K. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: A multicentre, prospective, test-negative design study. Lancet Infect. Dis. 2017, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Leidner, A.J. Summary of Three Economic Models Assessing Pneumococcal Vaccines in US Adults. 2021. Available online: https://stacks.cdc.gov/view/cdc/110717/cdc_110717_DS1.pdf (accessed on 17 March 2025).
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M. Vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 373, 93. [Google Scholar] [PubMed]
- Federal Statistic Bureau of Germany. Births. 2022. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Geburten/_inhalt.html#234040 (accessed on 4 December 2024).
- Federal Statistic Bureau of Germany. Deaths, Excluding Stillbirths, Subsequently Registered War Deaths, and Legal Declarations of Death. 2022. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Sterbefaelle-Lebenserwartung/_inhalt.html#234182 (accessed on 4 December 2024).
- De Miguel, S.; Latasa, P.; Yuste, J.; García, L.; Ordobás, M.; Ramos, B.; Pérez, M.; Ortiz, M.A.; Sanz, J.C. Age-dependent serotype-associated case-fatality rate in invasive pneumococcal disease in the autonomous community of madrid between 2007 and 2020. Microorganisms 2021, 9, 2286. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Heckl, D.; Boellisnger, T.; Weaver, J. Healthcare resource utilization and cost of pneumococcal disease in children in Germany, 2014–2019: A retrospective cohort study. Pneumonia 2023, 15, 7. [Google Scholar] [CrossRef]
- Destatis Statistisches Bundesamt. 4 Million More People Aged 67 or over Will Live in Germany in 2035. 2022. Available online: https://www.destatis.de/EN/Press/2022/12/PE22_511_124.html (accessed on 17 March 2025).
- Hethcote, H.W. The mathematics of infectious diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).