Global Perspectives on HPV Vaccination: Achievements, Challenges, and Lessons from the Brazilian Experience
Abstract
1. Introduction
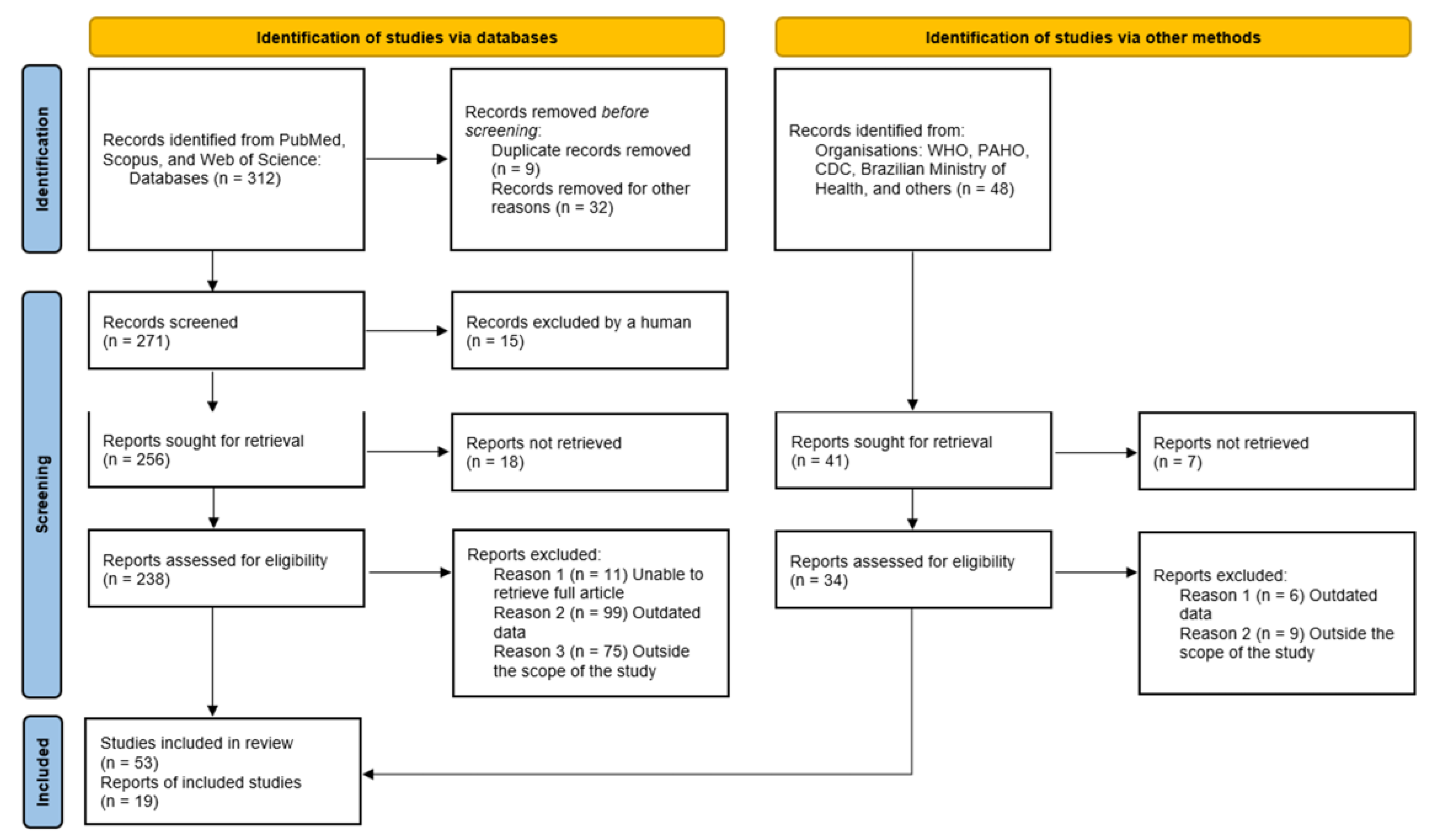
2. Methods
3. Results
3.1. Global Progress in HPV Vaccination
3.2. The Brazilian Experience: Achievements and Setbacks
4. Discussion
4.1. Vaccine Hesitancy, Misinformation, and Equity
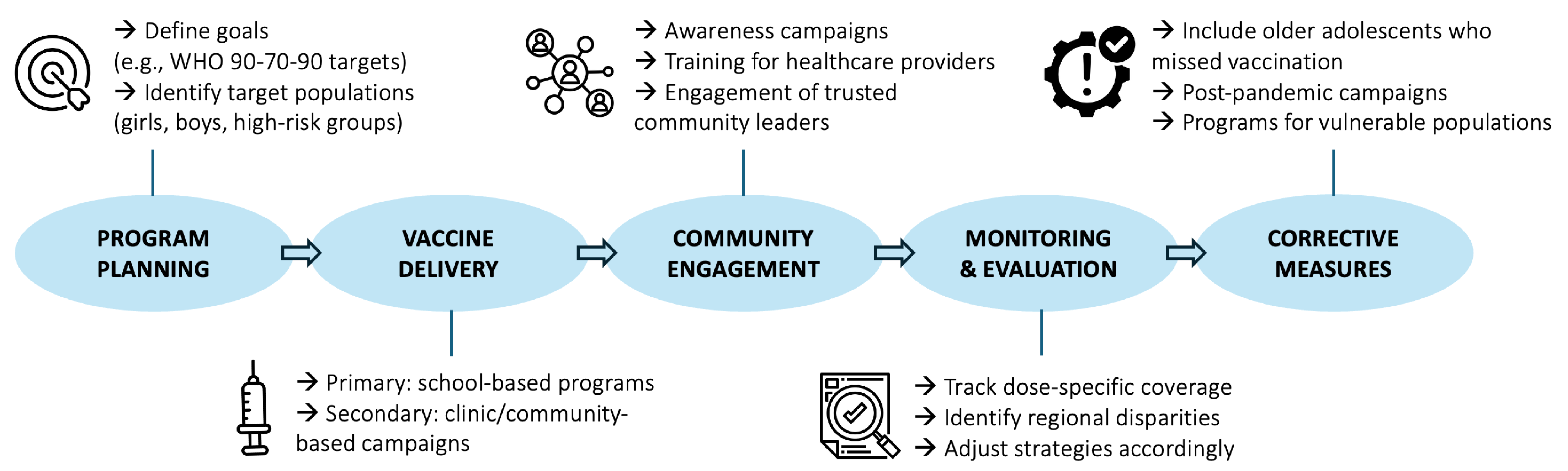
4.2. Policy Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Frazer, I.H. Development and implementation of papillomavirus prophylactic vaccines. J. Immunol. 2014, 192, 4007–4011. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and effectiveness of the quadrivalent HPV vaccine: A systematic review of 10 years of real-world experience. Vaccine 2016, 34, 4757–4767. [Google Scholar]
- Drolet, M.; Bénard, É.; Boily, M.-C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.L.; Cummings, T.; et al. Population-level impact and herd effects following HPV vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimates 2023: Cancer Incidence in Brazil [“Estimativa 2023: Incidência de Câncer no Brasil”]; INCA: Rio de Janeiro, Brazil, 2022. Available online: https://www.inca.gov.br/publicacoes/livros/estimativa-2023-incidencia-de-cancer-no-brasil (accessed on 18 October 2025).
- Vale, D.B.; Teixeira, J.C.; Bragança, J.F.; Derchain, S.; Zeferino, L.C. Trends in Cervical Cancer Mortality in Brazil: Regional Inequalities and Challenges for Screening. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1694–1701. [Google Scholar]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 30 September 2025).
- Ministério da Saúde (Brasil). HPV—Vacinação; Ministério da Saúde: Brasília, Brasil, 2025. Available online: https://www.gov.br/saude/pt-br/campanhas-da-saude/vacinacao/hpv (accessed on 17 October 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.L.; Murray, S.L.; A Hall, M.; Andrewartha, L.K.; A Banks, C.; Meijer, D.; Pitcher, H.C.; Scully, M.M.; Molchanoff, L. Human papillomavirus vaccine coverage among female Australian adolescents: Success of the school-based program. Med. J. Aust. 2013, 199, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.T.; Simms, K.T.; Lew, J.-B.; A Smith, M.; Brotherton, J.M.; Saville, M.; Frazer, I.H.; Canfell, K. The projected timeframe until cervical cancer elimination in Australia: A modelling study. Lancet Public Health 2019, 4, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Mesher, D.; Soldan, K.; Howell-Jones, R.; Panwar, K.; Manyenga, P.; Jit, M.; Beddows, S.; Gill, O. Reduction in HPV 16/18 prevalence among young women following the introduction of HPV immunisation in England. Vaccine 2013, 32, 26–32. [Google Scholar] [CrossRef]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 CIN incidence: A registry-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; Singleton, J.A.; Stokley, S. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2018. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 718–723. [Google Scholar] [CrossRef]
- Holman, D.M.; Benard, V.; Roland, K.B.; Watson, M.; Liddon, N.; Stokley, S. Barriers to Human Papillomavirus Vaccination Among US Adolescents: A Systematic Review of the Literature. JAMA Pediatr. 2014, 168, 76–82. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Prabhu, P.R.; Pawlita, M.; Gheit, T.; Bhatla, N.; Muwonge, R.; Nene, B.M.; Esmy, P.O.; Joshi, S.; Poli, U.R.R.; et al. Immunogenicity and HPV Infection After One, Two, and Three Doses of Quadrivalent HPV Vaccine in Girls in India: A Multicentre Prospective Cohort Study. Lancet Oncol. 2016, 17, 67–77. [Google Scholar] [CrossRef]
- Gallagher, K.E.; LaMontagne, D.S.; Watson-Jones, D. Status of HPV Vaccine Introduction and Barriers to Country Uptake. Vaccine 2018, 36, 4761–4767. [Google Scholar] [CrossRef]
- Murillo, R.; Herrero, R.; Sierra, M.S.; Forman, D. Cervical Cancer in Central and South America: Burden of Disease and Status of Disease Control. Cancer Epidemiol. 2016, 44, S121–S130. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Cervical Cancer Executive Summary V2—Analysis of the Situation of Cervical Cancer in the Region of the Americas; PAHO: Washington, DC, USA, 2024; Available online: https://www.paho.org/sites/default/files/2024-09/cervical-cancer-executive-summary-v2.pdf (accessed on 18 October 2025).
- Ministry of Health (Brazil). National Immunization Plan (PNI): Introduction of the HPV Vaccine (Plano Nacional de Imunizações (PNI): Introdução da Vacina HPV); Ministry of Health (Brazil): Brasília, Brazil, 2014. Available online: https://saude.es.gov.br/Media/sesa/PEI/Informe_Tecnico_Introducao_vacina_HPV_2014.pdf (accessed on 30 September 2025).
- Roteli-Martins, C.M.; Maranhão, A.G.K.; Fialho, S.C.A.V.; da Silva-Filho, A.L. The importance of the quadrivalent HPV vaccine in the elimination of cervical cancer in Brazil. Rev. Bras. Ginecol. Obstet. 2024, 46, e-rbgoedt4. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health (Brazil). National Immunization Plan (PNI): Introduction of the HPV Vaccine (Plano Nacional de Imunizações (PNI): Introdução da Vacina HPV); Ministry of Health (Brazil): Brasília, Brazil, 2014. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/notas-tecnicas/2024/nota-tecnica-no-41-2024-cgici-dpni-svsa-ms (accessed on 30 September 2025).
- Domingues, C.M.A.S.; Maranhão, A.G.K.; Teixeira, A.M.S.; Fantinato, F.F.S.; Domingues, R.A.S. The impact of the COVID-19 pandemic on immunization in Brazil: Disruptions and challenges. Cad. Saúde Pública 2020, 36, e00114120. [Google Scholar]
- Ministry of Health (Brazil). Technical Note No. 41/2024-CGICI/DPNI/SVSA/MS: Update on HPV Vaccination Within the Scope of the PNI. (Nota Técnica n 41/2024-CGICI/DPNI/SVSA/MS: Atualização Sobre a Vacinação Contra o HPV No Âmbito do PNI.); Ministry of Health (Brazil) (Brasil): Brasília, Brazil, 2024. Available online: https://sbim.org.br/images/files/notas-tecnicas/ms-svsa-dpni-cgici-nt-hpv-dose-unica-240402.pdf (accessed on 18 October 2025).
- Silveira, M.F.; Buffarini, R.; Bertoldi, A.D.; Santos, I.S.; Barros, A.J.D.; Matijasevich, A.; Menezes, A.M.B.; Gonçalves, H. Inequalities in HPV vaccination coverage: A nationwide analysis from Brazil. Vaccine 2021, 39, 331–337. [Google Scholar]
- World Health Organization. Ten Threats to Global Health in 2019; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 30 September 2025).
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef]
- Perkins, R.B.; Clark, J.A. What Affects Human Papillomavirus Vaccination Rates? A Qualitative Analysis of Providers’ Perceptions. Women’s Health Issues 2012, 22, e379–e386. [Google Scholar] [CrossRef]
- Cobos Muñoz, D.; Monzón Llamas, L.; Bosch-Capblanch, X. Exposing concerns about vaccination in low- and middle-income countries: A systematic review. Int. J. Public Health 2015, 60, 767–780. [Google Scholar] [CrossRef]
- Sato, A.P.S.; Andrade, F.B.; Passos, S.D. HPV Vaccine Coverage in Brazil: Trends and Regional Inequalities. Rev. Saúde Pública 2020, 54, 126. [Google Scholar]
- Moraes, C.L.; Ribeiro, D.C.; Silva, A.P. Social media and HPV vaccine refusal in Brazil: Qualitative insights. Vaccine 2021, 39, 3945–3952. [Google Scholar]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. Wkly. Epidemiol. Rec. 2022, 97, 645–672. [Google Scholar]
- Reisner, S.L.; Deutsch, M.B.; Bhasin, S.; Bockting, W.; Brown, G.R.; Feldman, J.; Garofalo, R.; Kreukels, B.; Radix, A.; Safer, J.D.; et al. Advancing methods for US transgender health research. Curr. Opin. Pediatr. 2016, 28, 103–107. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. HPV Vaccine—Fact Sheet Outlining Changes Under the National Immunisation Program in 2023; Department of Health and Aged Care: Canberra, ACT, Australia, 2023. Available online: https://www.health.gov.au/resources/publications/hpv-vaccine-fact-sheet-outlining-changes-under-the-national-immunisation-program-in-2023 (accessed on 18 October 2025).
- UK Health Security Agency. HPV Vaccination Programme Moves to Single Dose from September 2023; UKHSA: London, UK, 2023. Available online: https://www.gov.uk/government/news/hpv-vaccination-programme-moves-to-single-dose-from-september-2023 (accessed on 18 October 2025).
- NHS Wessex Cancer Alliance. HPV Vaccination Resources Toolkit v1.0 [Internet]; NHS: Winchester, UK, 2025; p. 4. Available online: https://wessexcanceralliance.nhs.uk/wp-content/uploads/2025/07/HPV_Vaccination_Resources_Toolkit_v1.0_2.pdf (accessed on 18 October 2025).
- Government of Ireland—Department of Health. Minister for Health Announces Expansion of the Laura Brennan HPV Vaccine Catch-up Programme: One Dose Required in Those Aged Under 25 [Internet]; Gov.ie: Dublin, Ireland, 2023. Available online: https://www.gov.ie/en/department-of-health/press-releases/minister-for-health-announces-expansion-of-the-laura-brennan-hpv-vaccine-catch-up-programme/ (accessed on 18 October 2025).
- National Advisory Committee on Immunization (NACI). Updated Recommendations on Human Papillomavirus (HPV) Vaccines [Internet]; Government of Canada: Ottawa, ON, Canada, 2024. Available online: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-summary-updated-recommendations-hpv-vaccines.html (accessed on 18 October 2025).
- Ministerio de Sanidad (España). Actualización de las recomendaciones de vacunación frente a VPH. In Revisión de la Estrategia de Una dosis [Internet]; Ponencia de Programa y Registro de Vacunaciones: Madrid, España, 2024. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/VPH_recomendaciones_vacunacion_estrategia1dosis.pdf (accessed on 18 October 2025).
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- Wilkin, T.; Lee, J.Y.; Lensing, S.Y.; Stier, E.A.; Goldstone, S.E.; Berry, J.M.; Jay, N.; Aboulafia, D.; Cohn, D.L.; Einstein, M.H.; et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1–infected men. J. Infect. Dis. 2010, 202, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC HPV Information Centre. In Human Papillomavirus and Related Diseases in the World: Summary Report 2021; ICO/IARC: Barcelona, Spain, 2021; Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 30 September 2025).
- Kiamba, E.W.; Goodier, M.R.; Clarke, E. Immune responses to human papillomavirus infection and vaccination. Front. Immunol. 2025, 16, 1591297. [Google Scholar] [CrossRef] [PubMed]
- Viscidi, R.P.; Lewis, R.M.; Beachler, D.C.; Bartels, H.C.; Lichter, K.; Kechagias, K.S. Human papillomavirus vaccine and risk of recurrence of high-grade cervical intraepithelial neoplasia: A meta-analysis. Vaccine 2021, 39, 2800–2809. [Google Scholar]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Dillner, J.; Tryggvadottir, L.; Munk, C.; Berger, S.; Enerly, E.; Hortlund, M.; Ágústsson, Á.I.; et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent HPV vaccine in Nordic women. EClinicalMedicine 2020, 23, 100401. [Google Scholar] [CrossRef]
- Olsson, S.E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D., Jr.; Ferris, D.; Block, S.; et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys aged 9–15 years: Interim analysis after 8 years. Papillomavirus Res. 2020, 10, 100203. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Falkenthal, T.E.H.; Sundström, K.; Munk, C.; Sture, T.; Bautista, O.; Thomas Group; Rawat, S.; Luxembourg, A. Long-term effectiveness of the nine-valent human papillomavirus vaccine: Interim results after 12 years of follow-up in Scandinavian women. Hum. Vaccines Immunother. 2024, 20, 2377903. [Google Scholar] [CrossRef]
- Jagu, S.; Kwak, K.; Schiller, J.T.; Lowy, D.R. Prospects for L2-based prophylactic HPV vaccines. Open Virol. J. 2017, 11, 97–113. [Google Scholar]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting HPV16 and HPV18 E6/E7 proteins for cervical intraepithelial neoplasia 2/3: A randomized, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Wirth, D.M.; Ortega-Rivera, O.A.; Steinmetz, N.F.; Pokorski, J.K. Dissolving microneedle delivery of a prophylactic HPV vaccine. Biomacromolecules 2022, 23, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Meites, E.; Markowitz, L.E.; Paz-Bailey, G.; Oster, A.M.; NHBS Study Group. HPV vaccination coverage among men who have sex with men—National HIV Behavioral Surveillance, United States, 2011. Vaccine 2011, 32, 6356–6359. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Kang, W.D.; Choi, H.S.; Kim, S.M. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol. Oncol. 2013, 130, 264–268. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Brotherton, J.M.L.; Canfell, K. Adjuvant prophylactic HPV vaccination for women undergoing treatment for cervical intraepithelial neoplasia: A systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar]
- De Vincenzo, R.; Conte, C.; Ricci, C.; Scambia, G.; Capelli, G. Long-term efficacy and safety of human papillomavirus vaccination. Int. J. Gynecol. Cancer 2014, 24, 1061–1067. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; et al. Adjuvant HPV vaccination to prevent recurrent cervical dysplasia after surgical treatment: A meta-analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef]
- Cherif, A.; Ovcinnikova, O.; Palmer, C.; Engelbrecht, K.; Reuschenbach, M.; Daniels, V. Cost-effectiveness of 9-valent HPV vaccination for patients treated for high-grade cervical intraepithelial neoplasia in the UK. JAMA Netw. Open 2024, 7, e2437703. [Google Scholar] [CrossRef]
- Zou, M.; Liu, H.; Liu, H.; Wang, M.; Zou, Z.; Zhang, L. Vaccinating women previously treated for human papillomavirus-related cervical precancerous lesions is highly cost-effective in China. Front. Immunol. 2023, 14, 1119566. [Google Scholar] [CrossRef] [PubMed]
- Chaiken, S.R.; Bruegl, A.S.; Caughey, A.B.; Emerson, J.; Munro, E.G. Adjuvant human papillomavirus vaccination after excisional procedure for cervical intraepithelial neoplasia: A cost-effectiveness analysis. Obstet. Gynecol. 2023, 141, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and low-er-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.R.; Smith, A.; Coyne-Beasley, T. A systematic literature review to examine the potential for social media to impact HPV vaccine uptake and awareness. Hum. Vaccin. Immunother. 2019, 15, 1465–1475. [Google Scholar] [CrossRef]
- Keim-Malpass, J.; Mitchell, E.M.; Camacho, F. HPV vaccination series completion and co-vaccination: Pairing vaccines may matter for adolescents. Vaccine 2015, 33, 5729–5732. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, C.F.; Giuliano, A.R.; Fernandez, M.E.; Ortiz, A.P.; Cazaban, C.G.; Li, R.; Deshmukh, A.A.; Sonawane, K. Tdap-HPV vaccination bundling in the USA: Trends, predictors, and implications for vaccine series completion. Prev. Med. 2022, 164, 107218. [Google Scholar] [CrossRef]
- Walling, E.B.; Dodd, S.; Bobenhouse, N.; Reis, E.C.; Sterkel, R.; Garbutt, J. Implementation of Strategies to Improve Human Papillomavirus Vaccine Coverage: A Provider Survey. Am. J. Prev. Med. 2019, 56, 74–83. [Google Scholar] [CrossRef]
- Perkins, R.B.; Humiston, S.; Oliver, K. Evidence supporting the initiation of HPV vaccination starting at age 9: Collection overview. Hum. Vaccin. Immunother. 2023, 19, 2269026. [Google Scholar] [CrossRef]
| Country | Program Start | Target Age | Delivery Strategy | Vaccine Type | 1st Dose Coverage (%) | Full Series Coverage (%) | Reported Impact | Equity/Funding | Source |
|---|---|---|---|---|---|---|---|---|---|
| United States | 2006 | 11–12 years | Clinic-based | Quadrivalent/9-valent (Gardasil®, Gardasil 9®) | ~60% | ~60% | Moderate progress; persistent geographic and sociodemographic gaps | Publicly funded through Vaccines for Children (VFC); gender-neutral (boys and girls) | CDC, National Immunization Survey–Teen, 2023 |
| Australia | 2007 | 12–13 years | School-based | Quadrivalent/9-valent (Gardasil 9®, single-dose since 2023) | >80% | >70% | Dramatic decline in HPV 16/18 infection and high-grade lesions; early evidence of cervical cancer reduction | National Immunisation Program; gender-neutral; single-dose policy since February 2023 | Australian Department of Health, 2023 |
| United Kingdom | 2008 | 12–13 years | School-based | Gardasil 9® (nonavalent, single-dose since 2023) | >85% | >80% | 86% reduction in HPV 16/18 among <21 years; projected elimination of cervical cancer within decades | NHS-funded; gender-neutral since 2019; single-dose Gardasil 9 since September 2023 | UK Health Security Agency, 2023 |
| Canada | 2007 | 9–26 years (routine 9–15) | School-based (primary), community clinics | Quadrivalent/9-valent (Gardasil®, Gardasil 9®) | 80–90% (first dose, girls) | 70–85% | Sharp decline in HPV prevalence and anogenital warts; early evidence of reduced cervical intraepithelial neoplasia (CIN2+) | Publicly funded via provincial programs; gender-neutral since 2015; school-based coverage > 85% in most provinces | Public Health Agency of Canada, Immunization Coverage Reports, 2023 |
| Spain | 2007 | 12 years (girls), 12–18 years (catch-up; boys since 2023) | School-based | Bivalent/9-valent (Cervarix®, Gardasil 9®) | ~90% (girls) | ~80% | Significant reduction in HPV 16/18 infection; rapid uptake of gender-neutral vaccination since 2023 | Fully funded under the National Health System; regional management by autonomous communities | Spanish Ministry of Health, Vaccination Calendar, 2024 |
| China | 2016 (national approval) | 9–14 years (recommended) | School/clinic | Cecolin® (bivalent, WHO PQ 2021); Walrinvax®/HPV-2 (bivalent, WHO PQ 2024); Cecolin-9® (nonavalent, 2025) | Expanding (20–60%) | Not available | Rapid uptake in pilot provinces; increasing domestic vaccine availability and single-dose implementation | Public–private partnership; domestic supply ensures affordability | National Health Commission of China, 2025; WHO PQ listings, 2024 |
| India | 2008 (state-level pilots); national rollout 2024 | 9–14 years | School/clinic | Cervavac® (quadrivalent); Cecolin® (bivalent) | Variable (pilot states 60–80%) | Variable | Single-dose evidence supports durable protection; national scale-up underway | Public programs expanding through state immunization systems; primarily girls 9–14 years | WHO/UNICEF Joint Reporting Form, 2023; Serum Institute of India, 2024 |
| Brazil | 2014 | 9–13 years (girls, later boys) | School-based | Quadrivalent (Gardasil®) via PNI | >80% (first dose) | <60% | Early success followed by decline due to misinformation and pandemic disruptions; recovery underway with expanded eligibility | National Immunization Program (SUS); gender-neutral since 2017; publicly funded | Brazilian Ministry of Health, PNI Report, 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, A.; Martins, C.A.d.O.; Paiva, G.; Barboza, É.d.A.; Chagas, M.; Callado, G.Y.; Araujo Júnior, E.; de Rezende-Filho, J.; Guimarães, I.C.C.d.V.; Granese, R.; et al. Global Perspectives on HPV Vaccination: Achievements, Challenges, and Lessons from the Brazilian Experience. Vaccines 2025, 13, 1106. https://doi.org/10.3390/vaccines13111106
Braga A, Martins CAdO, Paiva G, Barboza ÉdA, Chagas M, Callado GY, Araujo Júnior E, de Rezende-Filho J, Guimarães ICCdV, Granese R, et al. Global Perspectives on HPV Vaccination: Achievements, Challenges, and Lessons from the Brazilian Experience. Vaccines. 2025; 13(11):1106. https://doi.org/10.3390/vaccines13111106
Chicago/Turabian StyleBraga, Antonio, Caroline Alves de Oliveira Martins, Gabriela Paiva, Érica de Almeida Barboza, Marcela Chagas, Gustavo Yano Callado, Edward Araujo Júnior, Jorge de Rezende-Filho, Isabel Cristina Chulvis do Val Guimarães, Roberta Granese, and et al. 2025. "Global Perspectives on HPV Vaccination: Achievements, Challenges, and Lessons from the Brazilian Experience" Vaccines 13, no. 11: 1106. https://doi.org/10.3390/vaccines13111106
APA StyleBraga, A., Martins, C. A. d. O., Paiva, G., Barboza, É. d. A., Chagas, M., Callado, G. Y., Araujo Júnior, E., de Rezende-Filho, J., Guimarães, I. C. C. d. V., Granese, R., Calagna, G., & Fialho, S. C. A. V. (2025). Global Perspectives on HPV Vaccination: Achievements, Challenges, and Lessons from the Brazilian Experience. Vaccines, 13(11), 1106. https://doi.org/10.3390/vaccines13111106











