Double-Blind Randomized Phase 2 Trial Testing Personal Cancer Vaccines in Patients with Advanced Ovarian Cancer
Abstract
1. Introduction
2. Methods
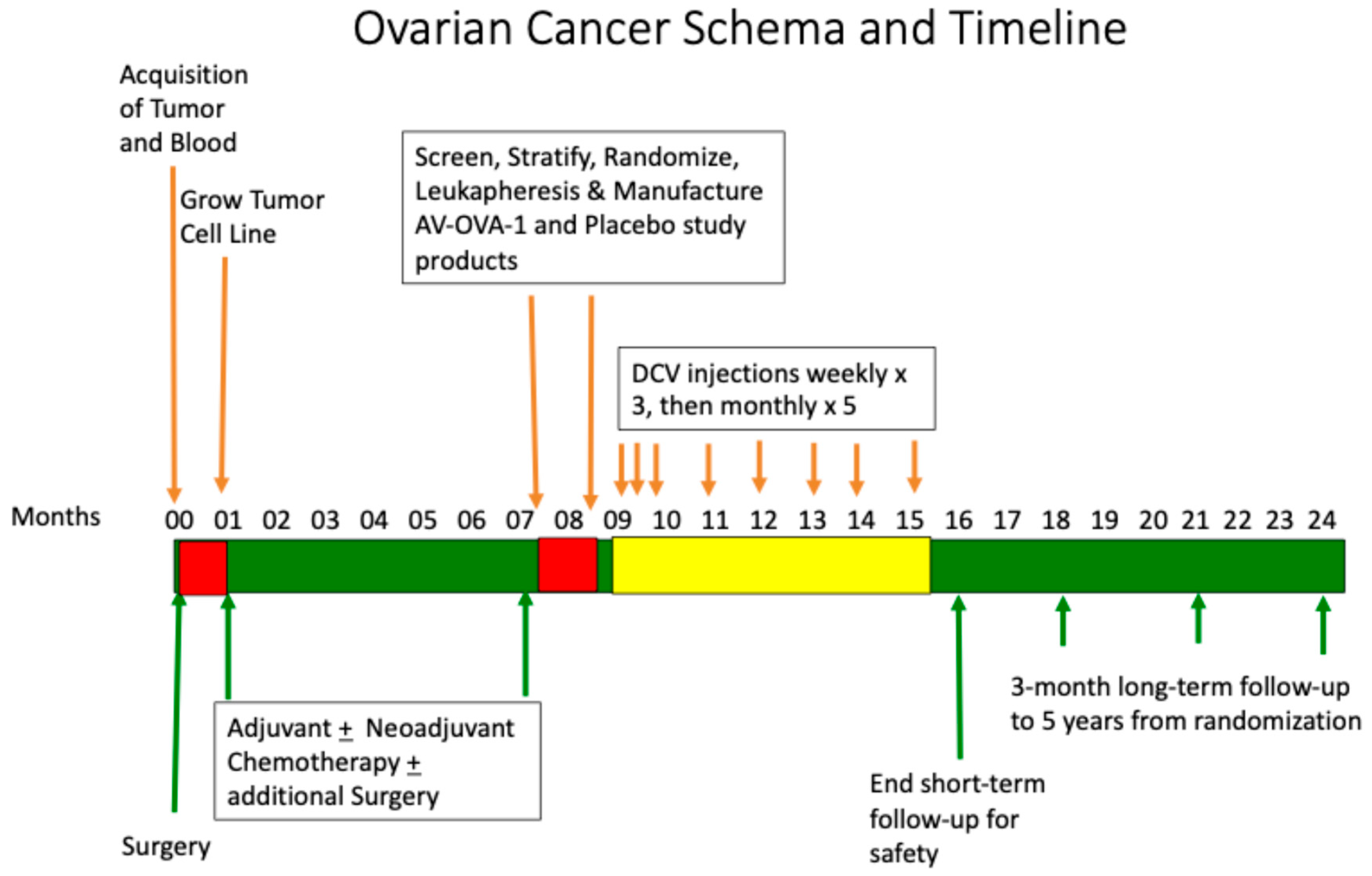
2.1. Trial Design and Oversight
2.2. Patients
2.2.1. Eligibility for Tumor Collection
2.2.2. Eligibility to Screen for Randomization
2.2.3. Stratification and Randomization
2.3. Manufacturing of AV-OVA-1 and MC
2.3.1. Autologous Tumor Antigens
2.3.2. Monocytes and Autologous Dendritic Cells
2.3.3. AV-OVA-1 (AV-OVA-1) and MC
2.4. Treatment
2.5. Endpoints
2.6. Statistical Methods
3. Results
3.1. Study Conduct
3.2. Patient Characteristics and Other Therapies
3.3. Study Product Manufacturing Success
3.4. Safety
3.5. Efficacy
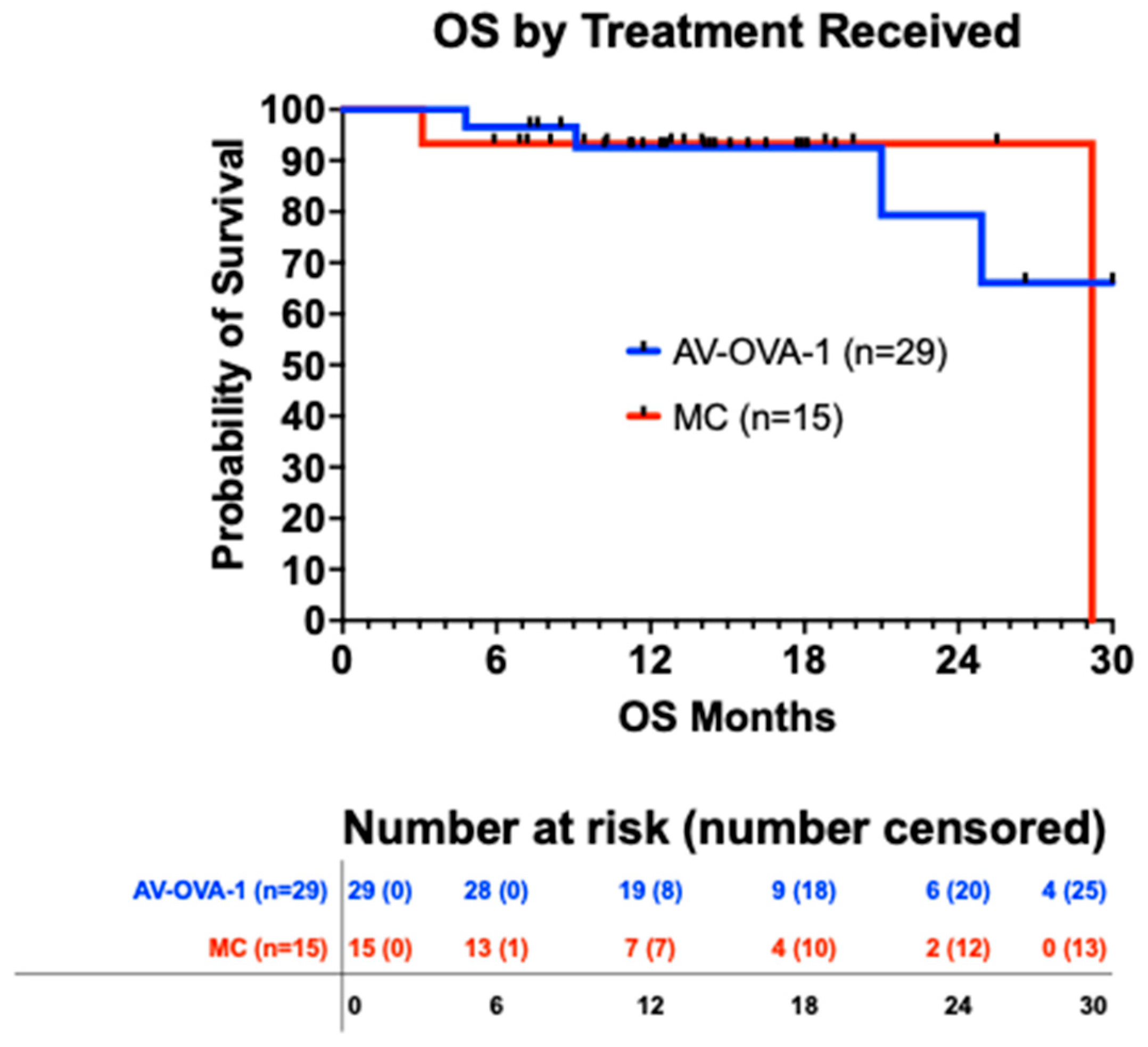
3.5.1. Overall Survival
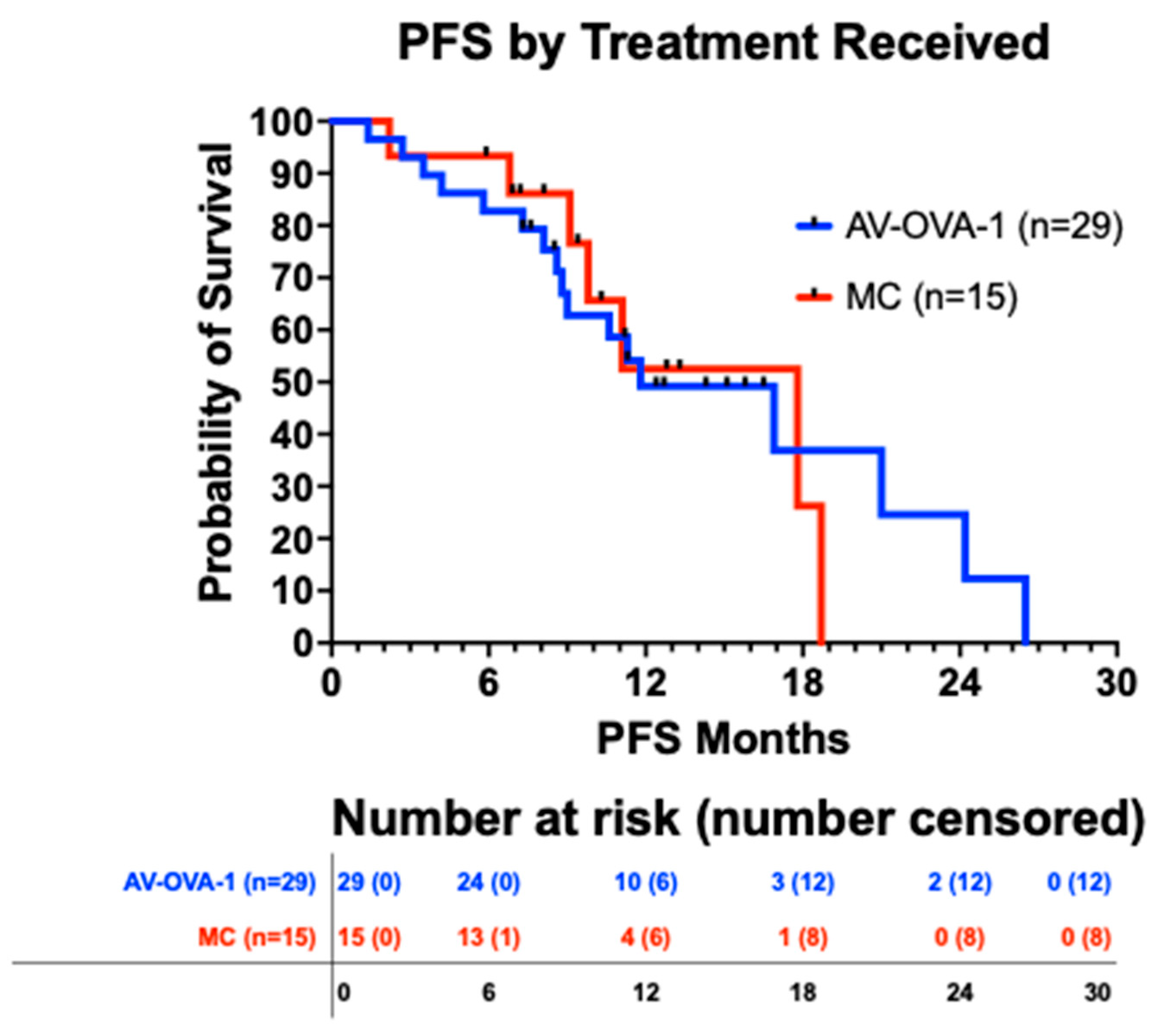
3.5.2. Progression-Free Survival
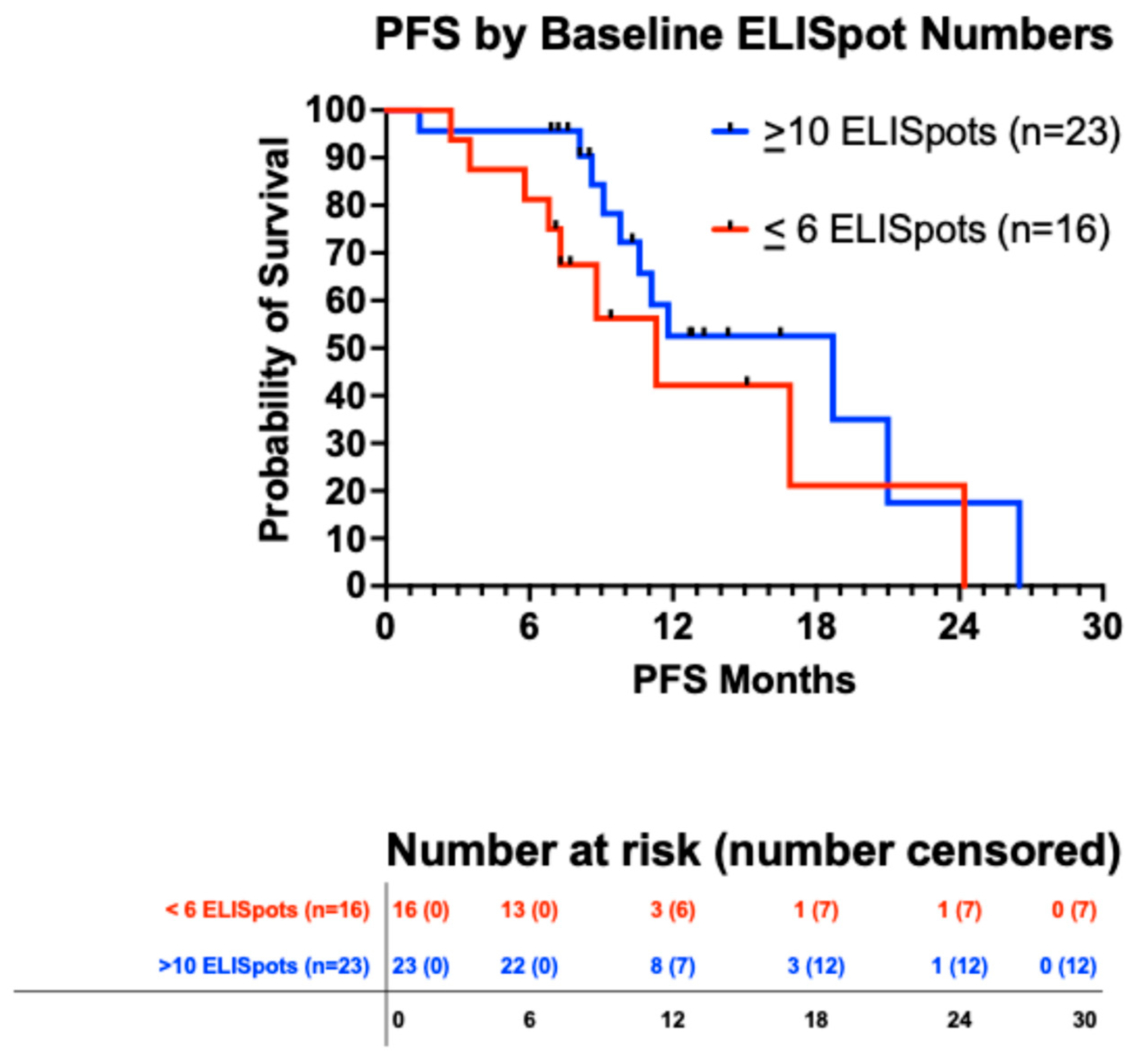
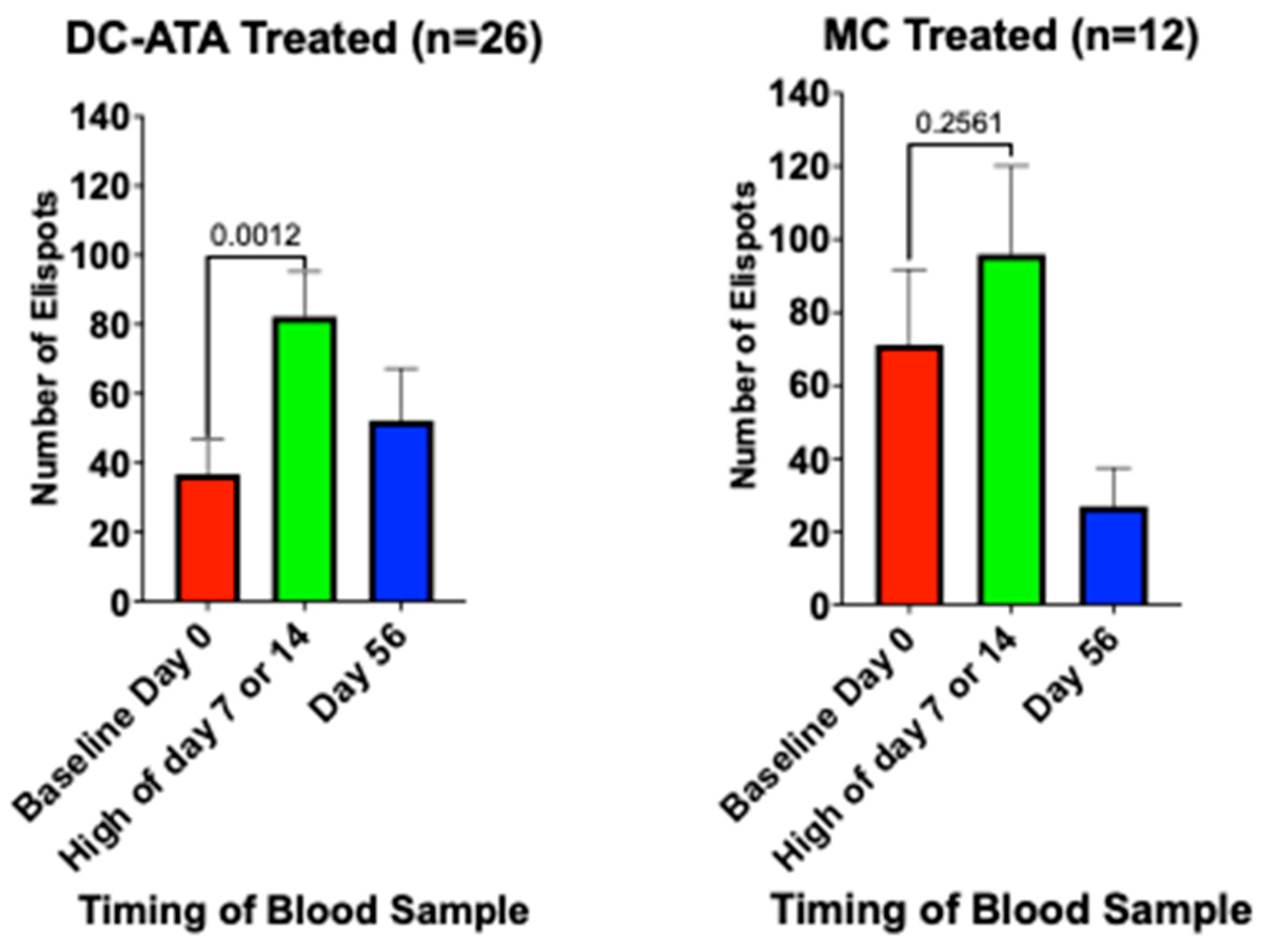
3.5.3. Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Plaxe, S.C.; Alvarez, R.D.; Bakkum-Galvez, J.N.; Barroilhet, L.; Behbakht, K.; Chen, L.-m.; Cristea, M.; DeRosa, M.; ElNaggar, A.C.; et al. NCCN Guidelines®: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer; Version 2.2018; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2017. [Google Scholar]
- Marth, C.; Abreu, M.H.; Andersen, K.K.; Aro, K.M.; Batarda, M.d.L.; Boll, D.; Ekmann-Gade, A.W.; Haltia, U.; Hansen, J.; Haug, A.J.; et al. Real-life data on treatment and outcomes in advanced ovarian cancer: An observational, multinational cohort study (RESPONSE trial). Cancer 2022, 128, 3080–3089. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Krivak, T.C.; Kabil, N.; Munley, J.; Moore, K.N. PARP Inhibitors in Ovarian Cancer: A Review. Target. Oncol. 2023, 18, 471–503. [Google Scholar] [CrossRef] [PubMed]
- Goldlust, I.S.; Guidice, E.; Lee, J.M. PARP inhibitors in ovarian cancer. Semin. Oncol. 2024, 51, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Lacchetti, C.; Armstrong, D.K.; Cliby, W.A.; Edelson, M.I.; Garcia, A.A.; Ghebre, R.G.; Gressel, G.M.; Lesnock, J.L.; Meyer, L.A.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update. J. Clin. Oncol. 2025, 43, 868–891. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.-M.; Cohen, J.; DeRosa, M.; Eisenhauer, E.L.; et al. NCCN Guidelines®: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer; Version 2.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Powell, D.J., Jr.; Singh, N.; Coukos, G. Immunotherapy for ovarian cancer: What’s next? J. Clin. Oncol. 2010, 29, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 2021, 39, 1842–1855. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Gubin, M.M.; Artyomov, M.N.; Mardis, E.R.; Schreiber, R.D. Tumor neoantigens: Building a framework for personalized cancer immunotherapy. J. Clin. Investig. 2015, 125, 3413–3421. [Google Scholar] [CrossRef]
- Zaidi, N.; Jaffee, E.M.; Yarchoan, M. Recent advances in therapeutic cancer vaccines. Nat. Rev. Cancer 2025, 25, 517–533. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.O.; Nistor, G.I.; Keirstead, H.S. Autologous dendritic cells loaded with antigens from self-renewing autologous tumor cells as patient-specific therapeutic cancer vaccines. Hum. Vaccines Immunother. 2023, 19, 2198467. [Google Scholar] [CrossRef]
- Kandalaft, L.E.; Chiang, C.L.; Tanyi, J.; Motz, G.; Balint, K.; Mick, R.; Coukos, G. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J. Transl. Med. 2013, 11, 149. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccne effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Buchroithner, J.; Erhart, F.; Pichler, J.; Widhalm, G.; Preusser, M.; Stockhammer, G.; Nowosielski, M.; Iglseder, S.; Freyschlag, C.F.; Oberndorfer, S.; et al. Audencel immunotherapy based on dendritic cells has no effect on overall and progression-free survival in newly diagnosed glioblastoma: A phase II randomized trial. Cancers 2018, 10, 372. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: A phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Figlin, R.A.; Tannir, N.M.; Uzzo, R.G.; Tykodi, S.S.; Chen, D.Y.; Master, V.; Kapoor, A.; Vaena, D.; Lowrance, W.T.; Bratslavsky, G.; et al. Results of the ADAPT phase 3 study of rocalpuldencel-T in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 2020, 26, 2327–2336. [Google Scholar] [CrossRef]
- DeBenedette, M.; Gamble, A.; Norris, M.; Horvatinovich, J.; Nicolette, C.A. A review of the clinical experience with CMN-001, a tumor RNA loaded dendritic cell immunotherapy for the treatment of metastatic renal cell carcinoma. Hum. Vaccines Immunother. 2023, 19, 2220629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bayer, M.E.; Chen, X.; Fredrickson, C.; Cornforth, A.N.; Liang, G.; Cannon, J.; He, J.; Fu, Q.; Liu, J.; et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J. Surg. Oncol. 2015, 111, 862–867. [Google Scholar] [CrossRef]
- Dillman, R.O.; Depriest, C. Dendritic cell vaccines presenting autologous tumor antigens from self-renewing cancer cells in metastatic renal cell cancer. J. Explor. Res. Pharmacol. 2018, 3, 93–101. [Google Scholar] [CrossRef]
- Dillman, R.O.; Selvan, S.R.; Schiltz, P.M.; McClay, E.F.; Barth, N.M.; DePriest, C.; de Leon, C.; Mayorga, C.; Cornforth, A.N.; Allen, K. Phase II trial of dendritic cells loaded with antigens from self-renewing, proliferating autologous tumor cells as patient-specific anti-tumor vaccines in patients with metastatic melanoma: Final Report. Cancer Biother. Radiopharm. 2009, 24, 311–319. [Google Scholar]
- Dillman, R.O.; Cornforth, A.N.; Nistor, G.I.; McClay, E.F.; Amatruda, T.T.; Depriest, C. Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in patients with metastatic melanoma: 5-year follow up and additional analyses. J. Immunother. Cancer. 2018, 6, 19. [Google Scholar] [CrossRef]
- Bota, D.A.; Piccioni, D.E.; Duma, C.M.; Kesari, S.; Carrillo, J.A.; LaRocca, R.V.; Aiken, R.D.; Taylor, T.H.; Abedi, M.; Robles, R.M.; et al. Phase 2 trial of personal dendritic cell vaccines in newly diagnosed glioblastoma: 3-year follow-up and correlations with survival. Hum. Vaccines Immunother. 2025, 21, 2556591. [Google Scholar] [CrossRef]
- Dillman, R.O.; Nayak, S.K.; Beutel, L. Establishing in vitro cultures of autologous tumor cells for use in active specific immunotherapy. J. Immunother. 1993, 14, 65–69. [Google Scholar] [CrossRef]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Zang, S.; Balch, C.; Chang, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.-M.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Cai, Y.; Ren, M.; Zhang, Y.; Shi, F.; Zhao, F.; He, X.; Pan, M.; Yan, C.; et al. Effect of ovarian cancer immunotherapy using ovarian cancer stem cell vaccine. J. Ovarian Res. 2015, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Sajdak, S.; Huczyński, A.; Rehlis, S.; Markowska, J. Ovarian cancer stem cells: A target for oncological therapy. Adv. Clin. Exp. Med. 2018, 27, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDcap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Zhao, F.; Lee, S.; Tarhini, A.A.; Margolin, K.A.; White, R.L.; Atkins, M.B.; Cohen, G.I.; Whiteside, T.L.; Kirkwood, J.M.; et al. Immune correlates of GM-CSF and melanoma peptide vaccination in a randomized trial for the adjuvant therapy of resected high-risk melanoma (E4697). Clin. Cancer Res. 2017, 23, 5034–5043. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, Q.; Li, J.; Liu, G.; Nikoulin, I.; Jia, S. Advanced clinical trials of dendritic cell vaccines in ovarian cancer. J. Investig. Med. 2020, 68, 1223–1227. [Google Scholar] [CrossRef]
- Zhang, X.; He, T.; Li, Y.; Chen, L.; Liu, H.; Wu, Y.; Guo, H. Dendritic cell vaccines in ovarian cancer. Front. Immunol. 2021, 11, 613773. [Google Scholar] [CrossRef]
- Caro, A.A.; Deschoemaeker, S.; Allonsius, L.; Coosemans, A.; Laoui, D. Dendritic cell vaccines: A promising approach in the fight against ovarian cancer. Cancers 2022, 14, 4037. [Google Scholar] [CrossRef]
- Loveland, B.E.; Zhao, A.; White, S.; Gan, H.; Hamilton, K.; Xing, P.-X.; Pietersz, G.A.; Apostolopoulos, V.; Vaughan, H.; Karanikas, V.; et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: A phase I trial in patients with adenocarcinoma. Clin. Cancer Res. 2006, 12 Pt 1, 869–877. [Google Scholar] [CrossRef]
- Rahma, O.E.; Ashtar, E.; Czystowska, M.; Szajnik, M.E.; Wieckowski, E.; Bernstein, S.; Herrin, V.E.; Shams, M.A.; Steinberg, S.M.; Merino, M.; et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: Subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol. Immunother. 2012, 61, 373–384. [Google Scholar] [CrossRef]
- Chu, C.S.; Boyer, J.; Schullery, D.S.; Gimotty, P.A.; Gamerman, V.; Bender, J.; Levine, B.L.; Coukos, G.; Rubin, S.C.; Morgan, M.A.; et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012, 61, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, X.; Cui, P.; Piao, C.; Xiao, M.; Liu, X.; Wang, Y.; Wu, X.; Liu, J.; Yang, L. Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol. Immunother. 2019, 68, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Dietz, A.B.; Gustafson, M.P.; Kalli, K.R.; Erskine, C.L.; Youssef, B.; Vijay, G.V.; Allred, J.B.; Pavelko, K.D.; Strausbauch, M.A.; et al. Th17-inducing autologous dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat. Commun. 2020, 11, 5173. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Cibula, D.; Knapp, P.; Mallmann, P.; Klat, J.; Minar, L.; Bartos, P.; Chovanec, J.; Valha, P.; Pluta, M.; et al. Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: A phase 2, open-label, multicenter, randomized trial. J. Immunother. Cancer. 2022, 10, e003190. [Google Scholar] [CrossRef] [PubMed]
- Sarivalasis, A.; Boudousquié, C.; Balint, K.; Stevenson, B.J.; Gannon, P.O.; Iancu, E.M.; Rossier, L.; Lluesma, S.M.; Mathevet, P.; Sempoux, C.; et al. A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma. J. Transl. Med. 2019, 17, 391. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Chiang, C.L.-L.; Chiffelle, J.; Thierry, A.-C.; Baumgartener, P.; Huber, F.; Goepfert, C.; Tarussio, D.; Tissot, S.; Torigian, D.A.; et al. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. npj Vaccines 2021, 6, 36. [Google Scholar] [CrossRef] [PubMed]

| Variable | AV-OVA-1 (n = 29) | MC (n = 16) |
|---|---|---|
| Age | ||
| Median | 61 years | 60 years |
| Range | 42 to 78 | 39 to 84 |
| <50 | 3 (10.3%) | 3 (18.8%) |
| 50–59 | 9 (31.0%) | 5 (31.2%) |
| 60–69 | 16 (55.2%) | 4 (25.0%) |
| 70–79 | 3 (10.3%) | 2 (12.5%) |
| >80 | 0 | 2 (12.5%) |
| Race/Ethnicity | ||
| Asian | 2 (6.9%) | 1 (6.2%) |
| Black | 0 (0%) | 0 (0%) |
| Hispanic | 4 (13.8%) | 3 (18.8%) |
| White | 23 (79.3%) | 12 (75%) |
| ECOG PFS at baseline | ||
| 0 | 19 (65.5%) | 11 (68.8%) |
| 1 | 9 (31.0%) | 4 (25.0%) |
| 2 | 1 (3.4%) | 1 (6.2%) |
| Stage at Diagnosis | ||
| Stage 3 | 23 (79.3%) | 9 (56.2%) |
| Stage 4 | 6 (20.7%) | 7 (43.8%) |
| Tissue of Origin | ||
| Ovary | 21 (72.4%) | 13 (68.8%) |
| Fallopian Tube | 6 (20.7%) | 2 (12.5%) |
| Primary Intraperitoneal | 2 (6.9%) | 1 (6.2%) |
| Histology | ||
| High-Grade Serous | 22 (75.9%) | 12 (75.0%) |
| Papillary Serous | 1 (3.4%) | 1 (6.2%) |
| Serous/Endometrioid | 2 6.9%) | 0 (0%) |
| Adenocarcinoma | 1 (3.4%) | 0 (0%0 |
| Poorly Differentiated | 3 (10.3%) | 1 (6.2%) |
| Clear Cell | 0 (0%) | 2 (12.5%) |
| Extent of surgical debulking | ||
| No residual disease | 11 (37.9%) | 7 (43.8%) |
| Optimally debulked 1 | 10 (34.5%) | 5 (31.2%) |
| Suboptimally debulked 2 | 1 (3.4%) | 2 (12.5%) |
| Not available | 6 (20.7%) | 2 (12.5%) |
| At randomization | ||
| No Evidence of Disease | 23 (79.3%) | 13 (81.2%) |
| Residual Disease | 6 (20.7%) | 3 (18.8%) |
| Time from Surgery to Randomization | ||
| Mean months | 6.1 | 6.4 |
| Median months | 5.9 | 6.3 |
| Range in months | 3.2 to 15.1 | 3.1 to 13.4 |
| Time from Surgery to First Injection | ||
| Mean months | 7.1 | 7.9 |
| Median months | 7.3 | 7.7 |
| Range in months | 4.6 to 16.3 | 4.5 to 14.6 |
| Time from Randomization to First injection | ||
| Mean months | 1.6 | 1.6 |
| Median months | 1.4 | 1.2 |
| Range in months | 1.0 to 3.4 | 1.0 to 5.2 |
| Primary Platinum-based Chemotherapy | AV-OVA-1 (n = 29) | MC (n = 16) |
| Neoadjuvant + adjuvant | 20 (69.0%) | 10 (62%) |
| Adjuvant | 9 (31.0%) | 6 (38%) |
| Treatment Agents | ||
| Taxane | 28 (96.4%) | 16 (100%) |
| Platinum | 29 (100%) | 16 (100%) |
| PARP Inhibitor | 12 (41.4%) | 7 (43.8%) |
| Bevacizumab | 11(37.9%) | 7 (43.8%) |
| Anti-PD-1 | 1 (3.4%) | 1 (6.2%) |
| Doxil | 0 | 1 (6.2%) |
| Gemcitabine | 0 | 1 (6.2%) |
| Therapy concurrent with vaccine therapy | AV-OVA-1 (n = 28) | MC (n = 16) |
| Any concurrent therapy | 20 (71.4%) | 5 (31.2%) |
| Taxane | 1 (3.6%) | 0 (0%) |
| Platinum | 3 (10.7%) | 0 (0%) |
| Doxil | 4 (14.3%) | 0 (0%) |
| Bevacizumab | 8 (28.6%) | 2 (12.5%) |
| Campothecin | 1 (3.6%) | 0 (0%) |
| Gemcitabine | 2 (7.2%) | 1 (6.2%) |
| PARP Inhibitor | 7 (25.0%) | 2 (12.5%) |
| Anti-PD-1 | 1 (3.6%) | 1 (6.2%) |
| Anti-Estrogen | 1 (3.6%) | 0 (0%) |
| Variable | Randomized to AV-OVA-1 (n = 29) | Randomized to MC (n = 16) | p Value |
|---|---|---|---|
| Tumor cell line | |||
| Mean days in culture | 24.1 | 24.8 | 0.87 |
| Median days in culture | 20.0 | 26.5 | |
| Range of days in culture | 8 to 62 | 10 to 67 | |
| In culture 28 days or less | 23 (79.3%) | 13 (81.2%) | |
| Tumor cells lysed | |||
| Mean × 106 | 66.7 | 45.0 | 0.071 |
| Median × 106 | 81.7 | 41.0 | |
| Range × 106 | 1.8 to 135 | 1.1 to 96.8 | |
| 10 million or more | 26 (89.7%) | 11 (68.8%) | |
| Monocytes cryopreserved | |||
| Mean × 109 | 1.70 | 1.13 | 0.022 |
| Median × 109 | 1.56 | 1.01 | |
| Range × 109 | 0.63 to 3.80 | 0.25 to 2.11 | |
| 450 million or more | 29 (100%) | 15/16 (93.8%) | |
| Final AV-OVA-1 product | AV-OVA-1 (n = 29) | MC (n = 15) | |
| Mean cell number × 106 | 95.7 | N/A | |
| Median cell number × 106 | 66.8 | N/A | |
| Cell number range × 106 | 17.8 to 508 | N/A | |
| 10 million or more cells | 29 (100%) | N/A | |
| Viable cells cryopreserved | DC-ATA (n = 29) | MC (n = 15) | |
| Mean × 106 per dose | 8.2 | 12.0 | |
| Median × 106 per dose | 6.0 | 9.2 | |
| Range × 106 per dose | 2.0 to 27.0 | 8.0 to 24.6 | |
| 1 million or more per dose | 29 (100%) | 15 (100%) |
| AV-OVA-1 | MC | AV-OVA-1 | MC | AV-OVA-1 | MC | |
|---|---|---|---|---|---|---|
| Body System | % of 29 Pts | % of 15 Pts | % of All 308 AE | % of All 141 AE | % of Related 87 AE | % of Related 57 AE |
| INJECTION SITE REACTION | 58.6 | 66.7 | 16.2 | 19.9 | 50.6 | 42.1 |
| GENERAL | 69.0 | 60.0 | 18.2 | 17.7 | 29.6 | 20.5 |
| Fatigue | 44.8 | 33.3 | 5.8 | 5.0 | 9.3 | 12.8 |
| Fever | 17.2 | 13.3 | 3.2 | 3.5 | 5.6 | 0.0 |
| Body aches | 20.7 | 6.7 | 1.0 | 0.7 | 3.7 | 2.6 |
| Chills | 10.3 | 20.0 | 2.6 | 3.5 | 1.9 | 2.0 |
| Flu-like symptoms | 13.8 | 6.7 | 3.5 | 4.3 | 3.7 | 0 |
| Malaise (lethargy) | 10.3 | 6.7 | 1.0 | 0.7 | 3.7 | 2.6 |
| DERMATOLOGIC | 41.4 | 46.7 | 8.4 | 9.9 | 12.6 | 12.3 |
| Pruritis | 27.6 | 20.0 | 4.5 | 3.5 | 5.3 | 8.3 |
| Rash | 13.8 | 26.7 | 1.6 | 5.0 | 5.3 | 2.1 |
| Hives (urticaria) | 6.9 | 13.3 | 1.0 | 1.4 | 0.0 | 1.8 |
| MUSCULOSKELETAL | 69.0 | 60.0 | 13.3 | 19.9 | 11.5 | 12.3 |
| Muscle aches, myalgias | 20.7 | 53.3 | 2.6 | 7.8 | 4.6 | 8.8 |
| Joint pain, discomfort, arthralgias | 24.1 | 13.3 | 3.2 | 2.1 | 3.4 | 0 |
| Back pain | 10.3 | 20.0 | 1.3 | 3.5 | 0.0 | 0.0 |
| Bone pain | 17.2 | 6.7 | 1.6 | 1.4 | 2.3 | 3.5 |
| GASTROINTESTINAL | 65.5 | 46.7 | 10.4 | 12.8 | 0.0 | 1.8 |
| Abdominal or pelvic pain | 34.5 | 33.3 | 3.2 | 4.9 | 0.0 | 0.0 |
| Constipation | 17.2 | 20.0 | 1.6 | 3.5 | 0.0 | 0.0 |
| Nausea | 24.1 | 20.0 | 2.3 | 2.1 | 0.0 | 0.0 |
| NEUROLOGIC | 41.4 | 26.7 | 6.2 | 5.7 | 1.1 | 0.0 |
| Headache | 37.9 | 20.0 | 4.2 | 5.0 | 1.1 | 0.0 |
| HEMATOLOGIC | 20.7 | 13.3 | 9.7 | 3.5 | 1.1 | 1.8 |
| Thrombocytopenia/petechiae | 13.8 | 6.7 | 2.6 | 1.4 | 1.1 | 1.8 |
| Neutropenia | 13.8 | 0.0 | 4.2 | 0.0 | 0.0 | 0.0 |
| Anemia | 6.9 | 6.7 | 1.6 | 2.1 | 0.0 | 0.0 |
| EAR NOSE AND THROAT | 41.4 | 20.0 | 6.5 | 2.1 | 1.1 | 0.0 |
| Nasal congestion, rhinitis, post-nasal drip | 17.2 | 0.0 | 2.6 | 0.0 | 0.0 | 0.0 |
| Oral pain (tongue or mouth, ulceration) | 10.3 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 |
| CARDIOVASCULAR | 24.1 | 0.0 | 2.9 | 0.0 | 1.9 | 0.0 |
| Hypertension | 13.8 | 0.0 | 2.0 | 0.0 | 1.9 | 0.0 |
| PSYCHOLOGIC | 17.2 | 13.3 | 2.3 | 2.1 | 0.0 | 0.0 |
| Depression, mood dysphoria | 3.4 | 13.3 | 0.3 | 1.4 | 0.0 | 0.0 |
| Insomnia | 6.9 | 6.7 | 0.6 | 0.7 | 0.0 | 0.0 |
| PULMONARY | 20.7 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 |
| Cough | 13.8 | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 |
| OCULAR | 13.8 | 6.7 | 1.3 | 2.1 | 1.1 | 0.0 |
| Blurred vision | 10.3 | 6.7 | 0.6 | 1.4 | 1.1 | 0.0 |
| AV-OVA-1 (n = 308) | MC (n = 141) | Total (n = 449) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade | Number and Percent of Highest AE per Patient | Number and Percent of All AE | Mean AE per Patient | Number and Percent of Highest AE per Patient | Number and Percent of All AE | Mean AE per Patient | Number and Percent of Highest AE per Patient | Number and Percent of All AE | Mean AE per Patient |
| 1 | 5 (17%) | 229 (74%) | 7.9 | 5 (33%) | 108 (77%) | 7.2 | 10 (23%) | 337 (75%) | 7.7 |
| 2 | 13 (45%) | 62 (20%) | 2.1 | 5 (33%) | 25 (18%) | 1.7 | 18 (41%) | 87 (19%) | 2.0 |
| 3 | 9 (31%) | 16 (5%) | 0.6 | 5 (33%) | 8 (6%) | 0.5 | 14 (32%) | 24 (5%) | 0.5 |
| 4 | 1 (3.4%) | 1 (0.3%) | 0.03 | 0 | 0 | 0 | 1 (2.3%) | 1 (0.2%) | 0.02 |
| Total | 28 (97%) | 308 (100%) | 10.6 | 15 (100%) | 141 (100%) | 9.4 | 43 (98%) | 449 (100%) | 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaid, L.N.; Corr, B.R.; Eskander, R.N.; Mason, J.R.; Lopez, K.L.; Godding, K.; Robles, R.M.; Keirstead, H.S.; Nistor, G.I.; Dillman, R.O. Double-Blind Randomized Phase 2 Trial Testing Personal Cancer Vaccines in Patients with Advanced Ovarian Cancer. Vaccines 2025, 13, 1099. https://doi.org/10.3390/vaccines13111099
Abaid LN, Corr BR, Eskander RN, Mason JR, Lopez KL, Godding K, Robles RM, Keirstead HS, Nistor GI, Dillman RO. Double-Blind Randomized Phase 2 Trial Testing Personal Cancer Vaccines in Patients with Advanced Ovarian Cancer. Vaccines. 2025; 13(11):1099. https://doi.org/10.3390/vaccines13111099
Chicago/Turabian StyleAbaid, Lisa N., Bradley R. Corr, Ramez N. Eskander, James R. Mason, Katrina L. Lopez, Krystal Godding, Rockelle M. Robles, Hans S. Keirstead, Gabriel I. Nistor, and Robert O. Dillman. 2025. "Double-Blind Randomized Phase 2 Trial Testing Personal Cancer Vaccines in Patients with Advanced Ovarian Cancer" Vaccines 13, no. 11: 1099. https://doi.org/10.3390/vaccines13111099
APA StyleAbaid, L. N., Corr, B. R., Eskander, R. N., Mason, J. R., Lopez, K. L., Godding, K., Robles, R. M., Keirstead, H. S., Nistor, G. I., & Dillman, R. O. (2025). Double-Blind Randomized Phase 2 Trial Testing Personal Cancer Vaccines in Patients with Advanced Ovarian Cancer. Vaccines, 13(11), 1099. https://doi.org/10.3390/vaccines13111099






