Regulating Immune Responses Induced by PEGylated Messenger RNA–Lipid Nanoparticle Vaccine
Abstract
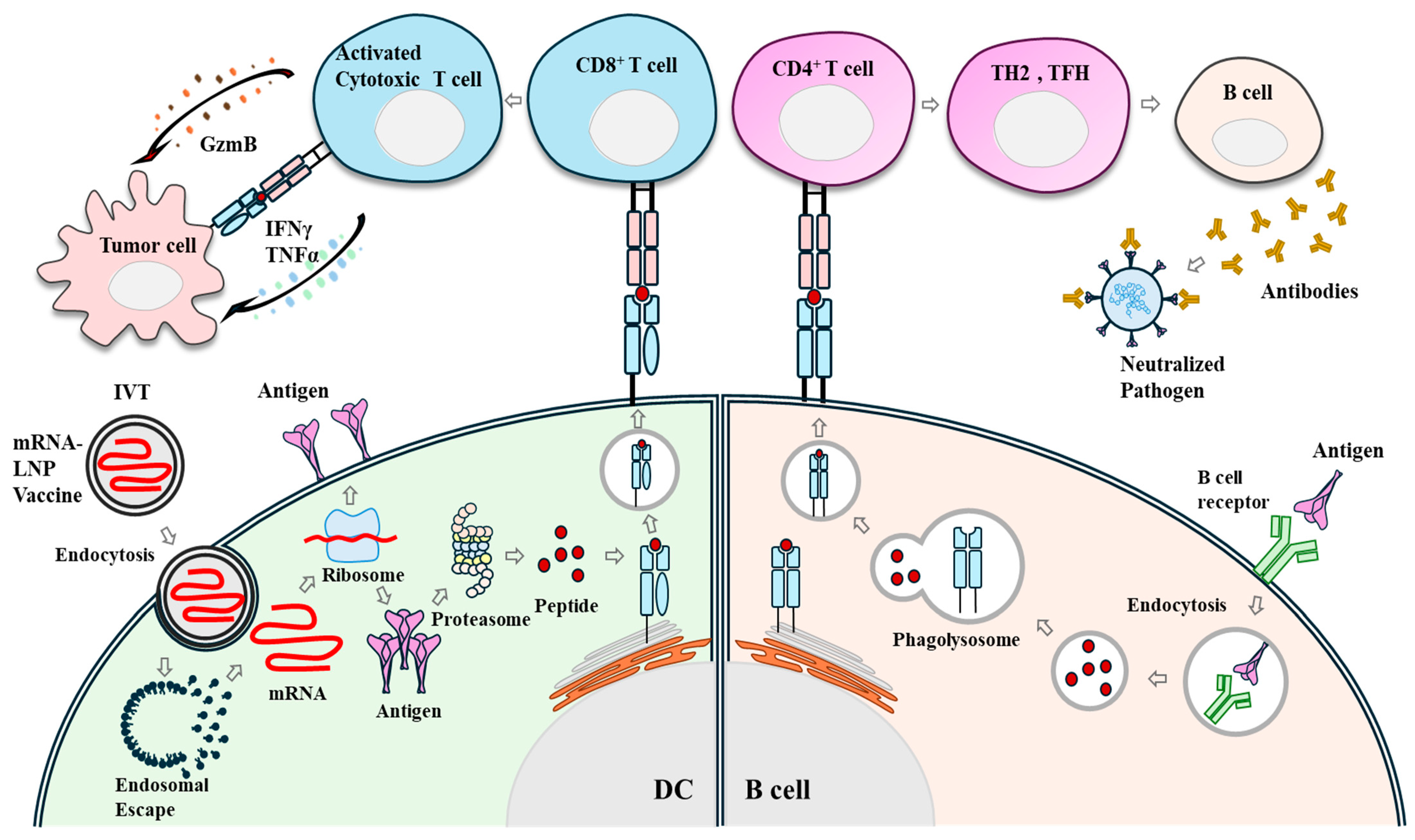
1. Immune Response Induced by mRNA Vaccines: Antigen Translation
2. Structure of mRNA-LNPs
3. Induction of Immunity by Lipid Nanoparticles
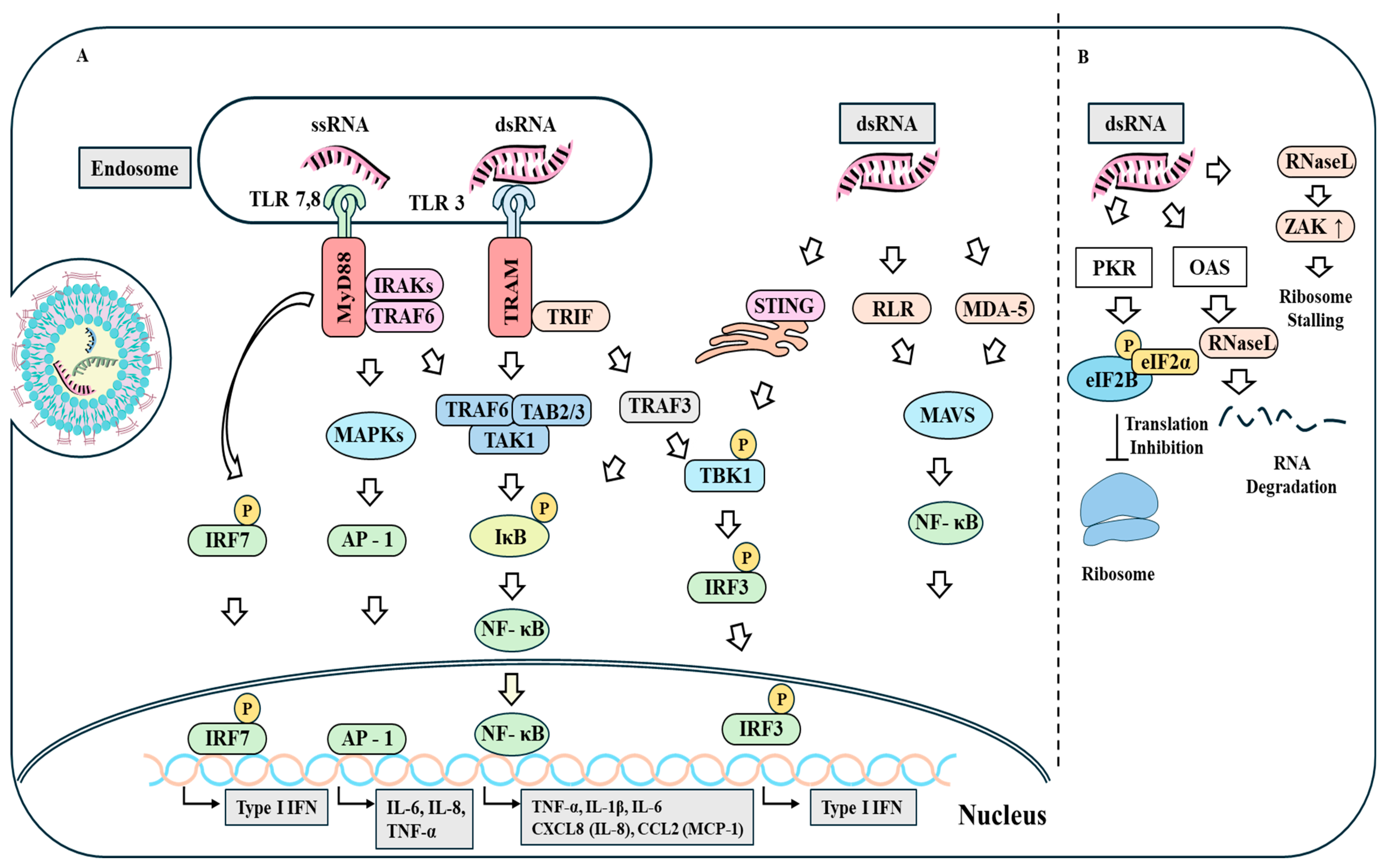
4. Innate RNA Sensing
5. Translational Inhibition of mRNA by dsRNA
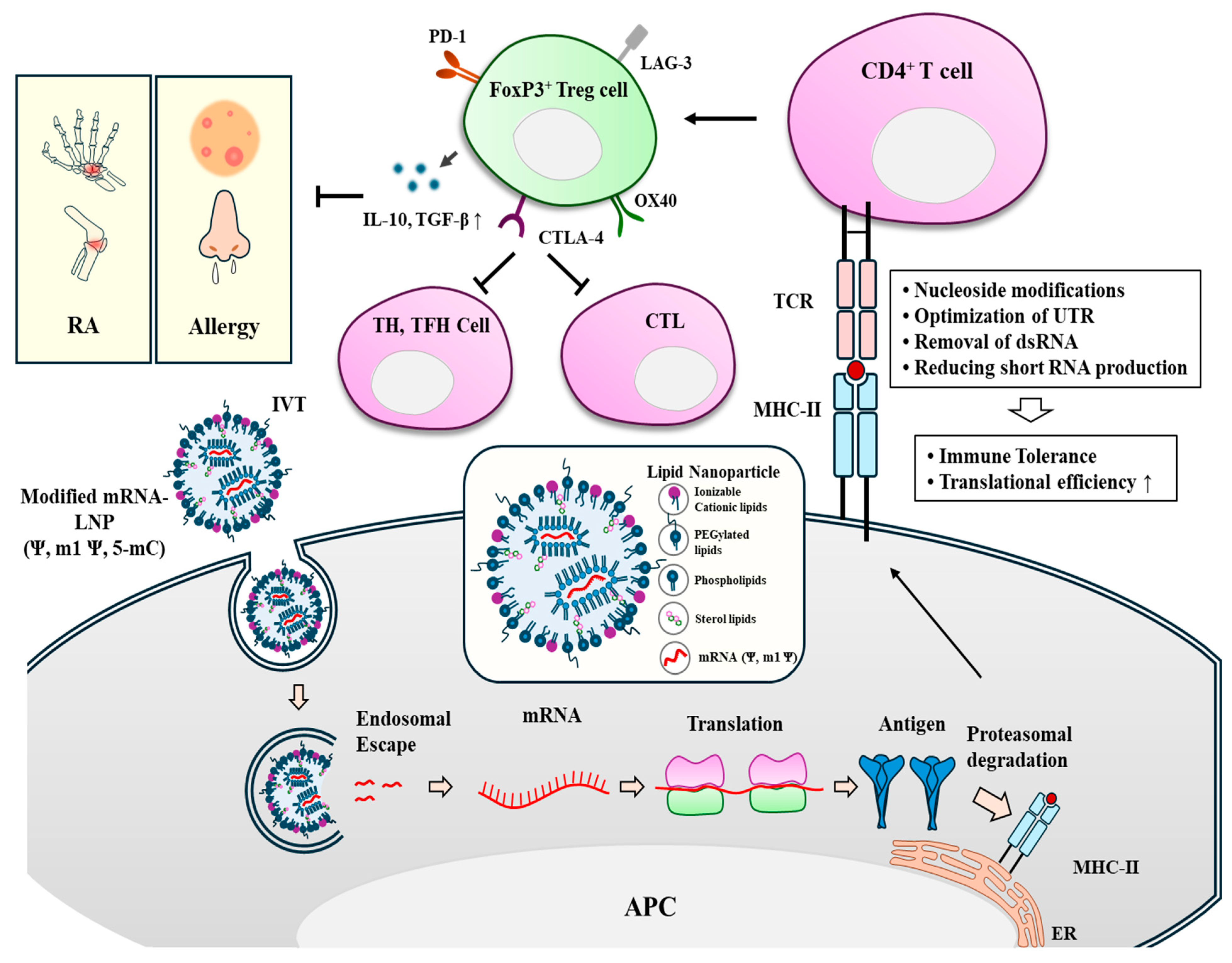
6. Effects of Modifications of mRNAs on Immunogenicity and Translation
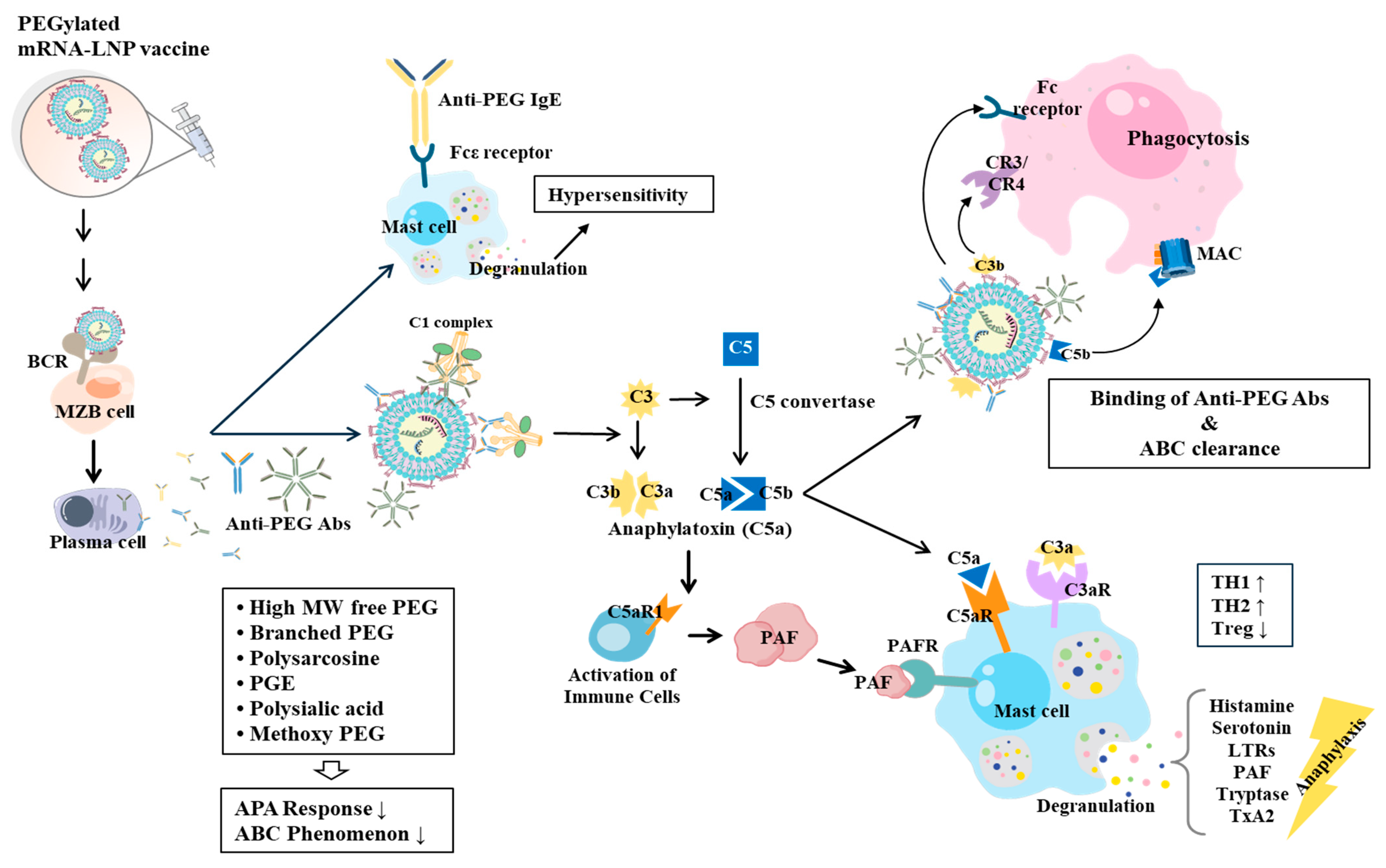
7. PEGylated mRNA-LNPs Cause Hypersensitivity Reactions, Allergies, and Complement Activation-Related Pseudoallergy (CARPA)
8. How to Reduce Immune Responses Associated with PEG
9. Tolerance-Inducing mRNAs Can Reduce Allergies and Autoimmunity
10. Discussion and Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berraondo, P.; Cuesta, R.; Sanmamed, M.F.; Melero, I. Immunogenicity and Efficacy of Personalized Adjuvant mRNA Cancer Vaccines. Cancer Discov. 2024, 14, 2021–2024. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Assaly, N.M.E.; Aly, D.M.; Atta, S.; Fteah, A.M.; Badawi, H.; Zahran, M.Y.; Kamel, M. Comparative study of neutralizing antibodies titers in response to different types of COVID-19 vaccines among a group of egyptian healthcare workers. Virol. J. 2024, 21, 277. [Google Scholar] [CrossRef]
- Kumar, S.; Hsiao, Y.W.; Wong, V.H.Y.; Aubin, D.; Wang, J.H.; Lisowski, L.; Rakoczy, E.P.; Li, F.; Alarcon-Martinez, L.; Gonzalez-Cordero, A.; et al. Characterization of RNA editing and gene therapy with a compact CRISPR-Cas13 in the retina. Proc. Natl. Acad. Sci. USA 2024, 121, e2408345121. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, L.; Li, Z.; Peng, X.; Li, H. mRNA vaccines in tumor targeted therapy: Mechanism, clinical application, and development trends. Biomark. Res. 2024, 12, 93. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of mRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Park, S.; Kim, G.; Choi, A.; Kim, S.; Yum, J.S.; Chun, E.; Shin, H. Comparative network-based analysis of toll-like receptor agonist, L-pampo signaling pathways in immune and cancer cells. Sci. Rep. 2024, 14, 17173. [Google Scholar] [CrossRef] [PubMed]
- Presnyak, V.; Alhusaini, N.; Chen, Y.H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, S.; Penna, R.R.; Frei, J.; Wyss, C.; Mellett, M.; Look, T.; Weiss, T.; Guenova, E.; Kündig, T.M.; Lauchli, S.; et al. Synthetic mRNAs Containing Minimalistic Untranslated Regions Are Highly Functional In Vitro and In Vivo. Cells 2024, 13, 1242. [Google Scholar] [CrossRef]
- Cheng, Z.; Islam, S.; Kanlong, J.G.; Sheppard, M.; Seo, H.; Nikolaitchik, O.A.; Kearse, M.G.; Pathak, V.K.; Musier-Forsyth, K.; Hu, W.S. Translation of HIV-1 unspliced RNA is regulated by 5′ untranslated region structure. J. Virol. 2024, 98, e0116024. [Google Scholar] [CrossRef]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Renauer, P.A.; Ökten, A.; Fang, Z.; Park, J.J.; Zhou, X.; Lin, Q.; Dong, M.B.; Filler, R.; Xiong, Q.; et al. Variant-specific vaccination induces systems immune responses and potent in vivo protection against SARS-CoV-2. Cell Rep. Med. 2022, 3, 100634. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Sun, J.; Liu, Z.; Shu, J.; Yan, H.; Kou, Z.; Wei, Y.; Jin, X. An mRNA vaccine encoding Chikungunya virus E2-E1 protein elicits robust neutralizing antibody responses and CTL immune responses. Virol. Sin. 2022, 37, 266–276. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Rivero Berti, I.; Gambaro, R.C.; Limeres, M.J.; Huck-Iriart, C.; Svensson, M.; Fraude-El Ghazi, S.; Pretsch, L.; Si, S.; Lieberwirth, I.; Landfester, K.; et al. Encapsulation of Dexamethasone into mRNA-Lipid Nanoparticles Is a Promising Approach for the Development of Liver-Targeted Anti-Inflammatory Therapies. Int. J. Mol. Sci. 2024, 25, 11254. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Q.B.; Xu, H.; Adi, D.; Ding, Y.W.; Luo, W.J.; Zhu, W.Z.; Xu, J.C.; Zhao, X.; Shi, X.J.; et al. siRNA nanoparticle targeting Usp20 lowers lipid levels and ameliorates metabolic syndrome in mice. J. Lipid Res. 2024, 65, 100626. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjug. Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Paun, R.A.; Jurchuk, S.; Tabrizian, M. A landscape of recent advances in lipid nanoparticles and their translational potential for the treatment of solid tumors. Bioeng. Transl. Med. 2023, 9, e10601. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Fuentes, S.; Arancibia, D.; Rojas, M.; Carmona, F.; Ortega, A.; Valenzuela, J.; Hernández-Álvarez, C.; Martín, I.R. Simultaneous Second Harmonic Generation and Multiphoton Excited Photoluminescence in Samarium-Doped BaTiO3 Nanoparticles Functionalized with Poly (ethylene glycol). ACS Omega 2024, 9, 28061–28071. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Kato, N.; Kodama, Y.; Mukai, H.; Kawakami, S. Influence of lipid composition of messenger RNA-loaded lipid nanoparticles on the protein expression via intratracheal administration in mice. Int. J. Pharm. 2023, 637, 122896. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Zheng, M.H.; Meng, X.T.; Ma, L.W.; Liang, H.Y.; Fan, H.Y. Role of toll-like receptors in the pathogenesis of COVID-19: Current and future perspectives. Scand. J. Immunol. 2023, 98, e13275. [Google Scholar] [CrossRef]
- Klein, C.R.; Heine, A.; Brossart, P.; Karakostas, P.; Schäfer, V.S. Anti-MDA5 autoantibodies predict clinical dynamics of dermatomyositis following SARS-CoV-2 mRNA vaccination: A retrospective statistical analysis of case reports. Rheumatol. Int. 2024, 44, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Villacampa, A.; Alfaro, E.; Morales, C.; Díaz-García, E.; López-Fernández, C.; Bartha, J.L.; López-Sánchez, F.; Lorenzo, Ó.; Moncada, S.; Sánchez-Ferrer, C.F.; et al. SARS-CoV-2 S protein activates NLRP3 inflammasome and deregulates coagulation factors in endothelial and immune cells. Cell Commun. Signal 2024, 22, 38. [Google Scholar] [CrossRef]
- Jeon, H.E.; Lee, S.; Lee, J.; Roh, G.; Park, H.J.; Lee, Y.S.; Kim, Y.J.; Kim, H.K.; Shin, J.H.; Lee, Y.J.; et al. SARS-CoV-2 mRNA vaccine intravenous administration induces myocarditis in chronic inflammation. PLoS ONE 2024, 19, e0311726. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, G.; Sævik, M.; Søyland, H.; Ueland, T.; Zhou, F.; Pathirana, R.; Madsen, A.; Vahokoski, J.; Lartey, S.; Halvorsen, B.E.; et al. Three doses of SARS-CoV-2 mRNA vaccine in older adults result in similar antibody responses but reduced cellular cytokine responses relative to younger adults. Vaccine X 2024, 20, 100564. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeon, J.H.; Kim, M.; Lee, Y.; Hwang, Y.H.; Park, M.; Li, C.H.; Lee, T.; Lee, J.A.; Kim, Y.M.; et al. Innate immune responses against mRNA vaccine promote cellular immunity through IFN-beta at the injection site. Nat. Commun. 2024, 15, 7226. [Google Scholar] [CrossRef] [PubMed]
- Gangaev, A.; van Sleen, Y.; Brandhorst, N.; Hoefakker, K.; Prajapati, B.; Singh, A.; Boerma, A.; van der Heiden, M.; Oosting, S.F.; van der Veldt, A.A.M.; et al. mRNA-1273 vaccination induces polyfunctional memory CD4 and CD8 T cell responses in patients with solid cancers undergoing immunotherapy or/and chemotherapy. Front. Immunol. 2024, 15, 1447555. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Korzun, T.; Moses, A.S.; Jozic, A.; Grigoriev, V.; Newton, S.; Kim, J.; Diba, P.; Sattler, A.; Levasseur, P.R.; Le, N.; et al. Lipid Nanoparticles Elicit Reactogenicity and Sickness Behavior in Mice Via Toll-Like Receptor 4 and Myeloid Differentiation Protein 88 Axis. ACS Nano 2024, 18, 24842–24859. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.M. Cationic lipid nanocarriers activate toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Bakos, T.; Mészáros, T.; Kozma, G.T.; Berényi, P.; Facskó, R.; Farkas, H.; Dézsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suzuoki, M.; Tanaka, H.; Sakurai, Y.; Hatakeyama, H.; Akita, H. Lymphatic Endothelial Cells Produce Chemokines in Response to the Lipid Nanoparticles Used in RNA Vaccines. Biol. Pharm. Bull. 2024, 47, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhang, L. Identifying RNA Sensors in Antiviral Innate Immunity. Methods Mol. Biol. 2025, 2854, 107–115. [Google Scholar] [PubMed]
- Kos, M.; Bojarski, K.; Mertowska, P.; Mertowski, S.; Tomaka, P.; Dziki, Ł.; Grywalska, E. Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors? Cells 2024, 13, 1708. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-López, A.; Garfias, Y. Cytokine profile of human limbal myofibroblasts: Key players in corneal antiviral response. Cytokine 2022, 160, 156047. [Google Scholar] [CrossRef] [PubMed]
- Roßmann, L.; Bagola, K.; Stephen, T.; Gerards, A.L.; Walber, B.; Ullrich, A.; Schülke, S.; Kamp, C.; Spreitzer, I.; Hasan, M.; et al. Distinct single-component adjuvants steer human DC-mediated T-cell polarization via Toll-like receptor signaling toward a potent antiviral immune response. Proc. Natl. Acad. Sci. USA 2021, 118, e2103651118. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.D.; Pokkali, S.; Kim, D.; Brammer, D.; Song, K.; McCarthy, E.; Lehman, C.; Todd, J.P.; Foulds, K.E.; Darrah, P.A.; et al. Immune Activation Profiles Elicited by Distinct, Repeated TLR Agonist Infusions in Rhesus Macaques. J. Immunol. 2023, 211, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef]
- Usero, L.; Leal, L.; Gómez, C.E.; Miralles, L.; Aurrecoechea, E.; Esteban, I.; Torres, B.; Inciarte, A.; Perdiguero, B.; Esteban, M.; et al. The Combination of an mRNA Immunogen, a TLR7 Agonist and a PD1 Blocking Agent Enhances In-Vitro HIV T-Cell Immune Responses. Vaccines 2023, 11, 286. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Hou, X.; Wang, C.; Kang, D.D.; Xue, Y.; Du, S.; Deng, B.; McComb, D.W.; Liu, S.L.; et al. STING Agonist-Derived LNP-mRNA Vaccine Enhances Protective Immunity Against SARS-CoV-2. Nano Lett. 2023, 23, 2593–2600. [Google Scholar] [CrossRef]
- Abo-Samaha, M.I.; Sharaf, M.M.; El-Nahas, A.F.; Odemuyiwa, S.O. Length-Dependent Modulation of B Cell Activating Factor Transcripts in Chicken Macrophage by Viral Double-Stranded RNA. Vaccines 2023, 11, 1561. [Google Scholar] [CrossRef]
- Choudhury, A.; Das, N.C.; Patra, R.; Mukherjee, S. In silico analyses on the comparative sensing of SARS-CoV-2 mRNA by the intracellular TLRs of humans. J. Med. Virol. 2021, 93, 2476–2486. [Google Scholar] [CrossRef]
- Miquel, C.H.; Abbas, F.; Cenac, C.; Foret-Lucas, C.; Guo, C.; Ducatez, M.; Joly, E.; Hou, B.; Guéry, J.C. B cell-intrinsic TLR7 signaling is required for neutralizing antibody responses to SARS-CoV-2 and pathogen-like COVID-19 vaccines. Eur. J. Immunol. 2023, 53, e2350437. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chi, Y.; Tao, X.; Yu, P.; Liu, Q.; Zhang, M.; Yang, N.; Liu, S.; Zhu, W. Rabies Virus Regulates Inflammatory Response in BV-2 Cells through Activation of Myd88 and NF-kappaB Signaling Pathways via TLR7. Int. J. Mol. Sci. 2024, 25, 9144. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.L.; Mayfield, J.; Barchiesi, R.; Salem, N.A.; Mayfield, R.D. Toll-like receptor 7: A novel neuroimmune target to reduce excessive alcohol consumption. Neurobiol. Stress 2024, 31, 100639. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Le, D.S.; Liu, L.; Zhang, X.X.; Yang, F.; Lai, G.R.; Zhang, C.; Zhao, M.L.; Shen, Y.P.; Liao, P.S.; et al. Targeting exosomal double-stranded RNA-TLR3 signaling pathway attenuates morphine tolerance and hyperalgesia. Cell Rep. Med. 2024, 5, 101782. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Zhao, X.; Liu, Z.; Shen, G.; Zhang, T.; Zhang, T.; Hu, G. Oxymatrine Modulation of TLR3 Signaling: A Dual-Action Mechanism for H9N2 Avian Influenza Virus Defense and Immune Regulation. Molecules 2024, 29, 1945. [Google Scholar] [CrossRef] [PubMed]
- Lamoot, A.; Jangra, S.; Laghlali, G.; Warang, P.; Singh, G.; Chang, L.A.; Park, S.C.; Singh, G.; De Swarte, K.; Zhong, Z.; et al. Lipid Nanoparticle Encapsulation Empowers Poly(I:C) to Activate Cytoplasmic RLRs and Thereby Increases Its Adjuvanticity. Small 2024, 20, e2306892. [Google Scholar] [CrossRef]
- Zhang, H.; Sandhu, P.K.; Damania, B. The Role of RNA Sensors in Regulating Innate Immunity to Gammaherpesviral Infections. Cells 2023, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Mikhalkevich, N.; Russ, E.; Iordanskiy, S. Cellular RNA and DNA sensing pathways are essential for the dose-dependent response of human monocytes to ionizing radiation. Front. Immunol. 2023, 14, 1235936. [Google Scholar] [CrossRef] [PubMed]
- Madaan, V.; Kollara, A.; Spaner, D.; Brown, T.J. ISGylation enhances dsRNA-induced interferon response and NFκB signaling in fallopian tube epithelial cells. J. Biol. Chem. 2024, 300, 107686. [Google Scholar] [CrossRef] [PubMed]
- Karasik, A.; Lorenzi, H.A.; DePass, A.V.; Guydosh, N.R. Endonucleolytic RNA cleavage drives changes in gene expression during the innate immune response. Cell Rep. 2024, 43, 114287. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, L.; Peruzzi, M.; Huetz, F.; Raffy, C.; Le Hir, J.; Minke, J.; Boudinot, P.; Collet, B. Salmonid Double-stranded RNA-Dependent Protein Kinase Activates Apoptosis and Inhibits Protein Synthesis. J. Immunol. 2024, 213, 700–717. [Google Scholar] [CrossRef]
- Yu, H.; Megawati, D.; Zheng, C.; Rothenberg, S. Protein Kinase R (PKR) as a Novel dsRNA Sensor in Antiviral Innate Immunity. Methods Mol. Biol. 2025, 2854, 265–282. [Google Scholar]
- Hu, J.; Hodgkinson, C.P.; Pratt, R.E.; Lee, J.; Sullenger, B.A.; Dzau, V.J. Enhancing cardiac reprogramming via synthetic RNA oligonucleotides. Mol. Ther. Nucleic Acids 2020, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wan, B.; Li, H.; He, J.; Chen, X.; Wang, L.; Wang, Y.; Xie, S.; Qiao, S.; Zhang, G. Porcine 2′,5′-oligoadenylate synthetase 2 inhibits porcine reproductive and respiratory syndrome virus replication in vitro. Microb. Pathog. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Aloise, C.; Schipper, J.G.; van Vliet, A.; Oymans, J.; Donselaar, T.; Hurdiss, D.L.; de Groot, R.J.; van Kuppeveld, F.J.M. SARS-CoV-2 nucleocapsid protein inhibits the PKR-mediated integrated stress response through RNA-binding domain N2b. PLoS Pathog. 2023, 19, e1011582. [Google Scholar] [CrossRef]
- Tahsin, A.; Bhattacharjee, P.; Al Saba, A.; Yasmin, T.; Nabi, A.H.M.N. Genetic and epigenetic analyses of IFN-γ gene proximal promoter region underlying positive correlation between persistently high anti-SARS-CoV-2 IgG and IFN-γ among COVID-19 vaccinated Bangladeshi adults. Vaccine 2024, 42, 126157. [Google Scholar] [CrossRef]
- Otter, C.J.; Bracci, N.; Parenti, N.A.; Ye, C.; Asthana, A.; Blomqvist, E.K.; Tan, L.H.; Pfannenstiel, J.J.; Jackson, N.; Fehr, A.R.; et al. SARS-CoV-2 nsp15 endoribonuclease antagonizes dsRNA-induced antiviral signaling. Proc. Natl. Acad. Sci. USA 2024, 121, e2320194121. [Google Scholar] [CrossRef] [PubMed]
- Cusic, R.; Burke, J.M. Condensation of RNase L promotes its rapid activation in response to viral infection in mammalian cells. Sci. Signal 2024, 17, eadi9844. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, B.; Asthana, A.; Silverman, R.H.; Yan, N. RNA helicase SKIV2L limits antiviral defense and autoinflammation elicited by the OAS-RNase L pathway. EMBO J. 2024, 43, 3876–3894. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Han, C.W.; Jeong, M.S.; Jang, S.B. Structural study of novel vaccinia virus E3L and dsRNA-dependent protein kinase complex. Biochem. Biophys. Res. Commun. 2023, 665, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Xu, L.; Gibson, T.M.; Gersbach, C.A.; Sullenger, B.A. Differential effects of toll-like receptor stimulation on mRNA-driven myogenic conversion of human and mouse fibroblasts. Biochem. Biophys. Res. Commun. 2016, 478, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.J.; Mancebo, C.; Garcinuño, S.; March, G.; Alvarez, Y.; Alonso, S.; Inglada, L.; Blanco, J.; Orduña, A.; Montero, O.; et al. Innate IRE1α-XBP1 activation by viral single-stranded RNA and its influence on lung cytokine production during SARS-CoV-2 pneumonia. Genes Immun. 2024, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kodigepalli, K.M.; Nanjundan, M. Induction of PLSCR1 in a STING/IRF3-dependent manner upon vector transfection in ovarian epithelial cells. PLoS ONE 2015, 10, e0117464. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jin, H.; Cui, Y.; Yang, F.; Chen, K.; Kuang, W.; Huo, C.; Xu, Z.; Li, Y.; Lin, A.; et al. PUS7-dependent pseudouridylation of ALKBH3 mRNA inhibits gastric cancer progression. Clin. Transl. Med. 2024, 14, e1811. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, C.; Fedorov, A.; Qiao, L.; Bao, H.; Beknazarov, N.; Wang, S.; Gautam, A.; Williams, R.M.; Crawford, J.C.; et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 2022, 606, 594–602. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Kariko, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Kariko, K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011, 39, 9329–9338. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Layhadi, J.A.; Hourcade, D.E.; Fulton, W.T.; Tan, T.J.; Dunham, D.; Chang, I.; Vel, M.S.; Fernandes, A.; Lee, A.S.; et al. Elucidating allergic reaction mechanisms in response to SARS-CoV-2 mRNA vaccination in adults. Allergy 2024, 79, 2502–2523. [Google Scholar] [CrossRef] [PubMed]
- Awaya, T.; Hara, H.; Moroi, M. Cytokine Storms and Anaphylaxis Following COVID-19 mRNA-LNP Vaccination: Mechanisms and Therapeutic Approaches. Diseases 2024, 12, 231. [Google Scholar] [CrossRef]
- Calzetta, L.; Chetta, A.; Aiello, M.; Frizzelli, A.; Ora, J.; Melis, E.; Facciolo, F.; Ippoliti, L.; Magrini, A.; Rogliani, P. The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma. Vaccines 2023, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T.; Sun, G.Y.; Lin, T.N.; The, D.B.L.; Ong, W.Y. COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines 2023, 11, 1181. [Google Scholar] [CrossRef]
- Suzuki, T.; Taketomi, Y.; Yanagida, K.; Yoshida-Hashidate, T.; Nagase, T.; Murakami, M.; Shimizu, T.; Shindou, H. Re-evaluation of the canonical PAF pathway in cutaneous anaphylaxis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1870, 159563. [Google Scholar] [CrossRef]
- Richter, A.W.; Akerblom, E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 1983, 70, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Han, H.; Liu, A.; Zhao, S.; Lawanprasert, A.; Nielsen, J.E.; Choudhary, H.; Liang, D.; Barron, A.E.; Murthy, N. Ethylene oxide graft copolymers reduce the immunogenicity of lipid nanoparticles. RSC Adv. 2024, 14, 30071–30076. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.B.; Tunbridge, M.J.; Hurtado, P.R.; Zuiani, J.; Mhatre, S.; Yip, K.H.; Le, T.A.; Yuson, C.; Kette, F.; Hissaria, P. PEGylated liposomes for diagnosis of polyethylene glycol allergy. J. Allergy Clin. Immunol. 2024, 154, 503–507.e1. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Mészáros, T.; Berényi, P.; Facskó, R.; Patkó, Z.; Oláh, C.Z.; Nagy, A.; Fülöp, T.G.; Glatter, K.A.; Radovits, T.; et al. Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions to PEG-containing COVID-19 vaccines: Evidence for immunogenicity of PEG. Vaccine 2023, 41, 4561–4570. [Google Scholar] [CrossRef]
- Miao, G.; He, Y.; Lai, K.; Zhao, Y.; He, P.; Tan, G.; Wang, X. Accelerated blood clearance of PEGylated nanoparticles induced by PEG-based pharmaceutical excipients. J. Control. Release 2023, 363, 12–26. [Google Scholar] [CrossRef]
- Carreño, J.M.; Singh, G.; Tcheou, J.; Srivastava, K.; Gleason, C.; Muramatsu, H.; Desai, P.; Aberg, J.A.; Miller, R.L.; Paris Study Group; et al. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine 2022, 40, 6114–6124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Saba, L.; Scheinman, R.I.; Banda, N.K.; Holers, M.; Monte, A.; Dylla, L.; Moghimi, S.M.; Simberg, D. Nanoparticle-Binding Immunoglobulins Predict Variable Complement Responses in Healthy and Diseased Cohorts. ACS Nano 2024, 18, 28649–28658. [Google Scholar] [CrossRef]
- Chen, W.A.; Chang, D.Y.; Chen, B.M.; Lin, Y.C.; Barenholz, Y.; Roffler, S.R. Antibodies against Poly (ethylene glycol) Activate Innate Immune Cells and Induce Hypersensitivity Reactions to PEGylated Nanomedicines. ACS Nano 2023, 17, 5757–5772. [Google Scholar] [CrossRef]
- Park, M.K.; Park, H.K.; Yu, H.S. Toll-like receptor 2 mediates Acanthamoeba-induced allergic airway inflammatory response in mice. PLoS Negl. Trop. Dis. 2023, 17, e0011085. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, G.; Jiang, Y.; Peng, C.; Li, W. TLR2-ERK signaling pathway regulates expression of galectin-3 in a murine model of OVA-induced allergic airway inflammation. Toxicol. Lett. 2024, 397, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Abu Lila, A.S.; Shimizu, T.; Ishida, T.; Kiwada, H. B cell-intrinsic toll-like receptor 7 is responsible for the enhanced anti-PEG IgM production following injection of siRNA-containing PEGylated lipoplex in mice. J. Control. Release 2014, 184, 1–8. [Google Scholar] [CrossRef]
- Stavnsbjerg, C.; Christensen, E.; Münter, R.; Henriksen, J.R.; Fach, M.; Parhamifar, L.; Christensen, C.; Kjaer, A.; Hansen, A.E.; Andresen, T.L. Accelerated blood clearance and hypersensitivity by PEGylated liposomes containing TLR agonists. J. Control. Release 2022, 342, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Barta, B.A.; Radovits, T.; Dobos, A.B.; Tibor Kozma, G.; Mészáros, T.; Berényi, P.; Facskó, R.; Fülöp, T.; Merkely, B.; Szebeni, J. Comirnaty-induced cardiopulmonary distress and other symptoms of complement-mediated pseudo-anaphylaxis in a hyperimmune pig model: Causal role of anti-PEG antibodies. Vaccine X 2024, 19, 100497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Luo, W.; Zhang, D.; Liu, R. An Assay for Immunogenic Detection of Anti-PEG Antibody. ChemBioChem 2024, 25, e202400316. [Google Scholar] [CrossRef]
- Davis, E.; Caparco, A.A.; Jones, E.; Steinmetz, N.F.; Pokorski, J.K. Study of uricase-polynorbornene conjugates derived from grafting-from ring-opening metathesis polymerization. J. Mater. Chem. B 2024, 12, 2197–2206. [Google Scholar] [CrossRef]
- Tsang, M.S.; Hou, T.; Chan, B.C.; Wong, C.K. Immunological Roles of NLR in Allergic Diseases and Its Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 1507. [Google Scholar] [CrossRef]
- Leven, T.; Coorevits, L.; Vandebotermet, M.; Tuyls, S.; Vanneste, H.; Santy, L.; Wets, D.; Proost, P.; Frans, G.; Devolder, D.; et al. Endotyping of IgE-Mediated Polyethylene Glycol and/or Polysorbate 80 Allergy. J. Allergy Clin. Immunol. Pract. 2023, 11, 3146–3160. [Google Scholar]
- Dézsi, L.; Mészáros, T.; Kozma, G.; H-Velkei, M.; Oláh, C.Z.; Szabó, M.; Patkó, Z.; Fülöp, T.; Hennies, M.; Szebeni, M.; et al. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: Complement activation as a possible contributing factor. Geroscience 2022, 44, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Ribak, Y.; Rubin, L.; Talmon, A.; Dranitzki, Z.; Shamriz, O.; Hershkowitz, I.; Tal, Y.; Hershko, A.Y. Administration of BNT162b2 mRNA COVID-19 vaccine to subjects with various allergic backgrounds. Front. Immunol. 2023, 14, 1172896. [Google Scholar] [CrossRef]
- Gao, P.; Tang, K.; Lu, Y.; Wang, M.; Wang, W.; Wang, T.; Sun, Y.; Zhao, J.; Mao, Y. Increased expression of ficolin-1 is associated with airway obstruction in asthma. BMC Pulm. Med. 2023, 23, 470. [Google Scholar] [CrossRef]
- Kokelj, S.; Östling, J.; Fromell, K.; Vanfleteren, L.E.G.W.; Olsson, H.K.; Nilsson Ekdahl, K.; Nilsson, B.; Olin, A.C. Activation of the Complement and Coagulation Systems in the Small Airways in Asthma. Respiration 2023, 102, 621–631. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef]
- Münter, R.; Stavnsbjerg, C.; Christensen, E.; Thomsen, M.E.; Stensballe, A.; Hansen, A.E.; Parhamifar, L.; Kristensen, K.; Simonsen, J.B.; Larsen, J.B.; et al. Unravelling Heterogeneities in Complement and Antibody Opsonization of Individual Liposomes as a Function of Surface Architecture. Small 2022, 18, e2106529. [Google Scholar] [CrossRef]
- Khunsuk, P.O.; Pongma, C.; Palaga, T.; Hoven, V.P. Zwitterionic Polymer-Decorated Lipid Nanoparticles for mRNA Delivery in Mammalian Cells. Biomacromolecules 2023, 24, 5654–5665. [Google Scholar] [CrossRef] [PubMed]
- Gabrielaitis, D.; Zitkute, V.; Saveikyte, L.; Labutyte, G.; Skapas, M.; Meskys, R.; Casaite, V.; Sasnauskiene, A.; Neniskyte, U. Nanotubes from bacteriophage tail sheath proteins: Internalisation by cancer cells and macrophages. Nanoscale Adv. 2023, 5, 3705–3716. [Google Scholar] [CrossRef] [PubMed]
- Barbey, C.; Wolf, H.; Wagner, R.; Pauly, D.; Breunig, M. A shift of paradigm: From avoiding nanoparticular complement activation in the field of nanomedicines to its exploitation in the context of vaccine development. Eur. J. Pharm. Biopharm. 2023, 193, 119–128. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.M.; Chen, E.; Lin, Y.C.; Tran, T.T.M.; Turjeman, K.; Yang, S.H.; Cheng, T.L.; Barenholz, Y.; Rofflerm, S.R. Liposomes with Low Levels of Grafted Poly (ethylene glycol) Remain Susceptible to Destabilization by Anti-Poly (ethylene glycol) Antibodies. ACS Nano 2024, 18, 22122–22138. [Google Scholar] [CrossRef]
- Son, K.; Ueda, M.; Taguchi, K.; Maruyama, T.; Takeoka, S.; Ito, Y. Evasion of the accelerated blood clearance phenomenon by polysarcosine coating of liposomes. J. Control. Release 2020, 322, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lila, A.S.A.; Kitayama, Y.; Abe, R.; Takata, H.; Ando, H.; Ishima, Y.; Ishida, T. Peritoneal B Cells Play a Role in the Production of Anti-polyethylene Glycol (PEG) IgM against Intravenously Injected siRNA-PEGylated Liposome Complexes. Biol. Pharm. Bull. 2024, 47, 469–477. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Ye, X.; Chen, Y.; Wang, L.; Meng, X.; Chen, W.; Wang, F. Activation of CYP3A by Accelerated Blood Clearance Phenomenon Potentiates the Hepatocellular Carcinoma-Targeting Therapeutic Effects of PEGylated Anticancer Prodrug Liposomes. Drug Metab. Dispos. 2023, 51, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Daily, K.P.; Gray, M.C.; Ragland, S.; Werner, L.M.; Brittany Johnson, M.; Eby, J.C.; Hewlett, E.L.; Taylor, R.P.; Criss, A.K. Phagocytosis via complement receptor 3 enables microbes to evade killing by neutrophils. J. Leukoc. Biol. 2023, 114, 1–20. [Google Scholar] [CrossRef]
- Zwarthoff, S.A.; Berends, E.T.M.; Mol, S.; Ruyken, M.; Aerts, P.C.; Józsi, M.; de Haas, C.J.C.; Rooijakkers, S.H.M.; Gorham, R.D., Jr. Functional Characterization of Alternative and Classical Pathway C3/C5 Convertase Activity and Inhibition Using Purified Models. Front. Immunol. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Laumonnier, Y.; Korkmaz, R.Ü.; Nowacka, A.A.; Köhl, J. Complement-mediated immune mechanisms in allergy. Eur. J. Immunol. 2023, 53, e2249979. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Köther, B.; Zwirner, J.; Dijkstra, D.; Purwar, R.; Wittmann, M.; Werfel, T. Human plasmacytoid dendritic cells express receptors for anaphylatoxins C3a and C5a and are chemoattracted to C3a and C5a. J. Investig. Dermatol. 2006, 126, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, B.; Piliponsky, A.M.; Oka, T.; Song, C.H.; Gerard, N.P.; Gerard, C.; Tsai, M.; Kalesnikoff, J.; Galli, S.J. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J. Allergy Clin. Immunol. 2013, 131, 541–548. [Google Scholar] [CrossRef]
- Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Subramanian, H. A Critical Role for Na(+)/H(+) Exchanger Regulatory Factor 1 in Modulating FcepsilonRI-Mediated Mast Cell Activation. J. Immunol. 2021, 206, 471–480. [Google Scholar] [CrossRef]
- West, P.W.; Bahri, R.; Garcia-Rodriguez, K.M.; Sweetland, G.; Wileman, G.; Shah, R.; Montero, A.; Rapley, L.; Bulfone-Paus, S. Interleukin-33 Amplifies Human Mast Cell Activities Induced by Complement Anaphylatoxins. Front. Immunol. 2021, 11, 615236. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Tanaka, Y.; Bando, K.; Sugawara, S.; Mizuta, K. Lipopolysaccharide Priming Exacerbates Anaphylatoxin C5a-Induced Anaphylaxis in Mice. Biol. Pharm. Bull. 2023, 46, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, K.Y.; Ma, N.; Wei, L.L.; Garstka, M.; Zhou, W.; Li, K. The C5a/C5aR2 axis promotes renal inflammation and tissue damage. JCI Insight 2020, 5, e134081. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, X.; Hu, C.; Qin, L.; He, R.; Luo, L.; Tang, W.; Feng, J. Respiratory Syncytial Virus Exacerbates OVA-mediated asthma in mice through C5a-C5aR regulating CD4(+)T cells Immune Responses. Sci. Rep. 2017, 7, 15207. [Google Scholar] [CrossRef]
- Karp, C.L.; Grupe, A.; Schadt, E.; Ewart, S.L.; Keane-Moore, M.; Cuomo, P.J.; Köhl, J.; Wahl, L.; Kuperman, D.; Germer, S.; et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat. Immunol. 2000, 1, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Rönnau, A.C.; Wulferink, M.; Gleichmann, E.; Unver, E.; Ruzicka, T.; Krutmann, J.; Grewe, M. Anaphylaxis to polyvinylpyrrolidone in an analgesic preparation. Br. J. Dermatol. 2000, 143, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Liccioli, G.; Mori, F.; Barni, S.; Pucci, N.; Novembre, E. Anaphylaxis to Polyvinylpyrrolidone in Eye Drops Administered to an Adolescent. J. Investig. Allergol. Clin. Immunol. 2018, 28, 263–265. [Google Scholar] [CrossRef]
- Baysal, S.; Anil, H.; Harmanci, K. A Case Report and Pediatric Literature Review: Povidone as a Rare Cause of Anaphylaxis in Children. Pediatr. Allergy Immunol. Pulmonol. 2024, 37, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, R.; Dairi, K.; Almadabgy, E.; Albiladi, A.; Gamal, L.; Almatrafi, D.; AlShariff, F.; Alsefri, A. New Onset of Neuro-Sjogren’s Syndrome Nine Months After the Third COVID-19 Vaccine Dose: A Case Report. Cureus 2024, 16, e69562. [Google Scholar] [CrossRef]
- Cahuapaza-Gutierrez, N.L. Systemic lupus erythematosus following COVID-19 vaccination. A systematic review of case reports and case series. Lupus 2024, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard-Mouritsen, M.A.; Jensen, B.M.; Poulsen, L.K.; Duus Johansen, J.; Garvey, L.H. Optimizing investigation of suspected allergy to polyethylene glycols. J. Allergy Clin. Immunol. 2022, 149, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, D.; Yan, N.; Li, J.; Zhang, H.; Liu, M.; Tang, X.; Liu, X.; Deng, Y.; Song, Y.; et al. Evasion of the accelerated blood clearance phenomenon by branched PEG lipid derivative coating of nanoemulsions. Int. J. Pharm. 2022, 612, 121365. [Google Scholar] [CrossRef]
- Sui, D.; Wang, Y.; Sun, W.; Wei, L.; Li, C.; Gui, Y.; Qi, Z.; Liu, X.; Song, Y.; Deng, Y. Cleavable-Branched Polymer-Modified Liposomes Reduce Accelerated Blood Clearance and Enhance Photothermal Therapy. ACS Appl. Mater. Interfaces 2023, 15, 32110–32120. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, M.D.; Shen, L.; DeWalle, A.C.; Joiner, J.B.; Ciociola, E.C.; Raghuwanshi, D.; Macauley, M.S.; Lai, S.K. Pre-treatment with high molecular weight free PEG effectively suppresses anti-PEG antibody induction by PEG-liposomes in mice. J. Control. Release 2021, 329, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, Z.; Ma, A.; Cruz-Teran, C.; Talkington, A.; Shipley, S.T.; Lai, S.K. Free PEG Suppresses Anaphylaxis to PEGylated Nanomedicine in Swine. ACS Nano 2024, 18, 8733–8744. [Google Scholar] [CrossRef]
- Hu, Y.; Hou, Y.; Wang, H.; Lu, H. Polysarcosine as an alternative to PEG for therapeutic protein conjugation. Bioconjug. Chem. 2018, 29, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Taguchi, K.; Matsumoto, K.; Kobatake, E.; Ito, Y.; Ueda, M. Polysarcosine-Coated liposomes attenuating immune response induction and prolonging blood circulation. J. Colloid Interface Sci. 2023, 651, 273–283. [Google Scholar] [CrossRef]
- Xia, J.; Chen, C.; Dong, M.; Zhu, Y.; Wang, A.; Li, S.; Zhang, R.; Feng, C.; Jiang, X.; Xu, X.; et al. Ginsenoside Rg3 endows liposomes with prolonged blood circulation and reduced accelerated blood clearance. J. Control. Release 2023, 364, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Toyofuku, W.M.; Chen, A.M.; Scott, M.D. Induction of immunotolerance via mPEG grafting to allogeneic leukocytes. Biomaterials 2011, 32, 9494–9503. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, S.; Zheng, Z.; Zhu, L.; Zhan, X. Modification with polysialic acid-PEG copolymer as a new method for improving the therapeutic efficacy of proteins. Prep. Biochem. Biotechnol. 2016, 46, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Qelliny, M.R.; Shimizu, T.; Elsadek, N.E.; Emam, S.E.; Takata, H.; Fathalla, Z.M.A.; Hussein, A.K.; Khaled, K.A.; Ando, H.; Ishima, Y.; et al. Incorporating Gangliosides into PEGylated Cationic Liposomes that Complexed DNA Attenuates Anti-PEG Antibody Production but Not Anti-DNA Antibody Production in Mice. Mol. Pharm. 2021, 18, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- van den Hoven, J.M.; Nemes, R.; Metselaar, J.M.; Nuijen, B.; Beijnen, J.H.; Storm, G.; Szebeni, J. Complement activation by PEGylated liposomes containing prednisolone. Eur. J. Pharm. Sci. 2013, 49, 265–271. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- La Gualana, F.; Maiorca, F.; Marrapodi, R.; Villani, F.; Miglionico, M.; Santini, S.A.; Pulcinelli, F.; Gragnani, L.; Piconese, S.; Fiorilli, M.; et al. Opposite Effects of mRNA-Based and Adenovirus-Vectored SARS-CoV-2 Vaccines on Regulatory T Cells: A Pilot Study. Biomedicines 2023, 11, 511. [Google Scholar] [CrossRef]
- Li, P.Y.; Bearoff, F.; Zhu, P.; Fan, Z.; Zhu, Y.; Fan, M.; Cort, L.; Kambayashi, T.; Blankenhorn, E.P.; Cheng, H. PEGylation enables subcutaneously administered nanoparticles to induce antigen-specific immune tolerance. J. Control. Release 2021, 331, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Liao, Y.P.; Luo, L.; Xia, T.; Nel, A.E. Use of a Liver-Targeting Immune-Tolerogenic mRNA Lipid Nanoparticle Platform to Treat Peanut-Induced Anaphylaxis by Single- and Multiple-Epitope Nucleotide Sequence Delivery. ACS Nano 2023, 17, 4942–4957. [Google Scholar] [CrossRef] [PubMed]
- Pfeil, J.; Simonetti, M.; Lauer, U.; Volkmer, R.; von Thülen, B.; Durek, P.; Krähmer, R.; Leenders, F.; Hamann, A.; Hoffmann, U. Tolerogenic Immunomodulation by PEGylated Antigenic Peptides. Front. Immunol. 2020, 11, 529035. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef]
- Pardi, N.; Krammer, F. mRNA vaccines for infectious diseases—Advances, challenges and opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef] [PubMed]
- Al Rahbani, G.K.; Woopen, C.; Dunsche, M.; Proschmann, U.; Ziemssen, T.; Akgün, K. SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma. Vaccines 2024, 12, 684. [Google Scholar] [CrossRef]
- Sittplangkoon, C.; Alameh, M.G.; Weissman, D.; Lin, P.J.C.; Tam, Y.K.; Prompetchara, E.; Palaga, T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 2022, 13, 983000. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xia, T. RNA lipid nanoparticles induce immune tolerance to treat human diseases. Med. Rev. 2023, 3, 180–183. [Google Scholar] [CrossRef]
- Wang, Z.; Jacobus, E.J.; Stirling, D.C.; Krumm, S.; Flight, K.E.; Cunliffe, R.F.; Mottl, J.; Singh, C.; Mosscrop, L.G.; Santiago, L.A.; et al. Reducing cell intrinsic immunity to mRNA vaccine alters adaptive immune responses in mice. Mol. Ther. Nucleic Acids 2023, 34, 102045. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Loomis, K.H.; Lindsay, K.E.; Zurla, C.; Bhosle, S.M.; Vanover, D.A.; Blanchard, E.L.; Kirschman, J.L.; Bellamkonda, R.V.; Santangelo, P.J. In Vitro Transcribed mRNA Vaccines with Programmable Stimulation of Innate Immunity. Bioconjug. Chem. 2018, 29, 3072–3083. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, Z.; Guo, J.; Luo, D.; Li, T.; Cao, Q.; Ren, X.; Fang, H.; Xu, D.; Cao, Y. Natural Wood-Derived Macroporous Cellulose for Highly Efficient and Ultrafast Elimination of Double-Stranded RNA from In Vitro-Transcribed mRNA. Adv. Mater. 2024, 36, e2303321. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Jeon, J.J.; Kim, Y.H.; Choe, S.J.; Lee, S. Long-Term risk of autoimmune diseases after mRNA-based SARS-CoV-2 vaccination in a Korean, nationwide, population-based cohort study. Nat. Commun. 2024, 15, 6181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Tong, X.; Chan, V.K.Y.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Wan, E.Y.F.; Chan, E.W.Y.; Lau, K.K.; et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: A descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J. Autoimmun. 2022, 130, 102830. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, X.; Zhou, J.; Li, Q.; Chu, L.; Di, G.; Xu, Z.; Chen, Q.; Wang, M.; Jiang, X.; et al. An in vitro-transcribed circular RNA targets the mitochondrial inner membrane cardiolipin to ablate EIF4G2+/PTBP1+ pan-adenocarcinoma. Nat. Cancer 2024, 5, 30–46. [Google Scholar] [CrossRef] [PubMed]
- McCallen, J.; Prybylski, J.; Yang, Q.; Lai, S.K. Cross-Reactivity of Select PEG-Binding Antibodies to Other Polymers Containing a C-C-O Backbone. ACS Biomater. Sci. Eng. 2017, 3, 1605–1615. [Google Scholar] [CrossRef]
- Li, M.; Huang, Y.; Wu, J.; Li, S.; Mei, M.; Chen, H.; Wang, N.; Wu, W.; Zhou, B.; Tan, X.; et al. A PEG-lipid-free COVID-19 mRNA vaccine triggers robust immune responses in mice. Mater. Horiz. 2023, 10, 466–472. [Google Scholar] [CrossRef]
- He, X.; Payne, T.J.; Takanashi, A.; Fang, Y.; Kerai, S.D.; Morrow, J.P.; Al-Wassiti, H.; Pouton, C.W.; Kempe, K. Tailored Monoacyl Poly (2-oxazoline)- and Poly (2-oxazine)-Lipids as PEG-Lipid Alternatives for Stabilization and Delivery of mRNA-Lipid Nanoparticles. Biomacromolecules 2024, 25, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Hassanel, D.N.B.P.; Pilkington, E.H.; Ju, Y.; Kent, S.J.; Pouton, C.W.; Truong, N.P. Replacing poly (ethylene glycol) with RAFT lipopolymers in mRNA lipid nanoparticle systems for effective gene delivery. Int. J. Pharm. 2024, 665, 124695. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Jeoung, J.; Kim, W.; Jeoung, D. Regulating Immune Responses Induced by PEGylated Messenger RNA–Lipid Nanoparticle Vaccine. Vaccines 2025, 13, 14. https://doi.org/10.3390/vaccines13010014
Jo H, Jeoung J, Kim W, Jeoung D. Regulating Immune Responses Induced by PEGylated Messenger RNA–Lipid Nanoparticle Vaccine. Vaccines. 2025; 13(1):14. https://doi.org/10.3390/vaccines13010014
Chicago/Turabian StyleJo, Hyein, Jaewhoon Jeoung, Wonho Kim, and Dooil Jeoung. 2025. "Regulating Immune Responses Induced by PEGylated Messenger RNA–Lipid Nanoparticle Vaccine" Vaccines 13, no. 1: 14. https://doi.org/10.3390/vaccines13010014
APA StyleJo, H., Jeoung, J., Kim, W., & Jeoung, D. (2025). Regulating Immune Responses Induced by PEGylated Messenger RNA–Lipid Nanoparticle Vaccine. Vaccines, 13(1), 14. https://doi.org/10.3390/vaccines13010014







