HPV Vaccine Uptake and Cervical Cancer Trends in Panama: A Reference Point for Future Impact Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Data Quality Assurance
2.4. Study Variables
2.5. Study Outcomes and Measures
- Vaccine uptake. In absolute numbers, measured by the number of first, second, and third doses (when applicable) of HPV administered by sex and year.
- Vaccine coverage. The EPI estimates the vaccine coverage by dividing the number of doses in the vaccination age group and province or comarca by the INEC’s population estimates for the same year, sex, and age group and province or comarca.
- CC screening. The SRH Department at the MoH provides aggregate data on Pap tests conducted, which are divided by INEC’s population estimates for women ≥18 years old.
- CC cumulative incidence rate. Estimated by dividing the counts of CC cases by the year’s population for each demographic subgroup.
- CC mortality rate. The mortality rate by CC was estimated by dividing the total number of deaths attributed to CC each year by the mid-year population estimate of women ≥18 years old. The counts of new CC cases are also described by age groups and by years of diagnosis.
- CC tumoral behavior at diagnosis. Tumoral behavior at the time of diagnosis of CC was classified as in situ or invasive and reported as counts per year. Cancer stages are unidentified in the current data.
2.6. Statistical Analyses
- ⚬
- Girls trend: (yearly change among girls).
- ⚬
- Boys trend: (yearly change among boys).
- ⚬
- Difference in trends: (boys’ vs. girls’ slopes).
3. Results
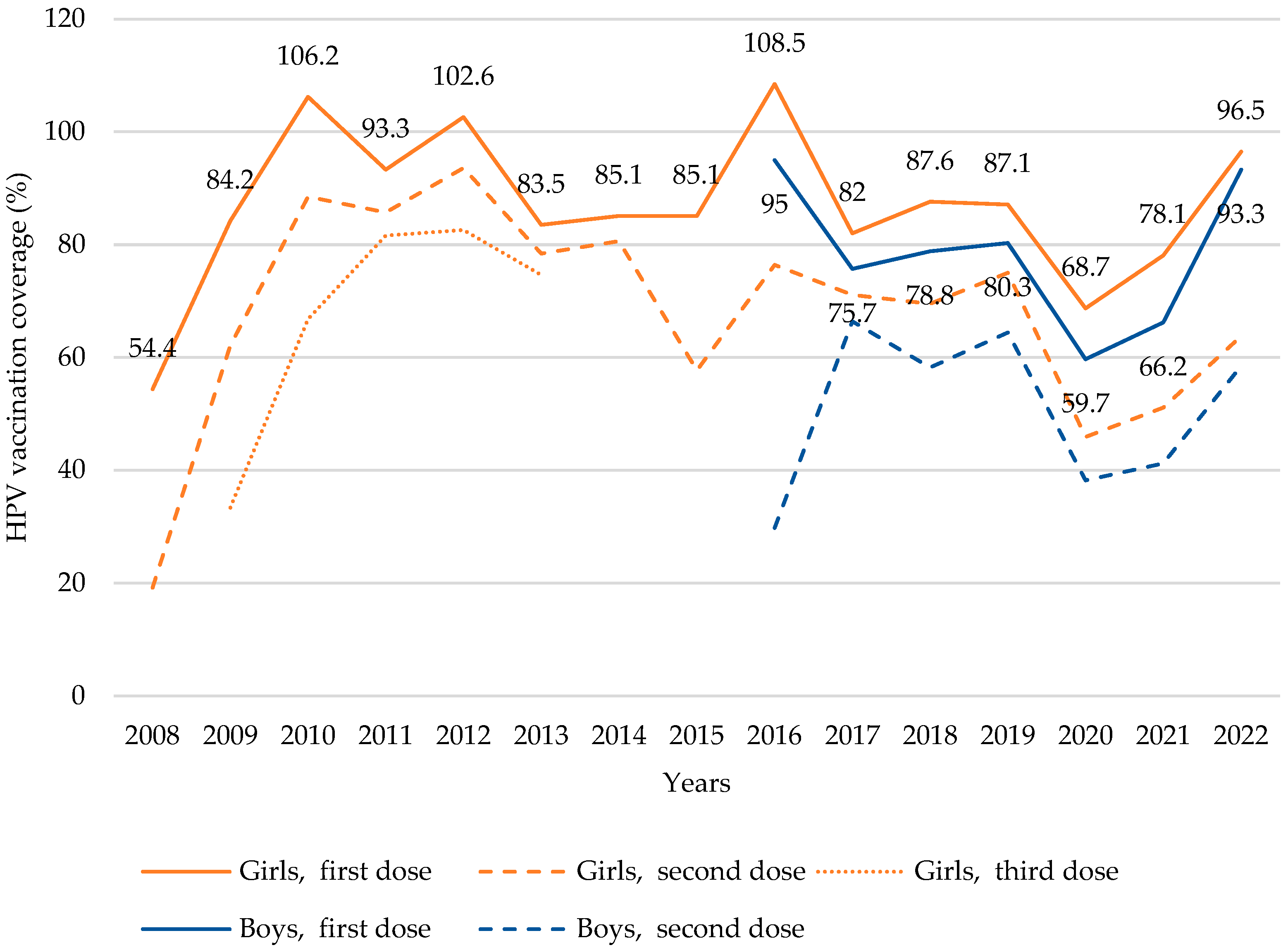
3.1. HPV Vaccine Uptake by Doses and Sex over Time
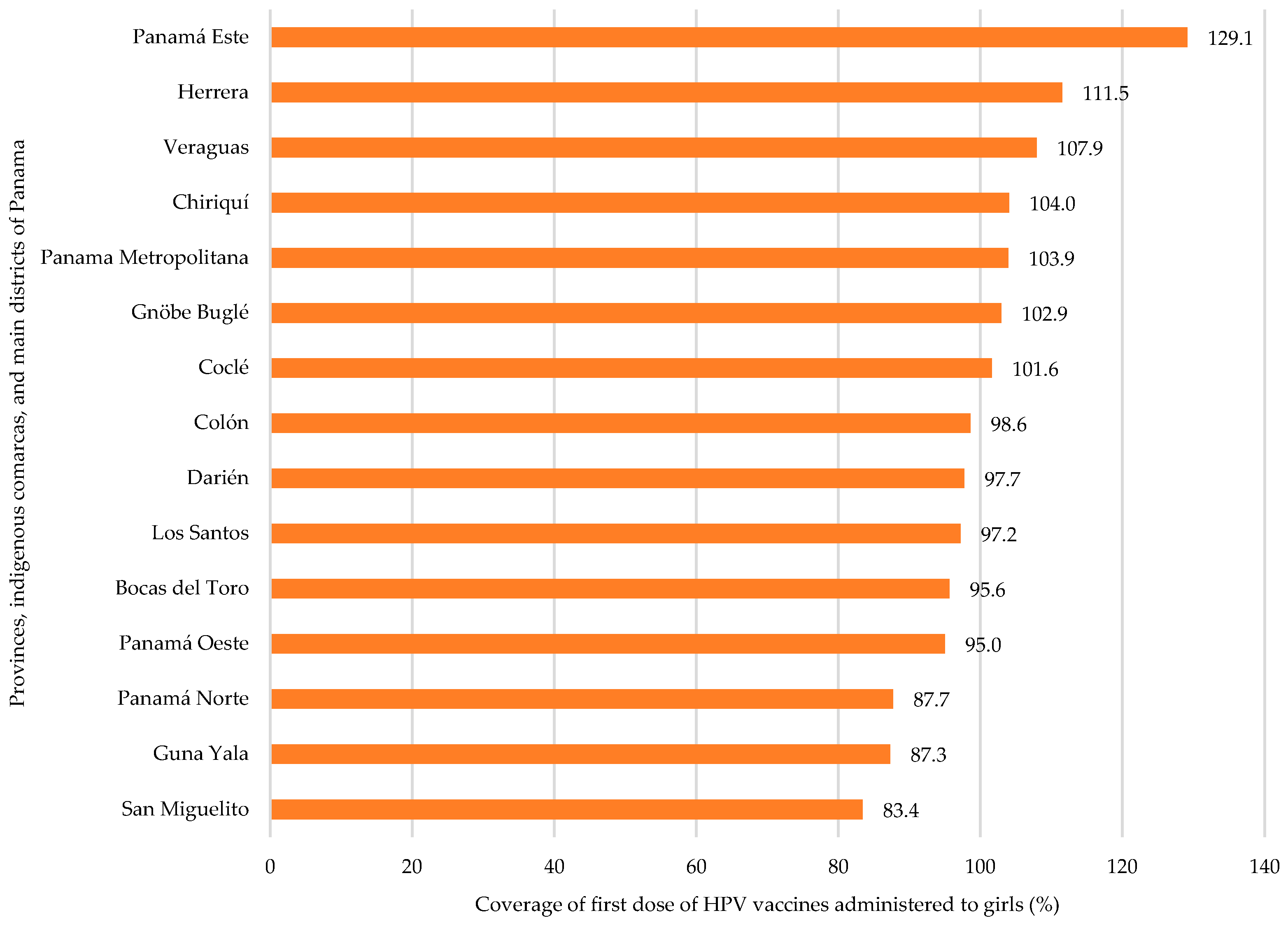
3.2. HPV Vaccine Coverage by Region
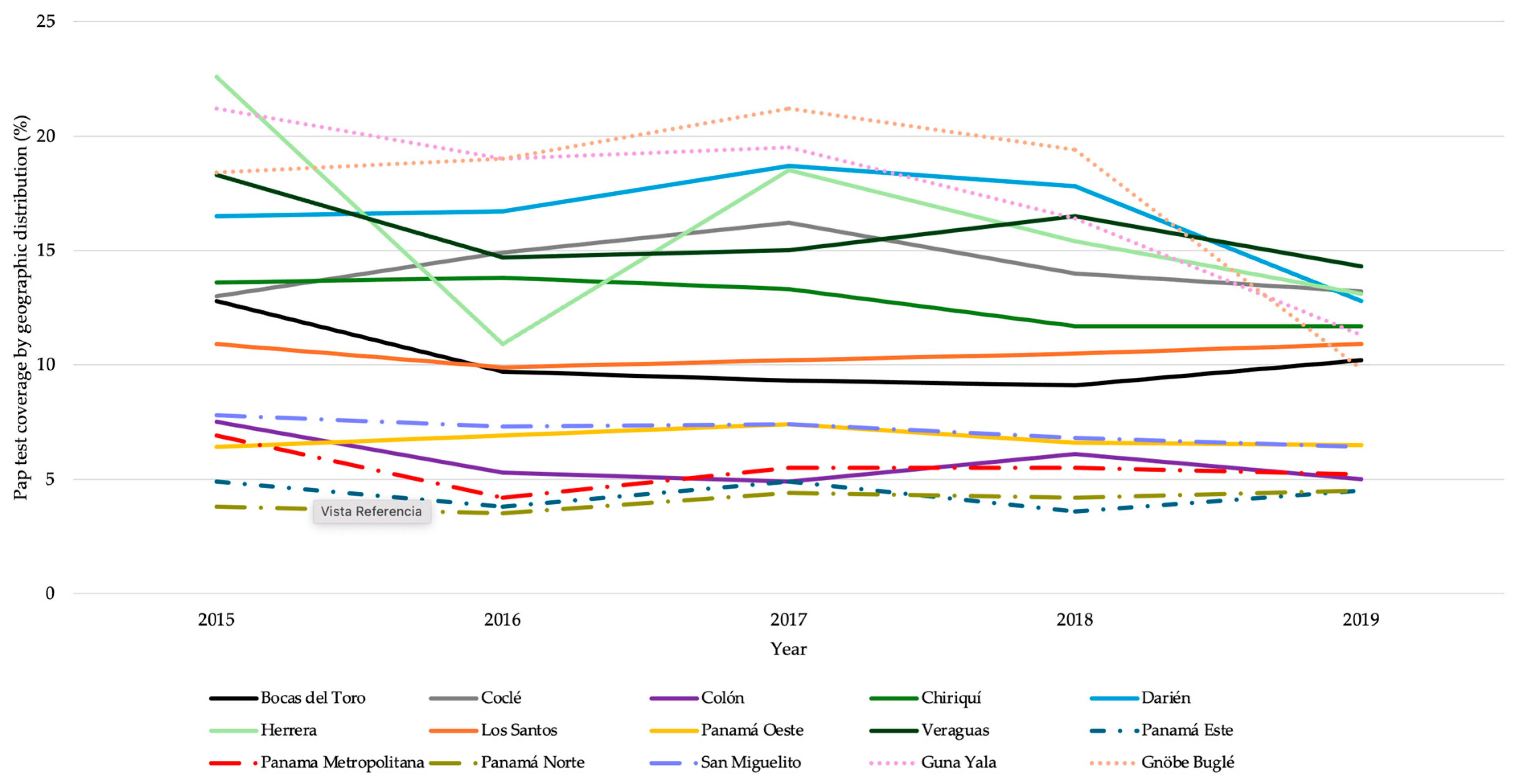
3.3. Cervical Cancer Screening
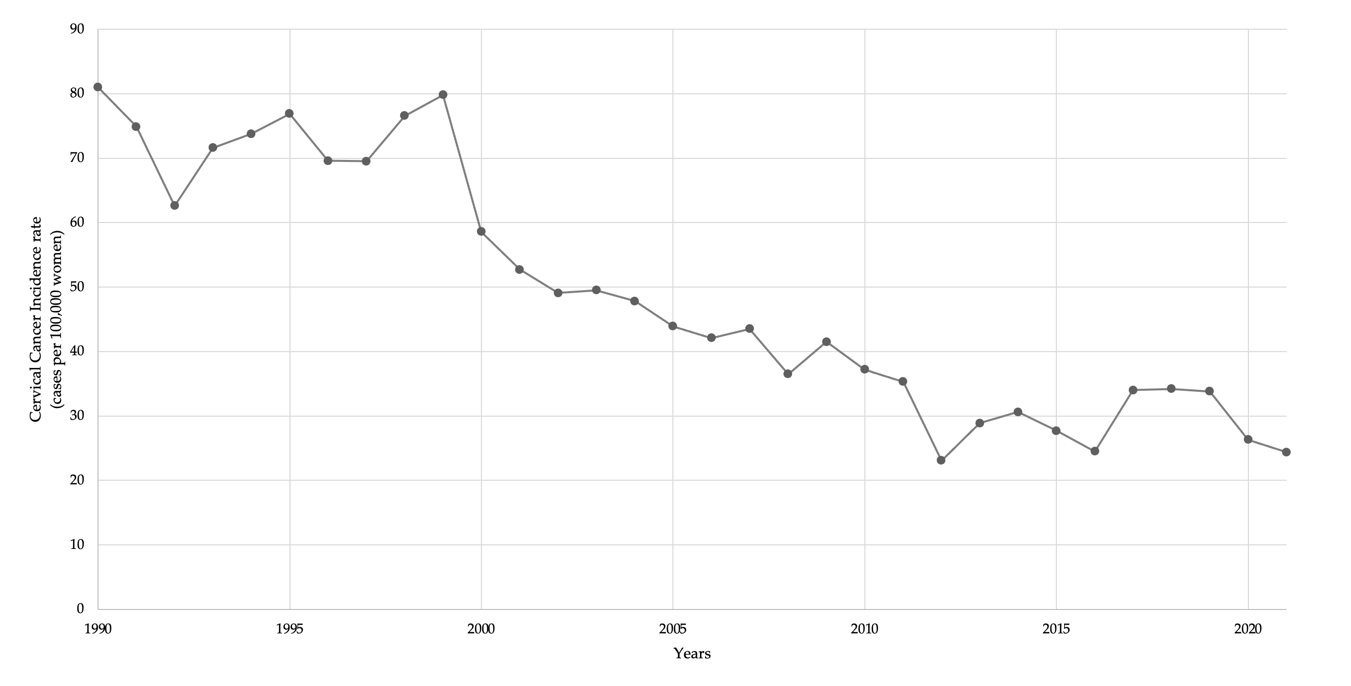
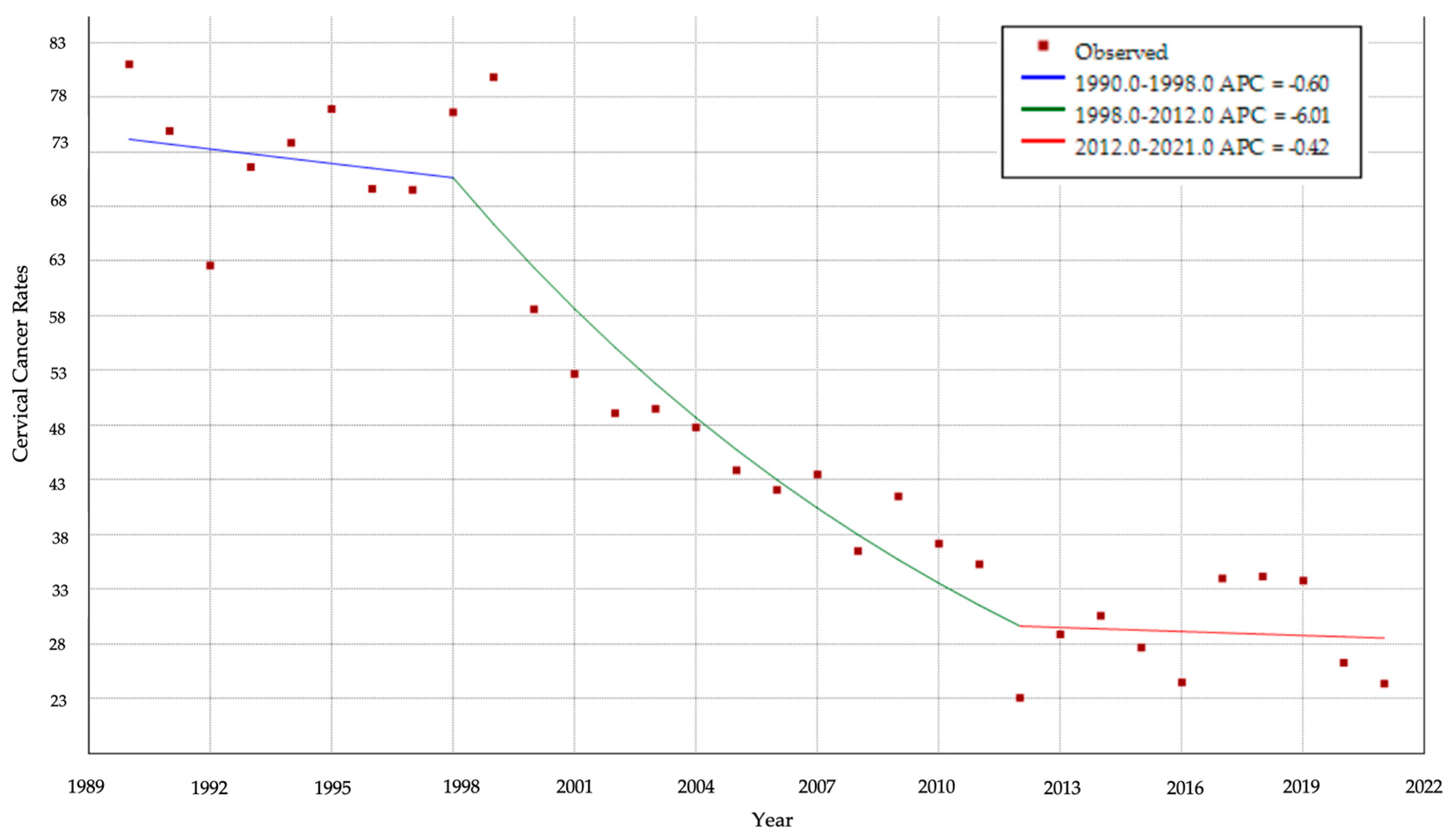
3.4. Cervical Cancer Incidence over Time
3.5. HPV Vaccination and Cervical Cancer Outcomes
3.5.1. CC Cumulative Incidence over Time
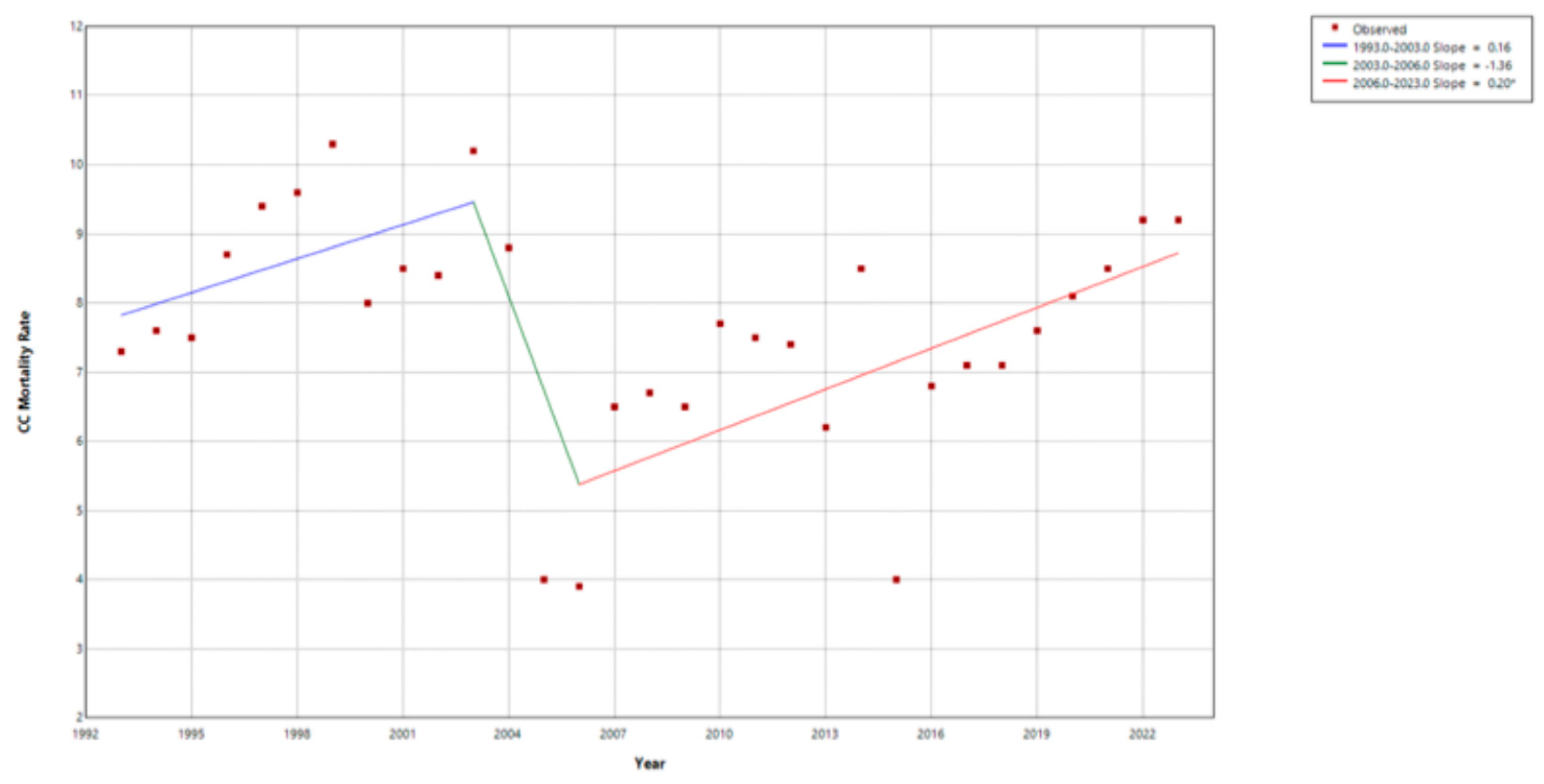
3.5.2. Cervical Cancer (CC) Mortality
4. Discussion
4.1. HPV Vaccine Uptake
4.2. Cervical Cancer
4.3. Health Education and Promotion
4.4. Sociocultural Considerations
4.5. Structural Considerations
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | annual percentage change |
| AR | autoregressive |
| CC | cervical cancer |
| CDC | Centers for Disease Control and Prevention |
| DFE | degrees of freedom for error |
| DW | Durbin–Watson statistic |
| EPI | Essential Program on Immunization |
| HPV | human papillomavirus |
| INEC | National Institute for Statistics and Census |
| ITS | Interrupted time series analysis |
| JRF | Joint Reporting Form on Immunizations |
| LAC | Latin America and the Caribbean |
| LMIC | low- and middle-income countries |
| MoH | Ministry of Health of Panama |
| MSE | mean standard error |
| PAHO | Pan-American Health Organization |
| Pap | Papanicolaou |
| rDF | residual degrees of freedom |
| SRH | sexual and reproductive health |
| STIs | sexually transmitted infections |
| VWA | Vaccination Week of the Americas |
| WHO | World Health Organization |
References
- Politis, M.; Higuera, G.; Chang, L.R.; Gomez, B.; Bares, J.; Motta, J. Trend Analysis of Cancer Mortality and Incidence in Panama, Using Joinpoint Regression Analysis. Medicine 2015, 94, e970. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Srivastava, S.; Manne, U. The Biology of Incipient, Pre-Invasive or Intraepithelial Neoplasia. Cancer Biomark. 2011, 9, 21–39. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical Cancer in Low and Middle Income Countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Rodrigues, A.; Flores, M.G.; Macedo Neto, A.O.; Braga, L.A.C.; Vieira, C.M.; Sousa Lima, R.M.; de Andrade, D.A.; Machado, K.K.; Guimarães, A.P. HPV Vaccination in Latin America: Coverage Status, Implementation Challenges and Strategies to Overcome It. Front. Oncol. 2022, 12, 984449. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.L.J.M.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019, 7, 176–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 29 July 2025).
- Okunade, K.S. Human Papillomavirus and Cervical Cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Basic Information about HPV and Cancer. Available online: https://www.cdc.gov/cancer/hpv/basic-information.html (accessed on 29 July 2025).
- National Cancer Institute. HPV and Cancer. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer (accessed on 29 July 2025).
- Joshi, S.; Muwonge, R.; Bhosale, R.; Chaudhari, P.; Kulkarni, V.; Mandolkar, M.; Deodhar, K.; Kand, S.; Phadke, N.; Rajan, S.; et al. A randomized controlled non-inferiority trial to compare the efficacy of ‘HPV screen, triage and treat’ with ‘HPV screen and treat’ approach for cervical cancer prevention among women living with HIV. Nat. Commun. 2025, 16, 1888. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tenorio, C.; Calle-Gómez, I.; Moya, R.; Omar, M.; Lopez-Hidalgo, J.; Rodriguez-Granges, J.; Muñoz, L.; García-Martinez, C. Prevalence and incidence of human papilloma virus-related dysplasia of oropharyngeal, cervical, and anal mucosae in Spanish people with HIV. AIDS 2025, 39, 649–657. [Google Scholar] [CrossRef]
- Osagie, E.; Akhigbe, P.; Idemudi, N.; Obuekwe, O.; Adebiyi, R.; Schlecht, N.; Liu, J.; Bromberg, Y.; E Eki-Udoko, F.; Osazuwa-Peters, N.; et al. Human Papillomavirus, Human Immunodeficiency Virus, and Oral Microbiota Interplay in Nigerian Youth (HOMINY): A Prospective Cohort Study Protocol. BMJ Open. 2025, 15, e091017. [Google Scholar] [CrossRef]
- Franco, E.L. Prevention of Cervical Cancer in Latin America: Future Challenges and Opportunities. Salud Publica Mex. 2018, 60, 609. [Google Scholar] [CrossRef]
- Sichero, L.; Picconi, M.A.; Villa, L.L. The Contribution of Latin American Research to HPV Epidemiology and Natural History Knowledge. Braz. J. Med. Biol. Res. 2020, 53, e9560. [Google Scholar] [CrossRef]
- Piñeros, M.; Laversanne, M.; Barrios, E.; Cancela, M.C.; de Vries, E.; Pardo, C.; Bray, F. An Updated Profile of the Cancer Burden, Patterns and Trends in Latin America and the Caribbean. Lancet Reg. Health Am. 2022, 13, 100294. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Cervical Cancer. Available online: https://www.paho.org/en/topics/cervical-cancer (accessed on 29 July 2025).
- Capote Negrin, L.G. Epidemiology of Cervical Cancer in Latin America. Ecancermedicalscience 2015, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Feliu, A.; Stern, M.C.; Villarreal-Garza, C.; Ferreccio, C.; Espina, C. The Latin America and the Caribbean Code Against Cancer: An Opportunity for Empowerment and Progress. Lancet Reg. Health Am. 2023, 28, 100644. [Google Scholar] [CrossRef]
- Pan American Health Organization. Progress Toward Implementation of Human Papillomavirus Vaccination—The Americas 2006–2010. Available online: https://www.paho.org/sites/default/files/SNE3305.pdf (accessed on 30 July 2025).
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global Estimates of Human Papillomavirus Vaccination Coverage by Region and Income Level: A Pooled Analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- HPV Information Centre. Human Papillomavirus and Related Cancers, Fact Sheet 2023. Available online: https://hpvcentre.net/statistics/reports/PAN_FS.pdf (accessed on 30 July 2025).
- Ferlay, J.; Shin, H.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Panama: Provinces & Major Urban Places—Population Statistics, Maps, Charts, Weather and Web Information. City Population. Available online: https://www.citypopulation.de/en/panama/cities/ (accessed on 30 July 2025).
- Boletín Estadístico del Programa Ampliado de Inmunizaciones 2022. SPP Panama. Available online: https://spp.com.pa/publicaciones/documentos-interes/vacunacion/boletin-estadistico-del-programa-ampliado-de-inmunizaciones-2022.pdf (accessed on 30 July 2025).
- Brinton, L.A.; Reeves, W.C.; Brenes, M.M.; Herrero, R.; Gaitan, E.; Tenorio, F.; de Britton, R.C.; Garcia, M.; Rawls, W.E. The male factor in the etiology of cervical cancer among sexually monogamous women. Int. J. Cancer 1989, 44, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Qendri, V.; Bogaards, J.A.; Berkhof, J. Who Will Benefit From Expanding HPV Vaccination Programs to Boys? JNCI Cancer Spectr. 2018, 2, pky076. [Google Scholar] [CrossRef]
- Luu, X.Q.; Jun, J.K.; Suh, M.; Oh, J.; Yu, S.; Choi, K.S. Cervical Cancer Screening, HPV Vaccination, and Cervical Cancer Elimination. JAMA Netw. Open 2025, 8, e2526683. [Google Scholar] [CrossRef]
- Pan American Health Organization. Vaccination Week in the Americas—VWA. Available online: https://www.paho.org/en/vaccination-week-americas (accessed on 28 July 2025).
- Pan American Health Organization (OPS/OMS). Panamá Lanza Estrategia de Eliminación del Cáncer Cervicouterino Hacia el 2030. Available online: https://www.paho.org/es/noticias/26-3-2025-panama-lanza-estrategia-eliminacion-cancer-cervicouterino-hacia-2029 (accessed on 30 July 2025).
- Ministerio de Salud de Panamá. Plan Nacional para la Prevención y Control del Cáncer 2010–2015. Available online: https://www.iccp-portal.org/sites/default/files/plans/Plan_nacional_para_la_prevención_y_control_del_cáncer_2010_-_2015.pdf (accessed on 30 July 2025).
- Ministerio de Salud de Panamá. Resolución No. 627 de 2 de Mayo de 2018. Normas de Prevención, Detección, y Seguimiento de las Lesiones Preinvasoras de Cuello Uterino y Guías de Manejo. Available online: https://www.minsa.gob.pa/sites/default/files/programas/resolucion_627.pdf (accessed on 30 July 2025).
- Ministerio de Salud de Panamá. Registro Nacional de Cáncer. Available online: https://www.minsa.gob.pa/contenido/registro-nacional-del-cancer (accessed on 30 July 2025).
- Quintana, H.K.; Velásquez, I.M.; Rodríguez, M.; Gómez, B.; Espino, M.; Valdés, P.; Roa, R. History of the National Cancer Registry of Panama. J. Regist. Manag. 2023, 50, 19–25. [Google Scholar]
- Contraloría General de la República. Instituto Nacional de Estadísticas y Censo. Available online: https://www.inec.gob.pa (accessed on 30 July 2025).
- World Health Organization-WHO. Data Quality Assurance. Module 1. Framework and Metrics; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bray, F.; Znaor, A.; Cueva, P.; Korir, A.; Swaminathan, R.; Ullrich, A.; Wang, S.A.; Parkin, D.M. Planning and Developing Population-Based Cancer Registration in Low- and Middle-Income Settings; International Agency for Research on Cancer-IARC: Lyon, France, 2014; ISBN -13978-92-832-0437-4. [Google Scholar]
- Calvo, A.E.; Tristán Urrutia, A.G.; Vargas-Zambrano, J.C.; López Castillo, H. Pertussis vaccine effectiveness following country-wide implementation of a hexavalent acellular pertussis immunization schedule in infants and children in Panama. Hum. Vaccines Immunother. 2024, 20, 2389577. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization-WHO; United Nations Children’s Fund-UNICEF. Joint Reporting Process. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/who-unicef-joint-reporting-process (accessed on 1 November 2025).
- PanAmerican Health Organization-PAHO. Immunization Data and Statistics. Available online: https://www.paho.org/en/topics/immunization/immunization-data-and-statistics (accessed on 16 November 2025).
- Wang, W.; Kothari, S.; Baay, M.; Garland, S.M.; Giuliano, A.R.; Nygård, M.; Velicer, C.; Tota, J.; Sinha, A.; Skufca, J.; et al. Real-world impact and effectiveness assessment of the quadrivalent HPV vaccine: A systematic review of study designs and data sources. Expert Rev. Vaccines 2022, 21, 227–240. [Google Scholar] [CrossRef]
- PanAmerican Health Organization. 16ª Reunión de la Comisión Regional de Certificación de la Erradicación de la Polio en la Región de las Américas. 2024. Available online: https://iris.paho.org/handle/10665.2/60232 (accessed on 1 November 2025).
- Lopez-Cortes, A.; Didonè, F.; Botta, L.; Hjalgrim, L.L.; Jakab, Z.; Cañete Nieto, A.; Stiller, C.; Zeller, B.; Gatta, G.; Pritchard-Jones, K.; et al. Cancer data quality and harmonization in Europe: The experience of the BENCHISTA Project—International benchmarking of childhood cancer survival by stage. Front. Oncol. 2023, 13, 1232451. [Google Scholar] [CrossRef]
- Tiangco, B.; Daguit, S.E.J.; Astrologo, N.C.; Flores, L.; Parma, R.N.; Celi, L.A. Challenges in the maintenance of an open hospital-based cancer registry system in a low-to-middle-income country (LMIC): 2017–2022 experience. PLoS Digit. Health 2024, 3, e0000328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vamos, C.A.; Calvo, A.E.; Daley, E.M.; Giuliano, A.R.; Castillo, H.L. Knowledge, Behavioral, and Sociocultural Factors Related to Human Papillomavirus Infection and Cervical Cancer Screening Among Inner-City Women in Panama. J. Community Health 2015, 40, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Nilbert, M.; Thomsen, L.A.; Winther Jensen, J.; Møller, H.; Borre, M.; Widenlou Nordmark, A.; Lambe, M.; Brändström, H.; Kørner, H.; Møller, B.; et al. The power of empirical data; lessons from the clinical registry initiatives in Scandinavian cancer care. Acta Oncol. 2020, 59, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Larønningen, S.; Skog, A.; Engholm, G.; Ferlay, J.; Johannesen, T.B.; Kristiansen, M.F.; Knoors, D.; Kønig, S.M.; Olafsdottir, E.J.; Pejicic, S.; et al. Nordcan.R: A new tool for federated analysis and quality assurance of cancer registry data. Front. Oncol. 2023, 13, 1098342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, C.; Messerschmidt, L.; Bravo, I.; Waldbauer, M.; Bhavikatti, R.; Schenk, C.; Grujic, V.; Model, T.; Kubinec, R.; Barceló, J. A General Primer for Data Harmonization. Sci Data. 2024, 11, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, E.L.; Akpan, I.N.; Taskin, T.; Alkhatib, S.; Grace, J.; Daley, E.M.; Wheldon, C.W. Clinician Perspectives on Implementing HPV Vaccination Guidelines into Practice. Med. Comport. 2024, 51, 233–240. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cytological Screening in the Control of Cervical Cancer: Technical Guidelines; WHO: Geneva, Switzerland, 1988; Available online: https://iris.who.int/handle/10665/41662 (accessed on 29 July 2025).
- Organización Mundial de la Salud. Control Integral del Cáncer Cervicouterino: Guía de Prácticas Esenciales; Organización Mundial de la Salud: Geneva, Switzerland, 2007; p. 279. ISBN 978-92-4-354700-8. [Google Scholar]
- Ministerio de Salud de Panamá. Normas de Prevención, Detección y Guías de Manejo y Seguimiento de las Lesiones Preinvasoras del Cuello Uterino; Ministerio de Salud: Panama city, Panamá; Organización Panamericana de la Salud: Washington, DC, USA, 2010; p. 113. ISBN 978-9962-642-60-2. [Google Scholar]
- Ministerio de Salud de Panamá/Caja de Seguro Social. Normas de Prevención, Detección y Seguimiento de las Lesiones Preinvasoras del Cuello Uterino y Guías de Manejo. Año: 2017. República de Panamá. p. 12. Available online: https://www.minsa.gob.pa/sites/default/files/programas/normas_de_prevencion_cacu.pdf (accessed on 16 November 2025).
- Garrido, J.L. Analysis of the Cancer Cases in the Republic of Panama 1982–1989. Eur. J. Gynaecol. Oncol. 1993, 14, 68–70. [Google Scholar]
- Garrido, J.L. Cervical Dysplasias 1982–2010 in the Republic of Panama: Diagnosis, Treatment, and Evolution. Eur. J. Gynaecol. Oncol. 2014, 35, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud de Panamá. Análisis de Situación de Citología Cervical a Nivel de las Regiones del MINSA, Pe riodo 2018–2019. Available online: https://www.minsa.gob.pa/sites/default/files/programas/citologia_cervical.pdf (accessed on 30 July 2025).
- Calvo, A. Social Construction of Cervical Cancer Screening Among Women in Panama City, Panama. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2005. Available online: https://digitalcommons.usf.edu/etd/2805/ (accessed on 29 July 2025).
- Calvo, A.; Brown, K.M.; McDermott, R.J.; Bryant, C.A.; Coreil, J.; Loseke, D. Social construction of cervical cancer screening among Panamanian women. Am. J. Health Educ. 2012, 43, 153–163. [Google Scholar] [CrossRef]
- Trigg, M.; Calvo, A. An Analysis of the COVID-19 Pandemic Control Measures on Latin American Women: A Systematic Review. SSRN Electron. J. 2022, 25, 1–25. [Google Scholar] [CrossRef]
- Ministerio de Salud de Panamá. Boletín Epidemiológico Semanal: Semana No. 21. Available online: https://www.minsa.gob.pa/sites/default/files/publicacion-general/boletin_epidemiologico_sem_21_2021.pdf (accessed on 30 July 2025).
- DeSieghardt, A.; Ding, L.; Ermel, A.; Franco, E.L.; Dagnall, C.; Brown, D.R.; Yao, S.; Kahn, J.A. Population-Level Effectiveness and Herd Protection 17 Years After HPV Vaccine Introduction. JAMA Pediatr. 2025, e253568. [Google Scholar] [CrossRef]
- Ministerio de Salud de Panamá. Análisis Epidemiológico de la Situación de la Infecciones de Transmisión Sexual, Año: 2021 República de Panamá. Available online: https://www.minsa.gob.pa/sites/default/files/publicacion-general/analisis_de_las_its.pdf (accessed on 31 July 2025).
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: A systematic review of 10 years of real-world experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Guo, F.; Cofie, L.E.; Berenson, A.B. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine introduction. Am. J. Prev. Med. 2018, 55, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Leval, A.; Herweijer, E.; Ploner, A.; Eloranta, S.; Fridman Simard, J.; Dillner, J.; Young, C.; Netterlid, E.; Sparén, P.; Arnheim-Dahlström, L. Quadrivalent human papillomavirus vaccine effectiveness: A Swedish national cohort study. J. Natl. Cancer Inst. 2013, 105, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.L.; Palmer, T.J.; Cuschieri, K.; Kavanagh, K.; Roy, K. Assessing real world vaccine effectiveness: A review of Scotland’s approach to monitoring human papillomavirus (HPV) vaccine impact on HPV infection and cervical disease. Vaccine 2024, 42, 126177. [Google Scholar] [CrossRef]
- López Castillo, H.; Hess-Holtz, M.; Jenkins, L.; Vega, N.; Eskildsen, G.; Torre, D.; Calvo, A. Estimating the Size, Sociodemographic Characteristics, and STI Status of Three Key Populations at Risk for HIV Infection in Panama Using Capture–Recapture Methods: The PEMAR Study. Ann. LGBTQ Public Popul. Health 2022, 3, 191–208. [Google Scholar] [CrossRef]
- Phillips, S.A.; Denoël, S.; Wentzensen, N.; Arbyn, M. Accuracy of HPV Self-Collection Compared with Clinician-Collected HPV Testing and Cytology: A Meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2025, 34, 1467–1471. [Google Scholar] [CrossRef]
- Ministerio de Salud de Panamá. Este Fin de Semana No Habrá Jornada de Vacunación. Available online: https://www.minsa.gob.pa/noticia/este-fin-de-semana-no-habra-jornada-de-vacunacion (accessed on 31 July 2025).
- Zammit, C.; Ashfaq, M.; Boyd, L.; Paton, C.; Jiang, J.; Brotherton, J.; Nightingale, C. Considerations in the development of an mHealth approach to increase cervical screening participation in primary care in Victoria, Australia. Aust. J. Prim. Health 2025, 31, PY25101. [Google Scholar] [CrossRef]
- Gantz, L.; Calvo, A.; Hess-Holtz, M.; Gonzales, F.; Alguero, L.; Murphy, S.; Moran, M.; Frank, L.B.; Chatterjee, J.S.; de Herrera, P.A.; et al. Predictors of HPV Knowledge and HPV Vaccine Awareness Among Women in Panama City, Panama. World Med. Health Policy 2019, 11, 95–118. [Google Scholar] [CrossRef]
- Secretariat of Science, Technology and Innovation-SENACYT of Panama. La Bancada Independiente VAMOS Entrega Subsidio Postelectoral a la Senacyt Para Financiar Proyectos de Investigación Científica, Especialmente en la Lucha Contra el Cáncer. República de Panamá. Available online: https://www.senacyt.gob.pa/la-bancada-independiente-vamos-entrega-subsidio-postelectoral-a-la-senacyt-para-financiar-proyectos-de-investigacion-cientifica (accessed on 1 August 2025).
- National Cancer Institute. Cancer Stat Facts: Cervical Cancer. Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 30 July 2025).
- American Cancer Society. Screening Leads to Cervical Cancer Decline in the United States. Available online: https://www.fightcancer.org/sites/default/files/FINAL%20-%20Cervical%20Cancer%20General%20Factsheet%2001.08.20.pdf (accessed on 28 July 2025).
- Choudhury, S.A.; Choudhury, N.A.; Humphrey, A.D.; Berthaud, V.; Ladson, G.; Tucker, V.A.; Vermund, S.H. Higher prevalence of human papillomavirus-related cervical precancerous abnormalities in HIV-infected compared to HIV-uninfected women. J. Natl. Med. Assoc. 2016, 108, 19–23. [Google Scholar] [CrossRef]


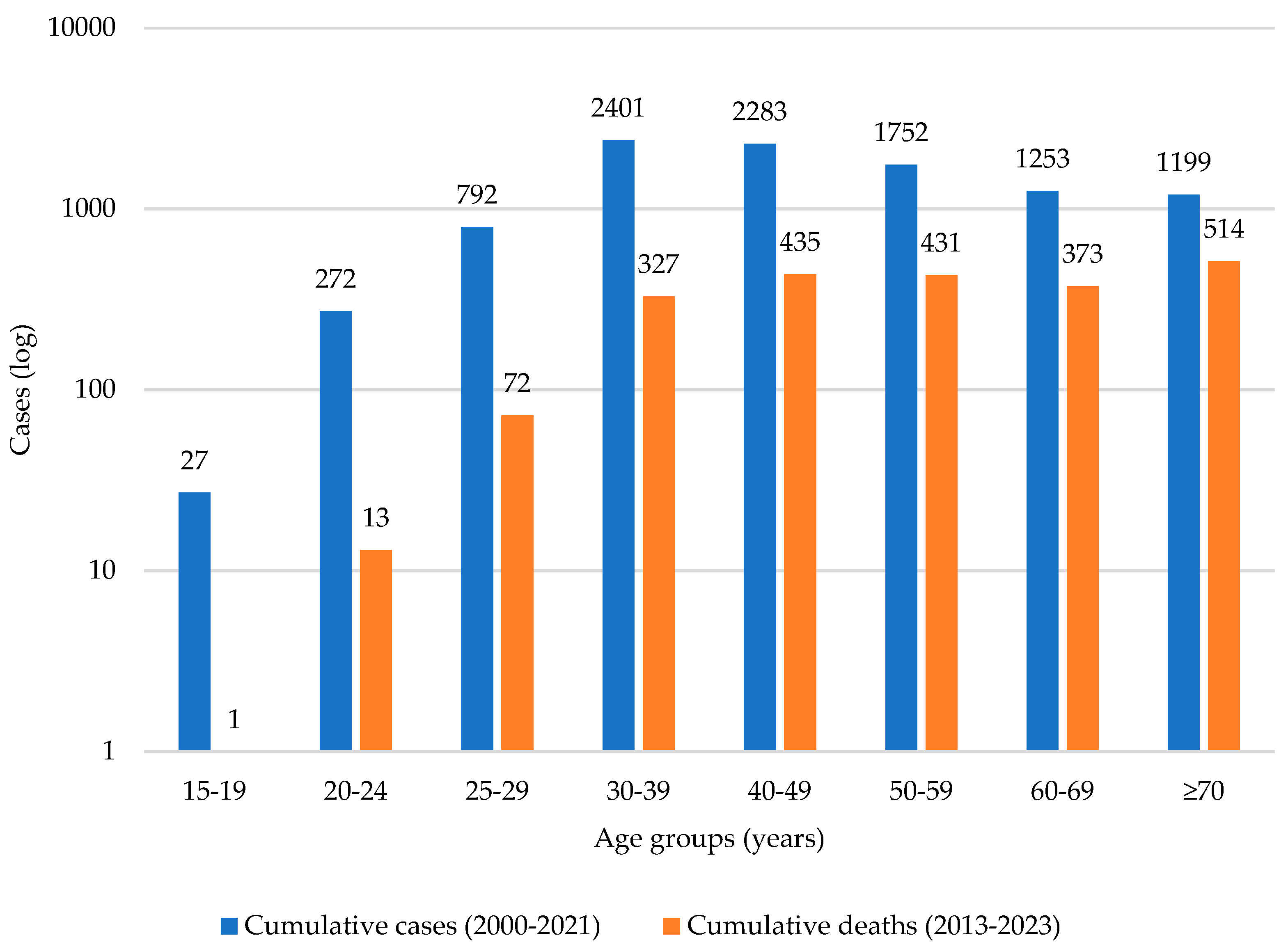
| Term | Estimate | Standard Error | p Value |
|---|---|---|---|
| 9.52 | 3.11 | 0.01 | |
| −0.44 | 0.43 | 0.32 | |
| −5.81 | 16.03 | 0.72 | |
| Time | 0.20 | 1.49 | 0.89 |
| Interrupted Time Series Analysis | ||
| Immediate Model Estimate (MSE = 32.20, rDF = 28, n = 32) | 10-Year Model Estimate (MSE = 36.65, rDF = 28, n = 32) | |
| Intercept | 81.30 (p < 0.001) | 80.12 (p < 0.001) |
| Time before intervention | −2.18 (p < 0.001) | −2.10 (p < 0.001) |
| Year of intervention | −8.86 (p = 0.12) | 8.37 (p = 0.33) |
| Time after intervention | 1.59 (p = 0.03) | 0.44 (p = 0.89) |
| Durbin–Watson Statistics | 2.03 (p = 0.34) | 1.83 (p = 0.18) |
| Time Series Regression with Vaccine Coverage | ||
| Immediate Model Estimate (MSE = 27.74, rDF = 12, n = 14) | 10-Year Model Estimate (MSE = 7.43, rDF = 2, n = 4) | |
| Intercept | 38.73 (p = 0.001) | 42.45 (p = 0.047) |
| Vaccination Rates | −0.09 (p = 0.39) | −0.15 (p = 0.30) |
| Durbin–Watson Statistics | 1.75 (p = 0.35) | 1.73 (p = 0.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, A.; Hall, S.; Melgar Cossich, V.B.; Andreadakis, J.; López Castillo, H.; Pinto, D.; Hewitt, I.d. HPV Vaccine Uptake and Cervical Cancer Trends in Panama: A Reference Point for Future Impact Studies. Vaccines 2025, 13, 1173. https://doi.org/10.3390/vaccines13111173
Calvo A, Hall S, Melgar Cossich VB, Andreadakis J, López Castillo H, Pinto D, Hewitt Id. HPV Vaccine Uptake and Cervical Cancer Trends in Panama: A Reference Point for Future Impact Studies. Vaccines. 2025; 13(11):1173. https://doi.org/10.3390/vaccines13111173
Chicago/Turabian StyleCalvo, Arlene, Sabrina Hall, Verónica B. Melgar Cossich, Jonathan Andreadakis, Humberto López Castillo, Dalys Pinto, and Itzel de Hewitt. 2025. "HPV Vaccine Uptake and Cervical Cancer Trends in Panama: A Reference Point for Future Impact Studies" Vaccines 13, no. 11: 1173. https://doi.org/10.3390/vaccines13111173
APA StyleCalvo, A., Hall, S., Melgar Cossich, V. B., Andreadakis, J., López Castillo, H., Pinto, D., & Hewitt, I. d. (2025). HPV Vaccine Uptake and Cervical Cancer Trends in Panama: A Reference Point for Future Impact Studies. Vaccines, 13(11), 1173. https://doi.org/10.3390/vaccines13111173






