Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle
Abstract
1. Introduction
2. Considerations for mRNA Product Design—The Beginning of the Lifecycle
Applications of Artificial Intelligence (AI) and Machine Learning
3. Clinical Trials of mRNA Products
Challenges with Emerging and Tropical Diseases
4. CMC Lifecycle Considerations in mRNA Product Manufacturing and Deployment
4.1. Manufacturing and Supply Chain Considerations
4.2. Increasing the Stability of mRNA-LNP Vaccines and Therapeutics
5. Regulatory, Lifecycle and Deployment Considerations of mRNA Therapeutics
5.1. Rare Genetic Diseases
5.2. New Approaches Will Be Needed to Keep Pace with Therapeutics Development for the Large Number of Rare Diseases
5.3. Lifecycle Considerations for mRNA Rare Disease Therapies
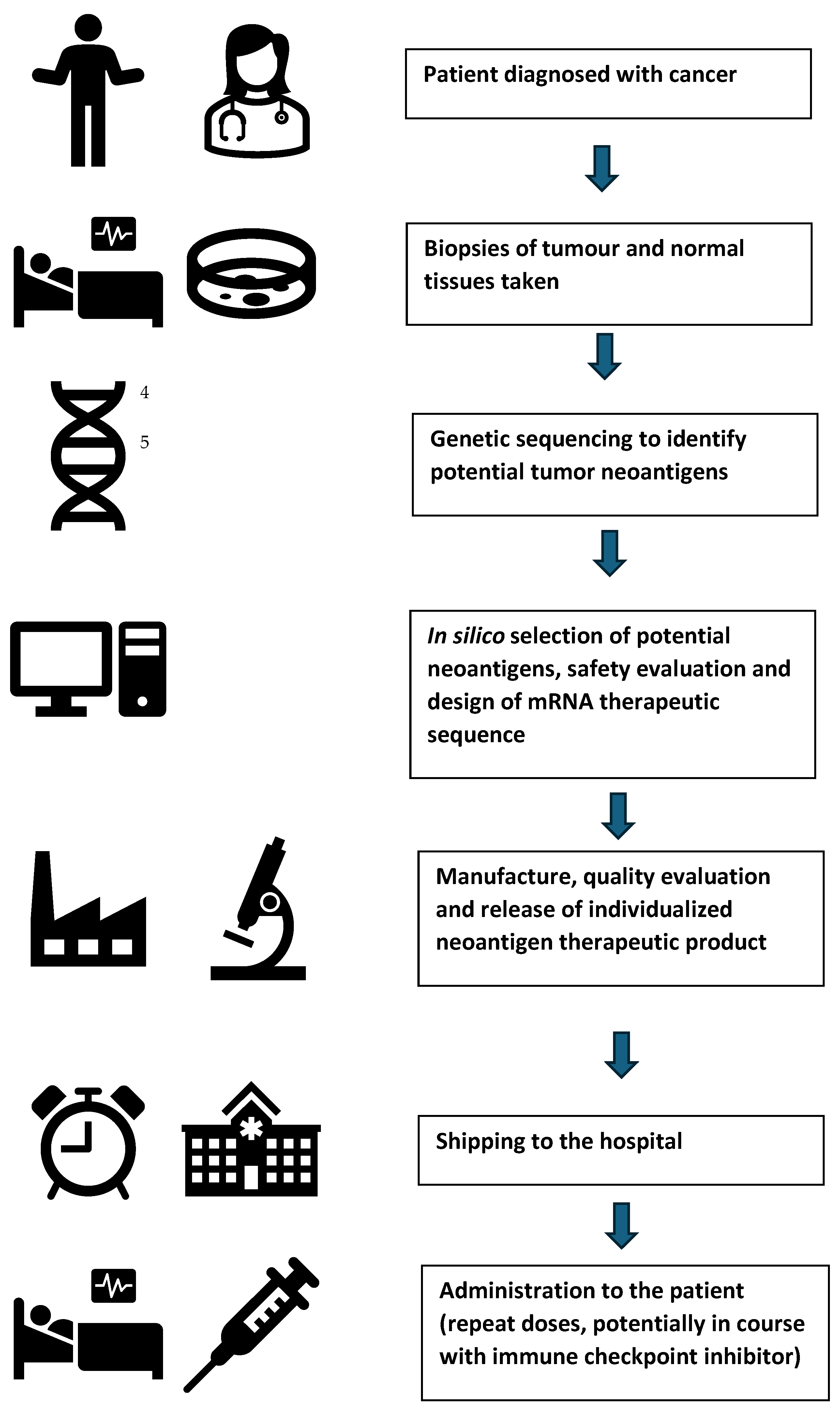
5.4. Oncology—Individualized Neoantigen Therapies (INTs)
- (1)
- Simultaneous delivery of multiple tumor antigens, reducing the risk of resistances or antigen loss or change;
- (2)
- Full-length antigens can be encoded if required, enabling multiple epitopes to be presented;
- (3)
- Induction of a broad T-cell response;
- (4)
- Manufacture is rapid and scalable compared with some of the other approaches.
5.5. Regulatory Considerations for Individualized Neoantigen Therapies (INTs)
- (1)
- Ensuring that the biopsy of the tumor tissue taken for sequencing is representative;
- (2)
- Justification of the algorithms used to select neoantigen peptides;
- (3)
- Assessment of controls over automated parallel manufacturing processes;
- (4)
- Establishing the consistency of a test product across multiple batches based on agreed representative quality attributes;
- (5)
- Using pooled stability data from multiple batches.
6. Emerging Regulatory Trends and Issues
6.1. Need for Greater International Alignment in Regulatory Pathways
6.2. Differing International Approaches to the Regulatory Application of Platform Technology
- (1)
- Starting materials: including DNA plasmids, enzymes, cell banks for expression systems;
- (2)
- mRNAs that encode antigens of interest;
- (3)
- mRNA-LNP control and testing of LNP size and mRNA encapsulation;
- (4)
- Unit operations throughout the manufacturing process;
- (5)
- Analytical techniques throughout the manufacturing process—identity, quantity, purity, integrity, characterization, potency and safety (contamination);
- (6)
- Approaches to the validation of processes and methods used for manufacture and analysis;
- (7)
- Understanding of the degradation pathways and metabolism of mRNA and LNP components in consideration of non-clinical assessment and determination of shelf life;
- (8)
- Clinical experience justifying specification limits for certain shared attributes between products, such as particle size and product-related impurities.
6.3. mRNA Platform Master Files
6.4. mRNA Vaccine Laboratory Lot or Batch Release
6.5. Critical Requirements for International Regulatory Alignment
- The need to exclude all conventional (non-self-amplifying) mRNA-LNP products from the definition of “gene therapy medicinal product” in the EU [127]. The definition should not encompass products that edit or alter the human genome;
- Agreement on a wider, more flexible definition of platform technologies;
- Prompt publication of detailed regulatory guidance on platform technology requirements, especially relating to chemistry, manufacturing and controls;
- Establishment of platform technology master file option for human mRNA products;
- Consensus on the definition of different mRNA-LNP vaccine components as starting materials or excipients [130] and of mRNA-LNP as drug substance or drug product;
- International consensus on lot release requirements for mRNA vaccines.
7. mRNA Product Safety
7.1. mRNA Vaccine Reactogenicity
7.2. Adverse Events of Special Interest
7.3. Attribution of Adverse Events
- (1)
- Bell’s palsy (temporary one-sided facial drooping);
- (2)
- Swelling of the face;
- (3)
- Severe allergic reaction;
- (4)
- Extensive swelling of the vaccinated limb;
- (5)
- Swelling of the face in patients who have had facial dermatological fillers;
- (6)
- A skin reaction (erythema multiforme);
- (7)
- Unusual (paraesthesia) or decreased sensation in the skin (hypoaesthesia);
- (8)
- Heavy menstrual bleeding.
7.4. Potential for Adverse Events with Therapeutic mRNA Products
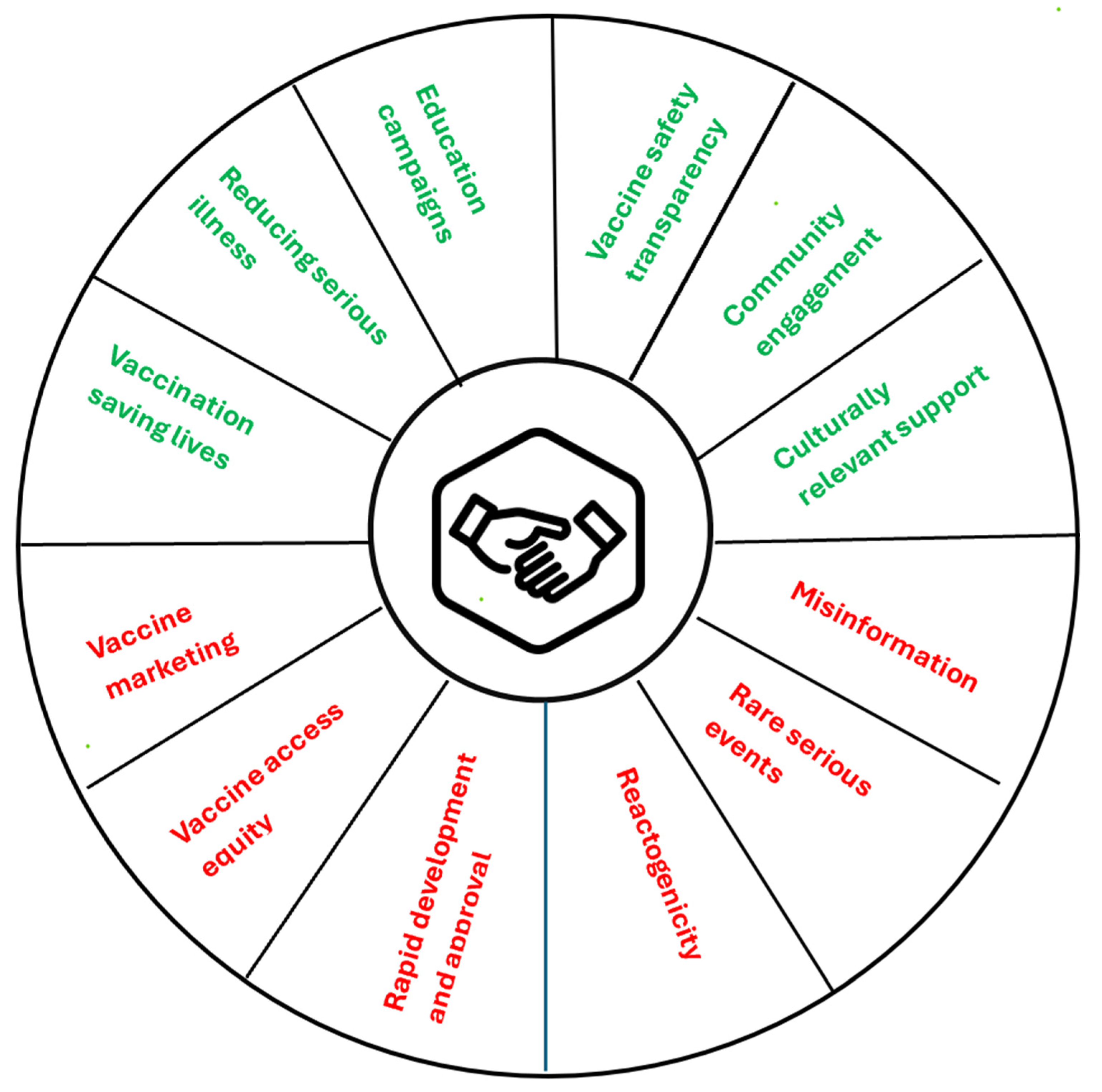
8. Social License to Operate for mRNA Products
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skerritt, J.H.; Tucek-Szabo, C.; Sutton, B.; Nolan, T. The Platform Technology Approach to mRNA Product Development and Regulation. Vaccines 2024, 12, 528. [Google Scholar] [CrossRef]
- Whitley, J.; Zwolinski, C.; Denis, C.; Maughan, M.; Hayles, L.; Clarke, D.; Snare, M.; Liao, H.; Shiou, S.; Marmura, T.; et al. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl. Res. 2021, 242, 38–55. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Harmonised Guideline Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (Q12) November 2019. Available online: https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf (accessed on 10 January 2025).
- Androsavich, J.R. Frameworks for the transformational breakthroughs in RNA-based medicines. Nat. Rev. Drug Discov. 2024, 23, 412–444. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wu, Y.; Lian, J. Circular RNA vaccine in disease prevention and treatment. Sig. Transduct. Target. Ther. 2023, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Shi, L.; Sheng, T.; Yan, X.; Lin, L.; Meng, C.; Wu, S.; Chen, Y.; Zhang, Y.; Wang, C.; et al. Reformulating lipid nanoparticles for organ-targeted mRNA accumulation and translation. Nat. Commun. 2024, 15, 5659. [Google Scholar] [CrossRef]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stosky-Oterin, L.; Peer, D. Targeting cancer with mRNA-lipid nanoparticles: Key considerations and future prospects. Nat. Rev. Clin. Oncol. 2023, 20, 739–0754. [Google Scholar] [CrossRef]
- Cullis, P.R.; Feigner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Kang, D.D.; Li, H.; Dong, Y. Advancements of in vitro transcribed mRNA (IVT mRNA) to enable translation into the clinics. Adv. Drug Deliv. Rev. 2023, 199, 114961. [Google Scholar] [CrossRef]
- Metkar, M.; Pepin, C.S.; Moore, M.J. Tailor made: The art of therapeutic mRNA design. Nat. Rev. Drug Discov. 2024, 23, 67–83. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Wei, H.-H.; Zheng, L.; Wang, Z. mRNA therapeutics: New vaccination and beyond. Fundam. Res. 2023, 3, 749–759. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA therapy positively regulates fasting blood glucose and decreases liver abnormalities in a mouse model of glycogen storage disease 1a. Mol. Ther. 2018, 26, 814–821. [Google Scholar] [CrossRef]
- Bicknell, A.A.; Reid, D.W.; Licata, M.C.; Jones, A.K.; Cheng, Y.M.; Li, M.; Hsiao, C.J.; Pepin, C.S.; Metkar, M.; Levdansky, Y.; et al. Attenuating ribosome load improves protein output from mRNA by limiting translation-dependent mRNA decay. Cell Rep. 2024, 43, 114098. [Google Scholar] [CrossRef]
- Vostrosablin, N.; Lim, S.; Gopal, P.; Brazdilova, K.; Parajuli, S.; Wei, X.; Gromek, A.; Prihoda, D.; Spale, M.; Muzdal, A.; et al. mRNAid, an open-source platform for therapeutic mRNA design and optimization strategies. NAR Genom. Bioinform. 2024, 6, Iqae028. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- Lv, H.; Shi, L.; Berkenpas, J.W.; Dao, F.-Y.; Zulfiqar, H.; Ding, H.; Zhang, Y.; Yang, L.; Cao, R. Application of artificial intelligence and machine learning for COVID-19 drug discovery and vaccine design. Brief. Bioinform. 2021, 22, bbab320. [Google Scholar] [CrossRef] [PubMed]
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 2022, 13, 1536–1557. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, P. Artificial intelligence for vaccine design. Methods Mol. Biol. 2022, 2412, 3–13. [Google Scholar]
- Thomas, S.; Abraham, A.; Baldwin, J.; Piplani, S.; Petrovsky, N. Artificial intelligence in vaccine and drug design. Methods Mol. Biol. 2022, 2410, 131–146. [Google Scholar]
- Dolgin, E. Personalized cancer vaccines pass first major clinical test. Nat. Rev. Drug Discov. 2023, 22, 607–609. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Mousavi, K.; Yazdi, A.; Zwierzyna, M.; Cardinali, M.; Fox, D.; Peel, T.; Coller, J.; Aggarwal, K.; Maruggi, G. Computational design of mRNA vaccines. Vaccine 2024, 42, 1831–1840. [Google Scholar] [CrossRef]
- He, S.; Gao, B.; Sabnis, R.; Sun, Q. RNA degformer: Accurate prediction of mRNA degradation at nucleotide resolution with deep learning. Brief. Bioinform. 2023, 24, bbac581. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y.; Zhang, S.; Chen, S.-J. mRNA vaccine sequence and structure design and optimization: Advances and challenges. J. Biol. Chem. 2025, 301, 108015. [Google Scholar] [CrossRef]
- Sharma, A.; Virmani, T.; Pathak, V.; Sharma, A.; Pathak, K.; Kumar, G.; Pathak, D. Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine. Biomed. Res. Int. 2022, 2022, 7205241. [Google Scholar] [CrossRef]
- Ghosh, A.; Larrondo-Petrie, M.M.; Pavlovic, M. Revolutionizing Vaccine Development for COVID-19: A Review of AI-Based Approaches. Information 2023, 14, 665. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Srinivasan, K.M.D.; Vincent, P.M.D.R. Personalized cancer vaccine design using AI-powered technologies. Front. Immunol. 2024, 15, 1357217. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Li, X.; Chen, K.; Maghsoudloo, M.; Kaboli, P.J.; Hashemi, M.; Khoushab, S.; Li, X. Compuational biology and artificial intelligence in mRNA vaccine design for cancer immunotherapy. Front. Cell. Infect. Microbiol. 2025, 14, 1501010. [Google Scholar] [CrossRef] [PubMed]
- Ras-Carmona, A.; Lehmann, A.A.; Lehmann, P.V.; Reche, P.A. Prediction of B cell epitopes in proteins using a novel sequence similarity-based methods. Sci. Rep. 2022, 12, 13739. [Google Scholar] [CrossRef]
- Hudson, D.; Fernandes, R.A.; Basham, M.; Ogg, G.; Koohy, H. Can we predict T cell specificity with digital biology and machine learning? Nat. Rev. Immunol. 2023, 23, 511–521. [Google Scholar] [CrossRef]
- Asediya, V.S.; Anjaria, P.A.; Mathakiya, R.A.; Koringa, P.G.; Nayak, J.B.; Bisht, D.; Fulmali, D.; Patel, V.A.; Desai, D.N. Vaccine development using artificial intelligence and machine learning: A review. Int. J. Biol. Macromol. 2024, 282, 136643. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Zeng, S.; Ren, X.; Yan, Y.; Gong, Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3393–3405. [Google Scholar] [CrossRef]
- Smith, C.C.; Chai, S.; Washington, A.R.; Lee, S.J.; Landoni, E.; Field, K.; Garness, J.; Bixby, L.M.; Selitsky, S.R.; Parker, J.S.; et al. Machine-learning prediction of tumor antigen immunogenicity in the selection of therapeutic epitopes. Cancer Immunol. Res. 2019, 7, 1591–1604. [Google Scholar] [CrossRef]
- Shao, X.M.; Bhattacharya, R.; Huang, J.; Sivakumar, I.K.A.; Tolkheim, C.; Zheng, L.; Hirsch, D.; Kaminow, B.; Omdahl, A.; Bonsack, M.; et al. High-throughput prediction of MHC class I and II neoantigens with MHCnuggets. Cancer Immunol. Res. 2020, 8, 396–408. [Google Scholar] [CrossRef]
- Lewis, M.M.; Beck, T.J.; Ghosh, D. Applying machine learning to identify ionizable lipids for nanoparticle-mediated delivery of mRNA. biorXiv 2023. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Cui, H.; Chen, J.; Xu, S.; Gong, F.; Golubovic, A.; Zhou, M.; Wang, K.C.; Varley, A.; et al. AGILE platform: A deep learning powered approach to accelerate LNP development for mRNA delivery. Nat. Commun. 2024, 15, 6305. [Google Scholar] [CrossRef]
- Van der Meel, R.; Grisoni, F.; Mulder, W.J.M. Lipid discovery for mRNA delivery guided by machine learning. Nat. Mater. 2024, 23, 880–881. [Google Scholar] [CrossRef]
- Our World in Data. “COVID-19, Vaccinations”. Available online: https://ourworldindata.org/grapher/cumulative-covid-vaccinations (accessed on 22 December 2024).
- Sparrow, E.; Hasso-Agopsowicz, M.; Kaslow, D.C.; Singh, K.; Rao, R.; Chibi, M.; Makubalo, L.E.; Reeder, J.C.; Kang, G.; Karron, R.A.; et al. Leveraging mRNA Platform Technology to Accelerate Development of Vaccines for Some Emerging and Neglected Tropical Diseases Through Local Vaccine Production. Front. Trop. Dis. 2022, 3, 844039. [Google Scholar] [CrossRef]
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.-S.; Chapon, C.; Tchitchek, N.; Cheraitia, L.; Lopez, E.M.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines 2021, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Expedited Program for Serious Conditions—Accelerated Approval of Drugs and Biologics Guidance for Industry. Draft Guidance December 2024. Available online: https://www.fda.gov/media/184120/download (accessed on 8 January 2025).
- Lo, M.K.; Spengler, J.R.; Welch, S.R.; Harmon, J.R.; Coleman-McCray, J.D.; Scholte, F.E.M.; Shrivastava-Ranjan, P.; Montgomery, J.M.; Nichol, S.T.; Weissman, D.; et al. Evaluation of a Single-Dose Nucleoside-Modified Messenger RNA Vaccine Encoding Hendra Virus-Soluble Glycoprotein Against Lethal Nipah virus Challenge in Syrian Hamsters. J. Infect. Dis. 2019, 221 (Suppl. 4), S493–S498. [Google Scholar] [CrossRef]
- Loomis, R.J.; DiPiazza, A.T.; Falcone, S.; Ruckwardt, T.J.; Morabito, K.M.; Abiona, O.M.; Chang, L.A.; Caringal, R.T.; Presnyak, V.; Narayanan, E.; et al. Chimeric Fusion (F) and Attachment (G) Glycoprotein Antigen Delivery by mRNA as a Candidate Nipah Vaccine. Front. Immunol. 2021, 12, 772864. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Bobb, K.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Cross, R.W.; Prasad, A.N.; Fenton, K.A.; Yu, H.; Fouts, T.R.; et al. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. npj Vaccines 2021, 6, 23. [Google Scholar] [CrossRef]
- Meyer, M.; Huang, E.; Yuzakov, O.; Ramanathan, P.; Ciaramella, G.; Bukreyev, A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J. Infect. Dis. 2018, 217, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Alberer, M.; Gand-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofino, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell 2024, 187, 5540–5553. [Google Scholar] [CrossRef]
- August, A.; Attarwala, H.Z.; Himansu, S.; Kalidindi, S.; Lu, S.; Pajon, R.; Han, S.; Lecerf, J.-M.; Tomassini, J.E.; Hard, M.; et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat. Med. 2021, 27, 2224–2233. [Google Scholar] [CrossRef]
- US Food and Drug Administration. New drug and biological drug products: Evidence needed to demonstrate effectiveness of new drug when human efficacy studies are not ethical or feasible, USFDA final rule, May 2002. Fed. Regist. 2002, 67, 37988–37998. [Google Scholar]
- US Food and Drug Administration. Product Development Under the Animal Rule Guidance for Industry, October 2015. Available online: www.fda.gov/media/88625/download (accessed on 3 August 2024).
- European Medicines Agency. Guideline on Procedures for the Granting of a Marketing Authorisation Under Exceptional Circumstances, Pursuant to Article 14 (8) of Regulation (EC) No 726/2004. Available online: www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-procedures-granting-marketing-authorisation-under-exceptional-circumstances-pursuant-article-14-8-regulation-ec-no-7262004_en.pdf (accessed on 17 April 2025).
- Mukonzo, J.K.; Ndagije, H.B.; Sabbiah, G.T.; Mathenge, W.; Price, D.A.; Grasela, T.H. Expanding regulatory science: Regulatory complementarity and reliance. Clin. Transl. Sci. 2024, 17, e13683. [Google Scholar] [CrossRef]
- Kis, Z.; Kontoravdi, C.; Dey, A.K.; Shattock, R.; Shah, N. Rapid development and deployment of high-volume vaccines for pandemic response. J. Adv. Manuf. Process. 2020, 2, e10060. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Challener, C.A. Meeting the demand for lipid excipients. BioPharm. Int. 2021, 34, 18–20. [Google Scholar]
- Webb, C.; Ip, S.; Bathula, N.V.; Popova, P.; Soriano, S.K.V.; Ly, H.H.; Eryilmaz, B.; Huu, V.A.N.; Broadhead, R.; Rabel, M.; et al. Current status and future perspectives on mRNA drug manufacturing. Mol. Pharm. 2022, 19, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef] [PubMed]
- Poveda, C.; Biter, A.B.; Bottazzi, M.E.; Strych, U. Establishing preferred product characterization for the evaluation of RNA vaccine antigens. Vaccines 2019, 7, 131. [Google Scholar] [CrossRef]
- Warne, N.; Ruesch, M.; Siwik, P.; Mensah, P.; Ludwig, J.; Hripcsak, M.; Godavarti, R.; Prigodich, A.; Dolsten, M. Delivering 3 billion doses of Comirnaty in 2021. Nat. Biotechnol. 2023, 41, 183–188. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Demongeot, J.; Fougere, C. mRNA COVID-19 Vaccines—Facts and hypotheses on fragmentation and encapsulation. Vaccines 2023, 11, 40. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research advances on the stability of mRNA vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef]
- Chheda, U.; Pradeepan, S.; Esposito, E.; Strezsak, S.; Fernadez-Delgado, O.; Kranz, J. Factors affecting stability of RNA–Temperature, length, concentration, pH and buffering species. J. Pharm. Sci. 2024, 113, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Binzel, D.W.; Li, X.; Burns, N.; Khan, E.; Lee, W.; Chen, L.-C.; Ellipilli, S.; Miles, W.; Soon Ho, Y.; Guo, P. Thermostability, tunability and tenacity of RNA as Rubbery Anionic Polymeric Materials in nanotechnology and nanomedicine—Specific cancer targeting with undetectable toxicity. Chem. Rev. 2021, 121, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Blenke, E.O.; Ornskov, E.; Schoneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, O.; Crommelin, D.J.A. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu, P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2023, 9, 9–24. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczko, D.; Kariko, K.; Screiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated nucleoside modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Panther, L.; Basnet, S.; Fierro, C.; Brune, D.; Legett, D.; Paterson, J.; Pickrell, P.; Lin, J.; Wu, K.; Lee, H.; et al. 2892. Safety and immunogenicity of mRNA-1647, an mRNA-based cytomegalovirus vaccine in healthy adults: Results of a phase 2, randomized, observer-blind, placebo-controlled, dose-finding trial. Open Forum Infect. Dis. 2023, 10 (Suppl. 2), ofad 500.2475. [Google Scholar] [CrossRef]
- Challener, C.A. Increasing mRNA product stability with lyophilization. BioPharm Int. 2024, 37, 22–25. [Google Scholar]
- Howard, M.D.; Lu, X.; Jay, M.; Dziubla, T.D. Optimization of the lyophilization process for long-term stability of solid–lipid nanoparticles. Drug Dev. Ind. Pharm. 2012, 38, 1270–1279. [Google Scholar] [CrossRef]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. Vaccines 2023, 8, 153. [Google Scholar] [CrossRef]
- Zhai, J.; Cote, T.; Chen, Y. Challenges and advances of the stability of mRNA delivery therapeutics. Nucleic Acid Insights 2024, 1, 101–112. [Google Scholar] [CrossRef]
- Stewart-Jones, G.B.E.; Elbashir, S.M.; Wu, K.; Lee, D.; Renzi, I.; Ying, B.; Kock, M.; Sein, C.E.; Choi, A.; Whitener, B.; et al. Domain-based mRNA vaccines encoding spike protein N-terminal and receptor binding domains confer protection against SARS-CoV-2. Sci. Transl. Med. 2023, 15, eadf4100. [Google Scholar] [CrossRef]
- Imani, S.; Tagit, O.; Pichon, C. Neoantigen vaccine nanoformulations based on chemically synthesized minimal mRNA (CmRNA): Small molecules, big impact. npj Vaccines 2024, 9, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.M.; Prins, C.; de Vries, J.J.C.; Viser, L.G.; Roukens, A.H.E. Establishing immunogenicity and safety of needle-free intradermal delivery by nanoporous ceramic skin patch of mRNA SARS-CoV-2 vaccine as a revaccination strategy in healthy volunteers. Virus Res. 2023, 334, 199175. [Google Scholar] [CrossRef]
- Van der Straeten, A.; Sarmadi, M.; Daristotle, J.L.; Kanelli, M.; Tostanoski, L.H.; Collins, J.; Pardeshi, A.; Han, J.; Varshney, D.; Eshaghi, B.; et al. A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines. Nat. Biotechnol. 2024, 42, 510–517. [Google Scholar] [CrossRef]
- Wu, J.; Zuo, J.; Dou, W.; Wang, K.; Long, J.; Yu, C.; Miao, Y.; Liao, Y.; Li, Y.; Cao, Y.; et al. Rapidly separable bubble microneedle-patch system present superior transdermal mRNA delivery efficiency. Int. J. Pharm. 2025, 674, 125427. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022, 25, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kang, S.; Won, C.; Min, D.-H. A Single-Dose mRNA Vaccine Employing Porous Silica Nanoparticles Induces Robust Immune Responses Against the Zika Virus. Adv. Sci. 2024, 11, e2404590. [Google Scholar] [CrossRef] [PubMed]
- Andretto, V.; Repellin, M.; Pujol, M.; Almouazen, E.; Sidi-Boumedine, J.; Granjon, T.; Zhang, H.; Remaut, K.; Jordheim, L.P.; Briançon, S.; et al. Hybrid core-shell particles for mRNA systemic delivery. J. Contr. Res. 2023, 353, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Blin, O.; Lefebvre, N.; Rascol, O.; Micallef, J. Orphan drug clinical development. Therapies 2020, 75, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Martini, P.G.V.; Avila, M.A.; Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 2019, 68, 1323–1330. [Google Scholar] [CrossRef]
- Córdoba, K.M.; Jericó, D.; Sampedro, A.; Jiang, L.; Iraburu, M.J.; Martini, P.G.V.; Berraondo, V.P.; Avila, M.A.; Fontanellas, A. Messenger RNA as a personalized therapy: The moment of truth for rare metabolic diseases. Int. Rev. Cell Mol. Biol. 2022, 372, 55–96. [Google Scholar]
- Martini, P.G.V.; Guey, L.T. A new era for rare genetic diseases: Messenger mRNA therapy. Hum. Gene Ther. 2019, 30, 1180–1188. [Google Scholar] [CrossRef]
- An, D.; Schneller, D.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017, 21, 3548–3558. [Google Scholar] [CrossRef]
- An, D.; Frassetto, A.; Jacquinet, E.; Eybye, M.; Milano, J.; DeAntonis, C.; Nguyen, V.; Laureano, R.; Milton, J.; Sabnis, S.; et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine 2019, 45, 519–528. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jerico, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaria, E.; Alegre, M.; et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018, 24, 1899–1909. [Google Scholar] [CrossRef]
- Yu, H.; Brewer, E.; Shields, M.; Crowder, M.; Sacchetti, C.; Soontornniyomkij, B.; Dou, D.; Clemente, B.; Sablad, M.; Chivukula, P.; et al. Restoring ornithine transcarbamylase (OTC) activity in an OTC-deficient mouse model using LUNAR-OTC mRNA. Clin. Transl. Discov. 2022, 2, e33. [Google Scholar] [CrossRef]
- Jiang, L.; Park, J.-S.; Yin, L.; Laureano, R.; Jacquinet, E.; Yang, J.; Liang, S.; Frassetto, A.; Zhuo, J.; Yan, X.; et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic aciduria. Nat. Commun. 2020, 11, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Truong, B.; Allegri, G.; Liu, X.B.; Burke, K.E.; Zhu, X.; Cederbaum, S.D.; Häberle, J.; Martini, P.G.V.; Lipshutz, G.S. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 21150–21159. [Google Scholar] [CrossRef]
- Khoja, S.; Liu, X.B.; Truong, B.; Nitzahn, M.; Lambert, J.; Eliav, A.; Nasser, E.; Randolph, E.; Burke, K.E.; White, R.; et al. Intermittent lipid nanoparticle mRNA administration prevents cortical dysmyelination associated with arginase deficiency. Mol. Ther.-Nucleic Acids 2022, 28, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Timmermand, O.V.; Perocheau, D.; Gil-Martinez, A.L.; Minnion, M.; Touramanidou, l.; Fang, S.; Messina, M.; Khalil, Y.; Spiewak, J.; et al. RNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical argininosuccinic aciduria. Sci. Transl. Med. 2024, 16, 729. [Google Scholar] [CrossRef] [PubMed]
- Koeberl, D.; Schulze, A.; Sondheimer, N.; Lipschultz, G.S.; Geberhiwot, T.; Li, R.; Saini, R.; Luo, J.; Sikirica, V.; Jin, L.; et al. Interim analysis of a first in human phase 1/2 mRNA trial for propionic aciduria. Nature 2024, 628, 872–877. [Google Scholar] [CrossRef]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef]
- Fermaglich, L.J.; Miller, K.L. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act. Orphanet J. Rare Dis. 2023, 18, 163–170. [Google Scholar] [CrossRef]
- Zanello, G.; Garrido-Estepa, M.; Crespo, A.; O’Connor, D.; Nabbout, R.; Waters, C.; Hall, A.; Taglialatela, M.; Chan, C.-H.; Pearce, D.A.; et al. Targeting shared molecular etiologies to accelerate drug development for rare diseases. EMBO Mol. Med. 2023, 1, e17159. [Google Scholar] [CrossRef]
- Muller, A.R.; Brands, M.M.M.G.; van de Ven, P.M.; Roes, K.C.B.; Cornel, M.C.; van Karnebeek, C.D.M.; Wijburg, F.A.; Daams, J.G.; Boot, E.; van Eeghen, A.M. Systematic Review of N-of-1 Studies in Rare Genetic Neurodevelopmental Disorders: The Power of 1. Neurology 2021, 96, 529–540. [Google Scholar] [CrossRef]
- Zablin, M.A.; Novack, G. N-of-1 Clinical Trials: A Scientific Approach to Personalized Medicine for Patients with Rare Retinal Diseases Such as Retinitis Pigmentosa. J. Ocul. Pharmacol. Ther. 2021, 37, 495–501. [Google Scholar]
- Lee, C.E.; Singleton, K.S.; Wallin, M.; Faundez, V. Rare Genetic Diseases: Nature’s Experiments on Human Development. iScience 2020, 23, 101123. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.R.; Praveen, P.; Kaduskar, B.D.; Moharir, S.C.; Mishra, R.K. mRNA biotherapeutics landscape for rare genetic disorders. J. Biosci. 2024, 49, 33–60. [Google Scholar] [CrossRef]
- Shen, G.; Liu, J.; Yang, H.; Xie, N.; Yang, Y. mRNA therapies: Pioneering a new era in rare genetic disease treatment. J. Control. Release 2024, 369, 696–721. [Google Scholar] [CrossRef]
- Brooks, P.J.; Ottinger, E.A.; Portero, D.; Lomash, R.M.; Alimardanov, A.; Terse, P.; Xu, X.; Chandler, R.J.; Hauserman, J.G.; Esposito, E.; et al. The Platform Vector Gene Therapies Project: Increasing the Efficiency of Adeno-Associated Virus Gene Therapy Clinical Trial Startup. Hum. Gene Ther. 2020, 31, 1034–1042. [Google Scholar] [CrossRef]
- Vervaeke, P.; Borgos, S.E.; Sanders, N.N.; Combes, F. Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv. Drug Deliv. Res. 2022, 184, 114236. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the promise of mRNA therapeutics. Nat. Biotech. 2022, 40, 1586–1600. [Google Scholar] [CrossRef]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Trivedi, V.; Yang, C.; Klippel, K.; Yegorov, O.; von Roemeling, C.; Hoang-Minh, L.; Fenton, G.; Ogando-Rivas, E.; Castillo, P.; Moore, G.; et al. mRNA-based precision targeting of neoantigens and tumor-associated antigens in malignant brain tumors. Genome Med. 2024, 16, 17. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9–46. [Google Scholar] [CrossRef]
- Wu, D.-W.; Jia, S.-P.; Xing, S.-J.; Ma, H.-I.; Wang, X.; Tang, Q.-Y.; Li, Z.-W.; Wu, Q.; Bai, M.; Zhang, X.-Y.; et al. Personalized neoantigen cancer vaccines: Current progression, challenges and a bright future. Clin. Exp. Med. 2024, 24, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine–induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomized, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Lang, F.; Schrors, B.; Lower, M.; Tureci, O.; Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- May, M. How mRNA is powering a personalized vaccine revolution. Nat. Med. 2024, 30, 2097–2098. [Google Scholar] [CrossRef]
- Jonker, A.H.; Taturu, E.-A.; Graessner, H.; Dimmock, D.; Jaffe, A.; Bayman, G.; Davies, J.; Mitkus, S.; Ilianch, O.; Horgan, R.; et al. The state-of-the-art of N-of-1 therapies and the IRDiRC N-of-1 development roadmap. Nat. Rev. Drug Discov. 2025, 24, 40–56. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines, October 2011. Available online: www.fda.gov/media/82312/download (accessed on 3 August 2024).
- Ministry of Food and Drug Safety, South Korea. Considerations on the Development of Personalized Neoantigen-Targeted Therapy Products (Guidance for Industry), September 2024. Available online: www.mfds.go.kr/eng/brd/m_1127/list.do (accessed on 12 November 2024).
- Medicines and Health Products Regulatory Agency. Draft Guideline on Individualised mRNA Cancer Immunotherapies, February 2025. Available online: https://assets.publishing.service.gov.uk/media/6799ef4d9a6dc0352ab34225/Individualised_mRNA_cancer_immunotherapies_0.6.5.pdf (accessed on 10 February 2025).
- Guerriaud, M.; Kohli, E. RNA-based drugs and regulation: Toward a necessary evolution of the definitions issued from the European union legislation. Front. Med. 2022, 9, 1012497. [Google Scholar] [CrossRef]
- von Fritschen, M.; Haber, C.; Straus, W.; Schneider, C.K.; Janosz, E.; Jägle, U.; Mendila, M.; Blume, C. Defining Gene Therapy Medicinal Products in the EU: Scientific and Regulatory Perspectives. DIA Global Forum, March 2024. Available online: https://globalforum.diaglobal.org/issue/march-2024/defining-gene-therapy-medicinal-products-in-the-eu/ (accessed on 8 January 2025).
- US Food and Drug Administration. Frequently Asked Questions—Developing Potential Cellular and Gene Therapy Products Draft Guidance for Industry, November 2024. Available online: www.fda.gov/media/183631/download (accessed on 8 January 2025).
- Aust, A. Regulatory strategies for developing and manufacturing RNA-LNPs. Nucleic Acid Insights 2024, 1, 139–145. [Google Scholar] [CrossRef]
- Djonova, J. Swiss regulatory aspects and evaluation considerations for ATMPs and other nucleic acid-based products such as mRNA vaccines. Vaccine Insights 2024, 3, 91–95. [Google Scholar] [CrossRef]
- Wan, K.-W.; Galway, F. MHRA regulatory considerations in the quality of mRNA products. Nucleic Acid Insights 2024, 1, 149–154. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities). Q11 Guideline, May 2012. Available online: https://database.ich.org/sites/default/files/Q11%20Guideline.pdf (accessed on 14 November 2024).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline Analytical Procedure Development Q14 Guideline, March 2022. Available online: https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf (accessed on 14 November 2024).
- European Medicines Agency. Guideline on Process Validation for the Manufacture of Biotechnology-Derived Active Substances and Data to be Provided in the Regulatory Submission. In EMA Guidance EMA/CHMP/BWP/187338/2014; European Medicines Agency: Amsterdam, The Netherlands, 2016. [Google Scholar]
- US Food and Drug Administration (Draft) Guidance Document: Platform Technology Designation Program for Drug Development, May 2024. Available online: www.fda.gov/regulatory-information/search-fda-guidance-documents/platform-technology-designation-program-drug-development (accessed on 3 August 2024).
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations. World Health Organization Expert Committee on Biological Standardization 74th Report, 2022, Annex 3. Available online: www.who.int/publications/m/item/evaluation-of-the-quality-safety-and-efficacy-of-messenger-rna-vaccines-for-the-prevention-of-infectious-diseases-regulatory-considerations (accessed on 11 November 2024).
- European Medicines Agency. Concept Paper on the Development of a Guideline on the 5 Quality Aspects of mRNA Vaccines, May 2023. Available online: www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-development-guideline-quality-aspects-mrna-vaccines_en.pdf (accessed on 26 March 2024).
- European Directorate for the Quality of Medicines and Healthcare. European Pharmacopoeia Commission Adopts First Three General Texts on mRNA Vaccines, January 2025. Available online: www.edqm.eu/en/-/european-pharmacopoeia-commission-adopts-first-three-general-texts-on-mrna-vaccines (accessed on 31 January 2025).
- European Medicines Agency. Procedural advice for vaccine platform technology master 5 file (vPTMF) certification. EMA Guidance EMA/CVMP/IWP/286631/2021, May 2022. Available online: www.ema.europa.eu/en/documents/scientific-guideline/draft-procedural-advice-vaccine-platform-technology-master-file-vptmf-certification_en.pdf (accessed on 3 August 2024).
- European Directorate for the Quality of Medicines and HealthCare, Human OCABR Guidelines. Available online: www.edqm.eu/en/omcl/human-ocabr-guidelines (accessed on 8 January 2025).
- Ball, G.; Reblin, T.; Buchanan, J.; Hendrickson, B.A.; Lewis, E.; Schnell, P.M.; Rockhold, F.W. A framework for safety evaluation throughout the product development life-cycle. Ther. Innov. Regul. Sci. 2020, 54, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Herve, C.; Laupeze, B.; Del Guidice, G.; Didierlaurent, A.M.; Da Silva, F.T. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39–49. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.-H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ishii, K.J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 2022, 23, 474–476. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, J.H.; Kim, M.; Lee, Y.; Hwang, Y.-H.; Park, M.; Li, C.H.; Lee, T.; Lee, J.-A.; Kim, Y.-M.; et al. Innate immune responses against mRNA vaccine promote cellular immunity through IFN-β at the injection site. Nat. Commun. 2024, 15, 7226–7240. [Google Scholar] [CrossRef] [PubMed]
- Laurini, G.S.; Montanaro, N.; Broccoli, M.; Bonaldo, G.; Motola, D. Real-life safety profile of mRNA vaccines for COVID-19: An analysis of VAERS database. Vaccine 2023, 41, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.S.F.; Sanchez, C.L.; Rose, T.B.; Smith, D.; Thorn, N.; Galiza, E.; Miah, T.; Pearce, J.; Hultin, C.; Cosgrove, C.; et al. Comparing reactogenicity of COVID-19 vaccine boosters: A systematic review and meta-analysis. Expert Rev. Vaccines 2024, 23, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Myers, T.; Gee, J.; Shay, D.K.; Marquez, P.; Baggs, J.; Zhang, B.; Licata, C.; Shimabukoro, T. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine 2021, 39, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 7, CD015477. [Google Scholar]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19 vaccinated and unvaccinated populations: A systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational global vaccine data network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Copland, E.; Patone, M.; Saatchi, D.; Handunnetthi, L.; Hirst, J.; Hunt, D.P.J.; Mills, N.L.; Moss, P.; Sheikh, A.; Coupland, C.A.A.; et al. Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England. Nat. Commun. 2024, 15, 3822. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Santosa, A.; Wettermark, B.; Fall, T.; Bjork, J.; Borjesson, M.; Gisslen, M.; Nyberg, F. Cardiovascular events following coronavirus disease 2019 vaccination in adults: A nationwide Swedish study. Eur. Heart J. 2024, 30, ehae639. [Google Scholar] [CrossRef]
- Barmada, A.; Klein, J.; Ranaswamy, A.; Brodsky, N.N.; Jaycox, J.R.; Sheikha, H.; Jones, K.M.; Habet, V.; Campbell, M.; Sumida, T.S.; et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Sci. Immunol. 2023, 8, eadh3455. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Follmann, D.; Hachigian, G.; Strout, C.; Overcash, J.S.; Doblecki-Lewis, S.; Whitaker, J.A.; Anderson, E.J.; et al. Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and booster phases. Nat. Commun. 2024, 15, 7469–7481. [Google Scholar] [CrossRef] [PubMed]
- Hulscher, N.; Hodgkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, L.; Le Vu, S.; Botton, J.; Bertrand, M.; Jabagi, M.-J.; Drouin, J.; Cuenot, F.; Zores, F.; Dray-Spira, R.; Weill, A.; et al. Long-term prognosis of patients with myocarditis attributed to COVID-19 mRNA vaccination, SARS-CoV-2 infection of conventional etiologies. JAMA 2024, 332, 1367–1377. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef]
- Kadkhoda, K. Post RNA-based COVID vaccines myocarditis: Proposed mechanisms. Vaccine 2022, 40, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.L.; Berning, A.A.; Mann, S.C.; Quaife, R.A.; Gill, E.A.; Auerbach, S.R.; Campbell, T.B.; Bristow, M. Vaccination-Associated Myocarditis and Myocardial Injury. Circ. Res. 2023, 132, 1338–1357. [Google Scholar] [CrossRef]
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis associated with COVID-19 vaccination. npj Vaccines 2024, 9, 122–129. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Saint-Gerons, D.M.; Ibarz, M.T.; Castro, J.L.; Fores-Martos, J.; Tabares-Seisdedsos, R. Myopericarditis associated with the Novavax COVID-19 vaccines (NVX-CoV2373): A retrospective analysis of individual case safety reports from VigiBase. Drugs-Real World Outcomes 2023, 10, 263–270. [Google Scholar] [CrossRef]
- Li, X.; Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.R.; et al. Characterising the background incidence rates of adverse events of special interest for COVID-19 vaccines in eight countries: Multinational network cohort study. BMJ 2021, 373, n1435. [Google Scholar] [CrossRef]
- World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI). User Manual for the Revised WHO Classification. Second Edition WHO/HIS/EMP/SAV. January 2018. Available online: https://iris.who.int/bitstream/handle/10665/259959/9789241513654-eng.pdf (accessed on 14 November 2024).
- European Medicines Agency. Pfizer XBB 1.5 Package Leaflet, Within Annex 1 Summary of Product Characteristics: Comirnaty, INN-Tozinameran, Tozinameran/Famtozinameran, Raxtozinameran, Bretovameran. 2023. Available online: www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 10 December 2024).
- European Medicines Agency. Moderna XBB 1.5 Package Leaflet, Within Annex 1 Summary of Product Characteristics: Spikevax, INN-Elasomeran, Elasomeran/Imelasomeran, Elasomeran/Davesomeran, Andusomeran. 2023. Available online: www.ema.europa.eu/en/documents/product-information/spikevax-epar-product-information_en.pdf (accessed on 10 December 2024).
- Morgan, H.J.; Clothier, H.J.; Kattan, G.S.; Boyd, J.H.; Buttery, J.P. Acute disseminated encephalomyelitis and transverse myelitis following COVID-18 vaccination–a self-controlled case series analysis. Vaccine 2024, 42, 2212–2219. [Google Scholar] [CrossRef]
- Kammerer, U.; Schulz, V.; Steger, K. BioNtech RNA-based COVID-19 injections contain large amounts of residual DNA including an SV40 promoter/enhancer sequence. Public Health Policy Law 2024, 5, 2019–2024. [Google Scholar]
- König, B.; Kirchner, J.O. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods Protoc. 2024, 7, 41. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration, Australia. Addressing Misinformation About Excessive DNA in the mRNA Vaccines, Media Release 18 October 2024. Available online: www.tga.gov.au/news/media-releases/addressing-misinformation-about-excessive-dna-mrna-vaccines (accessed on 14 November 2024).
- Global Vaccine Data Network. Debunking the DNA Contamination Claims in mRNA Vaccines. Available online: www.globalvaccinedatanetwork.org/news/plasmid-gate_debunking_the_DNA_contamination_claims_in_mRNA_vaccines (accessed on 14 November 2024).
- Therapeutic Goods Administration, Australia. Summary Report of Residual DNA and Endotoxin on COVID-19 mRNA Vaccines Conducted by TGA Laboratories, 11 November 2024. Available online: www.tga.gov.au/resources/publication/tga-laboratory-testing-reports/summary-report-residual-dna-and-endotoxin-covid-19-mrna-vaccines-conducted-tga-laboratories (accessed on 14 November 2024).
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J. Understanding vaccine hesitancy: A call for more social science in RNA vaccine research. Vaccine Insights 2024, 3, 107–110. [Google Scholar] [CrossRef]
- Leong, C.; Jin, L.; Kim, D.; Kim, J.; Teo, Y.Y.; Ho, T.-H. Assessing the impact of novelty and conformity on hesitancy towards COVID-19 vaccines using mRNA technology. Commun. Med. 2022, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. International Regulators and WHO Address Need to Boost COVID-19 Vaccine Confidence, June 2021. Available online: www.ema.europa.eu/en/news/international-regulators-and-who-address-need-boost-covid-19-vaccine-confidence (accessed on 14 November 2024).
- Peretti-Watel, P.; Verger, P.; Ward, J.K. To understand mRNA vaccine hesitancy, stop calling the public anti-science. Nat. Med. 2024, 30, 923–924. [Google Scholar] [CrossRef]
- Attwell, K.; Rizzi, M.; McKenzie, L.; Carlson, S.J.; Roberts, L.; Tomkinson, S.; Blyth, C.C. COVID-19 vaccine Mandates: An Australian attitudinal study. Vaccine 2021, 40, 7360–7369. [Google Scholar] [CrossRef]
- Medicines Patent Pool. mRNA Technology Transfer Programme. Available online: https://medicinespatentpool.org/what-we-do/mrna-technology-transfer-programme (accessed on 14 November 2024).
- Bussink-Voorend, D.; Hautvast, J.L.A.; Vandenberg, L.; Visser, O.; Hulscher, M.E.J.L. A systematic literature review to clarify the concept of vaccine hesitancy. Nat. Hum. Behav. 2022, 6, 1634–1648. [Google Scholar] [CrossRef]
- Attwell, K.; Hannah, A.; Leask, J. COVID-19: Talk of ‘vaccine hesitancy’ lets governments off the hook. Nature 2022, 602, 574–577. [Google Scholar] [CrossRef]
- de Miguel-Arribas, A.; Aleta, A.; Moreno, Y. Impact of vaccine hesitancy on secondary COVID-19 outbreaks in the US: An age-structured SIR model. BMC Infect. Dis. 2022, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Leigh, J.P.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 12, 3801. [Google Scholar] [CrossRef]
- Romer, D.; Winneg, K.M.; Jamieson, P.E.; Brensinger, C.; Jamieson, K.H. Misinformation about vaccine safety and uptake of COVID-19 vaccines among adults and 5–11 year olds in the United States. Vaccine 2022, 40, 6463–6470. [Google Scholar] [CrossRef]
- Jamieson, K.H.; Winneg, K.; Patterson, S., Jr.; Gibson, L.A.; Jamieson, P.E. Annenberg Science and Public Health Monitor–Summer 2024; University of Pennsylvania: Philadelphia, PA, USA, 2024; Available online: https://cdn.annenbergpublicpolicycenter.org/wp-content/uploads/2024/08/asaph-report-summer-2024.pdf (accessed on 14 November 2024).
- Ashfield, S.; Donelle, L.; Uppal, G.; Bauer, M.A.; Kothari, A. Community organization perspectives on COVID-19 vaccine hesitancy and how they increased COVID-19 vaccine confidence: A Canadian Immunization Research Network, social sciences and humanities network study. Front. Public Health 2023, 11, 1258742. [Google Scholar] [CrossRef]
- Shiman, L.J.; Diallo, F.; Nieves, C.I.; Brooks, B.; Dannefer, R.; Dorvil, S.; Lejano, M.; Pierre, J. “Be honest and gain trust”: A population health study to understand the factors associated with building trust in three historically disinvested neighbourhoods in New York City. Front. Public Health 2023, 11, 1285152. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.; Rosen, A.M.; Edwards, D.; Bolio, A.; Larson, H.J.; Servin, M.; Rudowitz, M.; Carfi, A.; Ceddia, F. Opportunities and challenges to implementing mRNA-based vaccines and medicines: Lessons from COVID-19. Front. Public Health 2024, 12, 1429265. [Google Scholar] [CrossRef]
- Van der Linden, S. Misinformation: Susceptibility, spread, and interventions to immunize the public. Nat. Med. 2022, 28, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, J.; van der Linden, S.; Goldberg, B.; Rathje, S.; Lewandowsky, S. Psychological inoculation improves resilience against misinformation on social media. Sci. Adv. 2024, 8, eabo6254. [Google Scholar] [CrossRef]
- Hu, J.; Rabin, K.; Constantinescu, C.; Larson, H.J.; Ratzan, S. Editorial: Building public confidence in innovative mRNA vaccines. Front. Public Health 2025, 13, 1557596. [Google Scholar] [CrossRef]
- Moffat, K.; Lacey, J.; Zhang, A.; Leipold, S. The social licence to operate: A critical review. For. Int. J. For. Res. 2016, 89, 477–488. [Google Scholar] [CrossRef]
- Kamenopoulos, S.; Agioutantis, Z. The importance of the social license to operate at the investment and operations stage of coal mining projects: Application using a decision support system. Extr. Ind. Soc. 2020, 8, 100740. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.H.A.; Kalkman, S.; van Thiel, G.J.M.W.; Mostert, M.; van Delden, J.J.M. The social licence for data-intensive health research: Towards co-creation, public value and trust. BMC Med. Ethics 2021, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Churchill, B.F.; Henkhaus, L.E.; Lawler, E.C. Effect of vaccine recommendations on consumer and firm behavior. J. Policy Anal. Manag. 2024, 44, 125–150. [Google Scholar] [CrossRef]
- Brewer, N.T. What Works to Increase Vaccination Uptake. Acta Pediatr. 2021, 21 (Suppl. 4), S9–S16. [Google Scholar] [CrossRef]
- Sergi, C.M.; Leung, A.K.C. Vaccination: A question of social responsibility. J. Prev. Med. Hyg. 2021, 62, E46–E47. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skerritt, J.H. Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle. Vaccines 2025, 13, 473. https://doi.org/10.3390/vaccines13050473
Skerritt JH. Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle. Vaccines. 2025; 13(5):473. https://doi.org/10.3390/vaccines13050473
Chicago/Turabian StyleSkerritt, John H. 2025. "Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle" Vaccines 13, no. 5: 473. https://doi.org/10.3390/vaccines13050473
APA StyleSkerritt, J. H. (2025). Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle. Vaccines, 13(5), 473. https://doi.org/10.3390/vaccines13050473





