Hepatitis Vaccines: Recent Advances and Challenges
Abstract
1. Introduction
2. Hepatitis A Vaccine
2.1. Hepatitis A
2.2. Types of Hepatitis A Vaccine
2.2.1. HepA-I
2.2.2. HepA-L
2.3. Challenges of Hepatitis A Vaccine Administration
3. Hepatitis B Vaccine
3.1. Hepatitis B
3.2. Preventive Hepatitis B Vaccines
3.2.1. Plasma-Derived Hepatitis B Vaccine
3.2.2. Recombinant Hepatitis B Vaccine
3.2.3. Next-Generation Recombinant Hepatitis B Vaccine
3.2.4. Novel Adjuvant Hepatitis B Vaccine
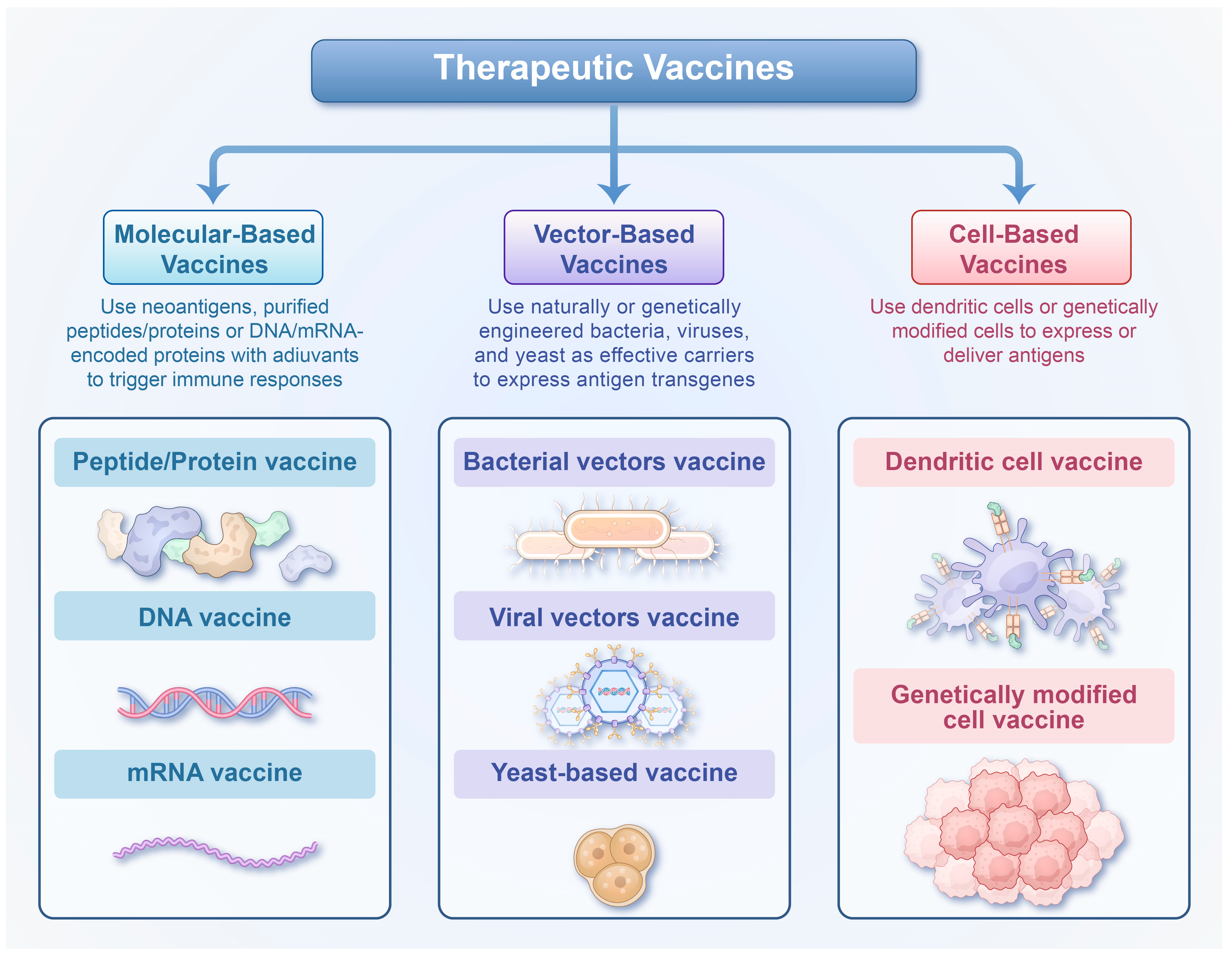
3.3. Therapeutic Hepatitis B Vaccines
3.4. Challenges of Hepatitis B Vaccine Administration
4. Hepatitis C Vaccine
4.1. Hepatitis C
4.2. Development and Challenges of the Hepatitis C Vaccine
5. Hepatitis D Vaccine
6. Hepatitis E Vaccine
6.1. Hepatitis E
6.2. Types of Hepatitis E Vaccine
6.3. Challenges of Hepatitis E Vaccine Administration
7. Combination Hepatitis Vaccines
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 27 September 2025).
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Xu, R.; Awan, M.U.N.; Song, Y.; Han, Q.; Xia, X.; Wei, J.; Xu, J.; Peng, J.; Zhang, J. HBV Vaccines: Advances and Development. Vaccines 2023, 11, 1862. [Google Scholar] [CrossRef]
- Costa, G.L.; Sautto, G.A. Towards an HCV vaccine: An overview of the immunization strategies for eliciting an effective B-cell response. Expert Rev. Vaccines 2025, 24, 96–120. [Google Scholar] [CrossRef] [PubMed]
- The Immunological Basis for Immunization Series: Module 18—Hepatitis A. Available online: https://www.who.int/publications/i/item/97892516327 (accessed on 27 September 2025).
- Hepatitis, A. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a (accessed on 27 September 2025).
- Kanda, T.; Sasaki, R.; Masuzaki, R.; Matsumoto, N.; Ogawa, M.; Moriyama, M. Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection. Viruses 2020, 12, 533. [Google Scholar] [CrossRef]
- Bakker, M.; Bunge, E.M.; Marano, C.; de Ridder, M.; De Moerlooze, L. Immunogenicity, effectiveness and safety of combined hepatitis A and B vaccine: A systematic literature review. Expert Rev. Vaccines 2016, 15, 829–851. [Google Scholar] [CrossRef]
- Cui, F.; Liang, X.; Wang, F.; Zheng, H.; Hutin, Y.J.; Yang, W. Development, production, and postmarketing surveillance of hepatitis A vaccines in China. J. Epidemiol. 2014, 24, 169–177. [Google Scholar] [CrossRef]
- Shi, J.; Shen, A.; Cheng, Y.; Zhang, C.; Yang, X. 30-Year Development of Inactivated Virus Vaccine in China. Pharmaceutics 2023, 15, 2721. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Zhang, L. Hepatitis A vaccination. Hum. Vaccin. Immunother. 2020, 16, 1565–1573. [Google Scholar] [CrossRef]
- Fukushima, S.; Kiyohara, T.; Ishii, K.; Nakano, T.; Hamada, A. Immunogenicity of aluminum-adsorbed hepatitis A vaccine (Havrix®) administered as a third dose after primary doses of Japanese aluminum-free hepatitis A vaccine (Aimmugen®) for Japanese travelers to endemic countries. Vaccine 2017, 35, 6412–6415. [Google Scholar] [CrossRef] [PubMed]
- Weekly Epidemiological Record. Available online: https://iris.who.int/server/api/core/bitstreams/61dbf312-2a7c-41d5-a2d2-782ec11cb711/content (accessed on 27 September 2025).
- Cohall, A.; Zucker, J.; Krieger, R.; Scott, C.; Guido, C.; Hakala, S.; Carnevale, C. Missed Opportunities for Hepatitis A Vaccination Among MSM Initiating PrEP. J. Community Health 2020, 45, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Theeten, H.; Van Herck, K.; Van Der Meeren, O.; Crasta, P.; Van Damme, P.; Hens, N. Long-term antibody persistence after vaccination with a 2-dose Havrix (inactivated hepatitis A vaccine): 20 years of observed data, and long-term model-based predictions. Vaccine 2015, 33, 5723–5727. [Google Scholar] [CrossRef]
- Chappuis, F.; Farinelli, T.; Deckx, H.; Sarnecki, M.; Go, O.; Salzgeber, Y.; Stals, C. Immunogenicity and estimation of antibody persistence following vaccination with an inactivated virosomal hepatitis A vaccine in adults: A 20-year follow-up study. Vaccine 2017, 35, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Wang, X.Y. Live attenuated hepatitis A vaccines developed in China. Hum. Vaccin. Immunother. 2014, 10, 659–666. [Google Scholar] [CrossRef]
- Shah, N.; Faridi, M.; Mitra, M.; Bavdekar, A.; Karadkhele, A.; Puppalwar, G.; Jain, R. Review of long term immunogenicity and tolerability of live hepatitis A vaccine. Hum. Vaccin. Immunother. 2020, 16, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Jiang, Q.; Gong, Y. Epidemiological effects of live attenuated hepatitis A vaccine (H(2)-strain): Results of A 10-year observation. Zhonghua Liu Xing Bing Xue Za Zhi 2001, 22, 188–190. [Google Scholar]
- Zhuang, F.C.; Mao, Z.A.; Jiang, L.M.; Wu, J.; Chen, Y.Q.; Jiang, Q.; Chen, N.L.; Chai, S.A.; Mao, J.S. [Long-term immunogenicity and effectiveness of live attenuated hepatitis A vaccine (H2-strain)-a study on the result of 15 years’ follow up]. Zhonghua Liu Xing Bing Xue Za Zhi 2010, 31, 1332–1335. [Google Scholar]
- Mayorga, O.; Bühler, S.; Jaeger, V.K.; Bally, S.; Hatz, C.; Frösner, G.; Protzer, U.; Van Damme, P.; Egger, M.; Herzog, C. Single-Dose Hepatitis A Immunization: 7.5-Year Observational Pilot Study in Nicaraguan Children to Assess Protective Effectiveness and Humoral Immune Memory Response. J. Infect. Dis. 2016, 214, 1498–1506. [Google Scholar] [CrossRef]
- Balogun, O.; Brown, A.; Angelo, K.M.; Hochberg, N.S.; Barnett, E.D.; Nicolini, L.A.; Asgeirsson, H.; Grobusch, M.P.; Leder, K.; Salvador, F.; et al. Acute hepatitis A in international travellers: A GeoSentinel analysis, 2008–2020. J. Travel Med. 2022, 29, taac013. [Google Scholar] [CrossRef]
- Brandl, M.; Schmidt, A.J.; Marcus, U.; Duffell, E.; Severi, E.; Mozalevskis, A.; Kivite-Urtane, A.; An der Heiden, M.; Dudareva, S. Self-reported hepatitis A and B vaccination coverage among men who have sex with men (MSM), associated factors and vaccination recommendations in 43 countries of the WHO European Region: Results from the European MSM Internet Survey, EMIS-2017. Eurosurveillance 2024, 29, 2400100. [Google Scholar] [CrossRef]
- Herzog, C.; Van Herck, K.; Van Damme, P. Hepatitis A vaccination and its immunological and epidemiological long-term effects - a review of the evidence. Hum. Vaccin. Immunother. 2021, 17, 1496–1519. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, J.L.; Garcia Garrido, H.M.; Tanck, M.W.; Maurer, I.; Harskamp, A.M.; Kootstra, N.; Grobusch, M.P.; Goorhuis, A. Hepatitis a vaccine immunogenicity and boostability in adults receiving immunosuppressive therapy and adults living with HIV: A prospective single-centre cohort study. J. Travel Med. 2025, 32, taae125. [Google Scholar] [CrossRef]
- World Health Statistics 2025. Available online: https://iris.who.int/bitstream/handle/10665/381418/9789240110496-eng.pdf?sequence=1 (accessed on 27 September 2025).
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, X.; Xi, Y. Intrauterine Infection and Mother-to-Child Transmission of Hepatitis B Virus: Route and Molecular Mechanism. Infect. Drug Resist. 2022, 15, 1743–1751. [Google Scholar] [CrossRef]
- Guidelines for the Prevention, Diagnosis, Care and Treatment for People with Chronic Hepatitis B Infection. Available online: https://www.who.int/publications/i/item/9789240090903 (accessed on 27 September 2025).
- Asandem, D.A.; Segbefia, S.P.; Kusi, K.A.; Bonney, J.H.K. Hepatitis B Virus Infection: A Mini Review. Viruses 2024, 16, 724. [Google Scholar] [CrossRef]
- Chien, R.N.; Liaw, Y.F. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses 2022, 14, 434. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, J.S.; Ahn, S.H. Hepatitis B Virus Cure: Targets and Future Therapies. Int. J. Mol. Sci. 2020, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Yeh, M.L.; Wong, G.L.; Chen, C.H.; Peng, C.Y.; Buti, M.; Enomoto, M.; Xie, Q.; Trinh, H.; Preda, C.; et al. Incidences and Determinants of Functional Cure During Entecavir or Tenofovir Disoproxil Fumarate for Chronic Hepatitis B. J. Infect. Dis. 2021, 224, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Edey, M.; Barraclough, K.; Johnson, D.W. Review article: Hepatitis B and dialysis. Nephrology 2010, 15, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Chang, M.H.; Chen, H.L.; Wu, J.F.; Chang, C.H.; Hsu, H.Y.; Ni, Y.H. Universal Infant Hepatitis B Virus (HBV) Vaccination for 35 Years: Moving Toward the Eradication of HBV. J. Infect. Dis. 2022, 225, 431–435. [Google Scholar] [CrossRef]
- Correction to: Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J. Infect. Dis. 2024, 230, e504. [CrossRef]
- Zhao, H.; Zhou, X.; Zhou, Y.H. Hepatitis B vaccine development and implementation. Hum. Vaccin. Immunother. 2020, 16, 1533–1544. [Google Scholar] [CrossRef]
- Immunization Coverage. Available online: https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage (accessed on 27 September 2025).
- Di Lello, F.A.; Martínez, A.P.; Flichman, D.M. Insights into induction of the immune response by the hepatitis B vaccine. World J. Gastroenterol. 2022, 28, 4249–4262. [Google Scholar] [CrossRef]
- Yan, R.; Sun, M.; Yang, H.; Du, S.; Sun, L.; Mao, Y. 2024 latest report on hepatitis B virus epidemiology in China: Current status, changing trajectory, and challenges. Hepatobiliary Surg. Nutr. 2025, 14, 66–77. [Google Scholar] [CrossRef]
- Cao, M.; Fan, J.; Lu, L.; Fan, C.; Wang, Y.; Chen, T.; Zhang, S.; Yu, Y.; Xia, C.; Lu, J.; et al. Long term outcome of prevention of liver cancer by hepatitis B vaccine: Results from an RCT with 37 years. Cancer Lett. 2022, 536, 215652. [Google Scholar] [CrossRef]
- Vesikari, T.; Langley, J.M.; Popovic, V.; Diaz-Mitoma, F. PreHevbrio: The first approved 3-antigen hepatitis B vaccine. Expert. Rev. Vaccines 2023, 22, 1041–1054. [Google Scholar] [CrossRef]
- Atsmon, J.; Machluf, N.; Yayon-Gur, V.; Sabbah, C.; Spaans, J.N.; Yassin-Rajkumar, B.; Anderson, D.E.; Popovic, V.; Diaz-Mitoma, F. Rapid and high seroprotection rates achieved with a tri-antigenic Hepatitis B vaccine in healthy young adults: Results from a Phase IV study. Vaccine 2021, 39, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Langley, J.M.; Segall, N.; Ward, B.J.; Cooper, C.; Poliquin, G.; Smith, B.; Gantt, S.; McElhaney, J.E.; Dionne, M.; et al. Immunogenicity and safety of a tri-antigenic versus a mono-antigenic hepatitis B vaccine in adults (PROTECT): A randomised, double-blind, phase 3 trial. Lancet Infect. Dis. 2021, 21, 1271–1281. [Google Scholar] [CrossRef]
- Alon, D.; Stein, G.Y.; Hadas-Golan, V.; Tau, L.; Brosh, T.; Turner, D. Immunogenicity of Sci-B-Vac (a Third-Generation Hepatitis B Vaccine) in HIV-Positive Adults. Isr. Med. Assoc. J. 2017, 19, 143–146. [Google Scholar] [PubMed]
- Weinstein, T.; Chagnac, A.; Boaz, M.; Ori, Y.; Herman, M.; Zevin, D.; Schmilovitz-Weiss, H.; Gafter, U. Improved immunogenicity of a novel third-generation recombinant hepatitis B vaccine in patients with end-stage renal disease. Nephron Clin. Pr. 2004, 97, c67–c72. [Google Scholar] [CrossRef] [PubMed]
- Beran, J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin. Biol. Ther. 2008, 8, 235–247. [Google Scholar] [CrossRef]
- Fendrix Suspension for Injection. Available online: https://www.medicines.org.uk/emc/files/pil.137.pdf (accessed on 27 September 2025).
- Horta, D.; Forné, M.; Agustí, A.; Raga, A.; Martín-Cardona, A.; Hernández-Soto, J.M.; Ruiz-Ramírez, P.; Esteve-Comas, M. Efficacy of Hepatitis B Virus Vaccines HBVaxpro40© and Fendrix© in Patients with Chronic Liver Disease in Clinical Practice. Vaccines 2022, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, M.; Zuccotti, G.; Pflomm, J.M. A Two-Dose Hepatitis B Vaccine for Adults (Heplisav-B). JAMA 2018, 319, 822–823. [Google Scholar] [CrossRef]
- Lee, G.H.; Lim, S.G. CpG-Adjuvanted Hepatitis B Vaccine (HEPLISAV-B®) Update. Expert Rev. Vaccines 2021, 20, 487–495. [Google Scholar] [CrossRef]
- Kosinska, A.D.; Bauer, T.; Protzer, U. Therapeutic vaccination for chronic hepatitis B. Curr. Opin. Virol. 2017, 23, 75–81. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, D.; Li, Y.; Yang, L. Development of therapeutic vaccines for the treatment of diseases. Mol. Biomed. 2022, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lim, T.H.; Leerapun, A.; Weltman, M.; Jia, J.; Lim, Y.S.; Tangkijvanich, P.; Sukeepaisarnjaroen, W.; Ji, Y.; Le Bert, N.; et al. Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep. 2021, 3, 100361. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Le Bert, N.; Lai-Hung Wong, G.; Douglas, M.W.; Lee, A.; Zhu, C.; Wang, B.; Lv, J.; Li, D.; Tan, Y.; et al. The Impact of Hepatitis B Surface Antigen Reduction via Small Interfering RNA Treatment on Natural and Vaccine (BRII-179)-Induced Hepatitis B Virus-Specific Humoral and Cellular Immune Responses. Gastroenterology 2025, 169, 136–149. [Google Scholar] [CrossRef]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS ONE 2018, 13, e0201236. [Google Scholar] [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Penton, E.; Gerardo, G.N.; Hiasa, Y. Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial. Pathogens 2021, 10, 1440. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.; Zhang, J.; Mao, Q.; Gong, G.; Sun, Y.; Chen, Y.; Wang, M.; Tan, D.; Gong, Z.; et al. Efficacy and safety of a nanoparticle therapeutic vaccine in patients with chronic hepatitis B: A randomized clinical trial. Hepatology 2022, 75, 182–195. [Google Scholar] [CrossRef]
- Fontaine, H.; Kahi, S.; Chazallon, C.; Bourgine, M.; Varaut, A.; Buffet, C.; Godon, O.; Meritet, J.F.; Saïdi, Y.; Michel, M.L.; et al. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: A randomised trial—ANRS HB02 VAC-ADN. Gut 2015, 64, 139–147. [Google Scholar] [CrossRef]
- Yang, F.Q.; Rao, G.R.; Wang, G.Q.; Li, Y.Q.; Xie, Y.; Zhang, Z.Q.; Deng, C.L.; Mao, Q.; Li, J.; Zhao, W.; et al. Phase IIb trial of in vivo electroporation mediated dual-plasmid hepatitis B virus DNA vaccine in chronic hepatitis B patients under lamivudine therapy. World J. Gastroenterol. 2017, 23, 306–317. [Google Scholar] [CrossRef]
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; Ma, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial. Hum. Vaccin. Immunother. 2020, 16, 388–399. [Google Scholar] [CrossRef]
- Lok, A.S.; Pan, C.Q.; Han, S.H.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Janssen, H.L.A.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Yoshida, E.M.; Trinh, H.; Rodell, T.C.; Laccabue, D.; et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients with Chronic Hepatitis. Gastroenterology 2019, 157, 227–241.e227. [Google Scholar] [CrossRef]
- Buti, M.; Bonanni, P.; Ladep, N.; Papatheodoridis, G.; Frühwein, M.; James, C.; Ward, J.W.; Vetter, V.; Cacciatore, P.; Kesters, D.; et al. Toward elimination of hepatitis A and B in Europe: Vaccination successes, challenges, and opportunities. Expert Rev. Vaccines 2025, 24, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Shamarina, D.; Sluga-O’Callaghan, M.; Kassianos, G.; Marijam, A.; Dave, V.; Davenport, E.; Andani, A.; Curran, D.; Dewda, P.; Steffen, R. Knowledge, Attitudes, and Practices of European Healthcare Professionals towards Hepatitis A and Hepatitis B Vaccination in at-Risk Adults. Vaccines 2023, 11, 1645. [Google Scholar] [CrossRef]
- Meier, M.A.; Berger, C.T. A simple clinical score to identify likely hepatitis B vaccination non-responders—Data from a retrospective single center study. BMC Infect. Dis. 2020, 20, 891. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tian, G.; Cui, Y.; Ding, C.; Deng, M.; Yu, C.; Xu, K.; Ren, J.; Yao, J.; Li, Y.; et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 2016, 6, 27251. [Google Scholar] [CrossRef]
- Hassnine, A.A.; Saber, M.A.; Fouad, Y.M.; Sarhan, H.; Elsayed, M.M.; Zaki, Z.M.; Abdelraheem, E.M.; Abdelhalim, S.M.; Elsayed, A.M. Clinical study on the efficacy of hepatitis B vaccination in hepatitis C virus related chronic liver diseases in Egypt. Virus Res. 2023, 323, 198953. [Google Scholar] [CrossRef]
- Tahir, A.; Shinkafi, S.H.; Alshrari, A.S.; Yunusa, A.; Umar, M.T.; Hudu, S.A.; Jimoh, A.O. A Comprehensive Review of Hepatitis B Vaccine Nonresponse and Associated Risk Factors. Vaccines 2024, 12, 710. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, G.U.; Maponga, T.; Rabie, H.; Taljaard, J. The role of new hepatitis B vaccines in South Africa. S. Afr. Med. J. 2024, 114, e1473. [Google Scholar] [CrossRef]
- Van Den Ende, C.; Marano, C.; Van Ahee, A.; Bunge, E.M.; De Moerlooze, L. The immunogenicity and safety of GSK’s recombinant hepatitis B vaccine in adults: A systematic review of 30 years of experience. Expert Rev. Vaccines 2017, 16, 811–832. [Google Scholar] [CrossRef]
- Hepatitis, C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 27 September 2025).
- Haga, Y.; Coates, S.; Ray, R. Hepatitis C virus chronicity and oncogenic potential: Vaccine development progress. Mol. Asp. Med. 2024, 99, 101305. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Ieva, F.; Ciarallo, M.; Caccianotti, B.; Santantonio, T.A. Acute Hepatitis C: Current Status and Future Perspectives. Viruses 2024, 16, 1739. [Google Scholar] [CrossRef]
- Pérez Jiménez, R.D.; Granados Monzón, R.; Hernández Febles, M.; Pena López, M.J. Acute hepatitis due to the hepatitisC virus: Where are the transmission occurring? Gastroenterol. Hepatol. 2022, 45, 192–197. [Google Scholar] [CrossRef]
- Sreekumar, B.K.; Taha, T.Y.; Ott, M. Taking cues from convalescence to improve vaccines against hepatitis C virus. J. Clin. Investig. 2022, 132, e161819. [Google Scholar] [CrossRef] [PubMed]
- Wolfisberg, R.; Holmbeck, K.; Billerbeck, E.; Thorselius, C.E.; Batista, M.N.; Fahnøe, U.; Lundsgaard, E.A.; Kennedy, M.J.; Nielsen, L.; Rice, C.M.; et al. Molecular Determinants of Mouse Adaptation of Rat Hepacivirus. J. Virol. 2023, 97, e0181222. [Google Scholar] [CrossRef]
- Vercauteren, K.; de Jong, Y.P.; Meuleman, P. Animal models for the study of HCV. Curr. Opin. Virol. 2015, 13, 67–74. [Google Scholar] [CrossRef]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Tabll, A.A.; Sohrab, S.S.; Ali, A.A.; Petrovic, A.; Steiner Srdarevic, S.; Siber, S.; Glasnovic, M.; Smolic, R.; Smolic, M. Future Prospects, Approaches, and the Government’s Role in the Development of a Hepatitis C Virus Vaccine. Pathogens 2023, 13, 38. [Google Scholar] [CrossRef]
- Skinner, N.E. Broadly Neutralizing Antibody Characteristics in Hepatitis C Virus Infection and Implications for Vaccine Design. Vaccines 2025, 13, 612. [Google Scholar] [CrossRef]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef]
- Costa, G.L.; Sautto, G.A. Exploring T-Cell Immunity to Hepatitis C Virus: Insights from Different Vaccine and Antigen Presentation Strategies. Vaccines 2024, 12, 890. [Google Scholar] [CrossRef]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef]
- Czarnota, A.; Offersgaard, A.; Owsianka, A.; Alzua, G.P.; Bukh, J.; Gottwein, J.M.; Patel, A.H.; Bieńkowska-Szewczyk, K.; Grzyb, K. Effect of Glycan Shift on Antibodies against Hepatitis C Virus E2 412-425 Epitope Elicited by Chimeric sHBsAg-Based Virus-Like Particles. Microbiol. Spectr. 2023, 11, e0254622. [Google Scholar] [CrossRef]
- Dustin, L.B.; Cashman, S.B.; Laidlaw, S.M. Immune control and failure in HCV infection—Tipping the balance. J. Leukoc. Biol. 2014, 96, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Lavie, M.; Hanoulle, X.; Dubuisson, J. Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies. Front. Immunol. 2018, 9, 910. [Google Scholar] [CrossRef]
- Sidorkiewicz, M. Hepatitis C Virus Uses Host Lipids to Its Own Advantage. Metabolites 2021, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vargas, J.; Pereira de Oliveira, R.; Jacquet, S.; Pontier, D.; Cosset, F.L.; Freitas, N. HDV-Like Viruses. Viruses 2021, 13, 1207. [Google Scholar] [CrossRef]
- Negro, F.; Lok, A.S. Hepatitis D: A Review. Jama 2023, 330, 2376–2387. [Google Scholar] [CrossRef]
- Peron, J.M.; Larrue, H.; Izopet, J.; Buti, M. The pressing need for a global HEV vaccine. J. Hepatol. 2023, 79, 876–880. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, H.; Huang, W.; T, J.H.; Geng, K.; Li, Z.; Wang, Y. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat. Mon. 2014, 14, e15618. [Google Scholar] [CrossRef]
- Hepatitis, E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 27 September 2025).
- Shrestha, M.P.; Scott, R.M.; Joshi, D.M.; Mammen, M.P., Jr.; Thapa, G.B.; Thapa, N.; Myint, K.S.; Fourneau, M.; Kuschner, R.A.; Shrestha, S.K.; et al. Safety and efficacy of a recombinant hepatitis E vaccine. N. Engl. J. Med. 2007, 356, 895–903. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, H.; Hu, Y.M.; Shi, C.B.; Wu, X.; Liang, Q.; Chi, C.P.; Li, L.; Liang, Z.L.; Meng, J.H.; et al. A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine 2017, 35, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Zhang, J.; Li, Y.M.; Ou, S.H.; Huang, G.Y.; He, Z.Q.; Ge, S.X.; Xian, Y.L.; Pang, S.Q.; Ng, M.H.; et al. A bacterially expressed particulate hepatitis E vaccine: Antigenicity, immunogenicity and protectivity on primates. Vaccine 2005, 23, 2893–2901. [Google Scholar] [CrossRef]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S. Hecolin vaccine: Long-term efficacy against HEV for a three-dose regimen. Lancet 2024, 403, 782–783. [Google Scholar] [CrossRef]
- WHO Position Paper on Hepatitis E Vaccines—May 2015. Available online: https://www.who.int/publications/i/item/WER9018-185-200 (accessed on 27 September 2025).
- Zhuang, C.; Wu, T.; Zhang, J.; Xia, N. Hecolin vaccination strategies for hepatitis E outbreak control in resource-constrained settings. Lancet Infect. Dis. 2025, 25, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.B.; Dudman, S.; Julin, C.H.; Ahmmed, F.; Stene-Johansen, K.; Sandbu, S.; Øverbø, J.; Dembinski, J.L.; Wisløff, T.; Rana, S.; et al. Receipt of hepatitis E vaccine and fetal loss in rural Bangladesh: Further analysis of a double-blind, cluster-randomised, controlled trial. Lancet Glob. Health 2024, 12, e1300–e1311. [Google Scholar] [CrossRef]
- Zhong, G.; Zhuang, C.; Hu, X.; Chen, Q.; Bi, Z.; Jia, X.; Peng, S.; Li, Y.; Huang, Y.; Zhang, Q.; et al. Safety of hepatitis E vaccination for pregnancy: A post-hoc analysis of a randomized, double-blind, controlled phase 3 clinical trial. Emerg. Microbes Infect. 2023, 12, 2185456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, J.; Wu, W.; Jiang, Z.; Yan, B.; Cao, Q.; Liu, H.; Pan, H.; Lv, J.; et al. Immunogenicity and safety of HepE Hecolin® in chronic hepatitis B patients at clinically stable stage: An open-label study in China. Hum. Vaccin. Immunother. 2025, 21, 2448882. [Google Scholar] [CrossRef] [PubMed]
- Boisnard, F.; Manson, C.; Serradell, L.; Macina, D. DTaP-IPV-HB-Hib vaccine (Hexaxim): An update 10 years after first licensure. Expert Rev. Vaccines 2023, 22, 1196–1213. [Google Scholar] [CrossRef]
- Agrawal, A.; Kolhapure, S.; Andani, A.; Ota, M.O.C.; Badur, S.; Karkada, N.; Mitra, M. Long-Term Persistence of Antibody Response with Two Doses of Inactivated Hepatitis A Vaccine in Children. Infect. Dis. Ther. 2020, 9, 785–796. [Google Scholar] [CrossRef]
- Beran, J.; Beutels, M.; Levie, K.; Van Damme, P.; Dieussaert, I.; Gillet, M.; Van Hoecke, C.; Tornieporth, N. A single dose, combined vaccine against typhoid fever and hepatitis A: Consistency, immunogenicity and reactogenicity. J. Travel Med. 2000, 7, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Beeching, N.J.; Clarke, P.D.; Kitchin, N.R.; Pirmohamed, J.; Veitch, K.; Weber, F. Comparison of two combined vaccines against typhoid fever and hepatitis A in healthy adults. Vaccine 2004, 23, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. DTaP-IPV-HepB-Hib Vaccine (Hexyon(®)): An Updated Review of its Use in Primary and Booster Vaccination. Paediatr. Drugs 2019, 21, 397–408. [Google Scholar] [CrossRef]

| Type | Trade Name | Year Licensed | HAV Strain | Adjuvant | Manufacturers |
|---|---|---|---|---|---|
| HepA-I | Havrix | 1992 | HM-175 | Aluminium hydroxide | GlaxoSmithKline Biologicals, London, UK |
| Vaqta | 1993 | CR-326 | Aluminium hydroxide | Merck, Sharp & Dohme Corporation, Kennywood, NJ, USA | |
| Avaxim | 1996 | GBM | Aluminium hydroxide | Sanofi Pasteur, Paris, France | |
| Epaxal | 1997 | RG-SB | Virosome | Crucell/Berna Biotech/Janssen Cilag Ltd., Bern, Switzerland | |
| Healive | 2002 | TZ84 | Aluminium hydroxide | Sinovac Biotch Co., Ltd., Beijing, China | |
| Weisairuian | 2006 | Lv-8 | Aluminium hydroxide | Institute of Medical Biology of the Chinese Academy of Medical Sciences, Kunming, China | |
| Veraxim | 2009 | YN5 | Aluminium hydroxide | Shanghai Wilson Bioengineering Inc., Shanghai, China | |
| Aimmugen | 1994 | KRM003 | Aluminium-free | KM Biologics Co., Ltd., Kumamoto, Japan | |
| HepA-L | Weisairuiji (Freeze-dried) | 2003 | H2 | None | Institute of Medical Biology of the Chinese Academy of Medical Sciences, Kunming, China |
| Freeze-dried live attenuated hepatitis A vaccine | 2003 | H2 | None | Zheijiang Pukang Biotechnology Company Limited, Zhejiang, China; Academy of Medical Sciences, Hangzhou, China | |
| Havac Weisairuiji (Freeze-dried) | 2000 | LA-1 | None | Changchun Institute of Biological Products, Changchun, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Liu, Y.; Li, L.; Liu, X.; Wu, B.; Wu, Y. Hepatitis Vaccines: Recent Advances and Challenges. Vaccines 2025, 13, 1174. https://doi.org/10.3390/vaccines13111174
Lu M, Liu Y, Li L, Liu X, Wu B, Wu Y. Hepatitis Vaccines: Recent Advances and Challenges. Vaccines. 2025; 13(11):1174. https://doi.org/10.3390/vaccines13111174
Chicago/Turabian StyleLu, Mei, Yakun Liu, Lele Li, Xueke Liu, Bin Wu, and Yingping Wu. 2025. "Hepatitis Vaccines: Recent Advances and Challenges" Vaccines 13, no. 11: 1174. https://doi.org/10.3390/vaccines13111174
APA StyleLu, M., Liu, Y., Li, L., Liu, X., Wu, B., & Wu, Y. (2025). Hepatitis Vaccines: Recent Advances and Challenges. Vaccines, 13(11), 1174. https://doi.org/10.3390/vaccines13111174






