Pichia pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid DNA
2.2. Yeast Cultivation
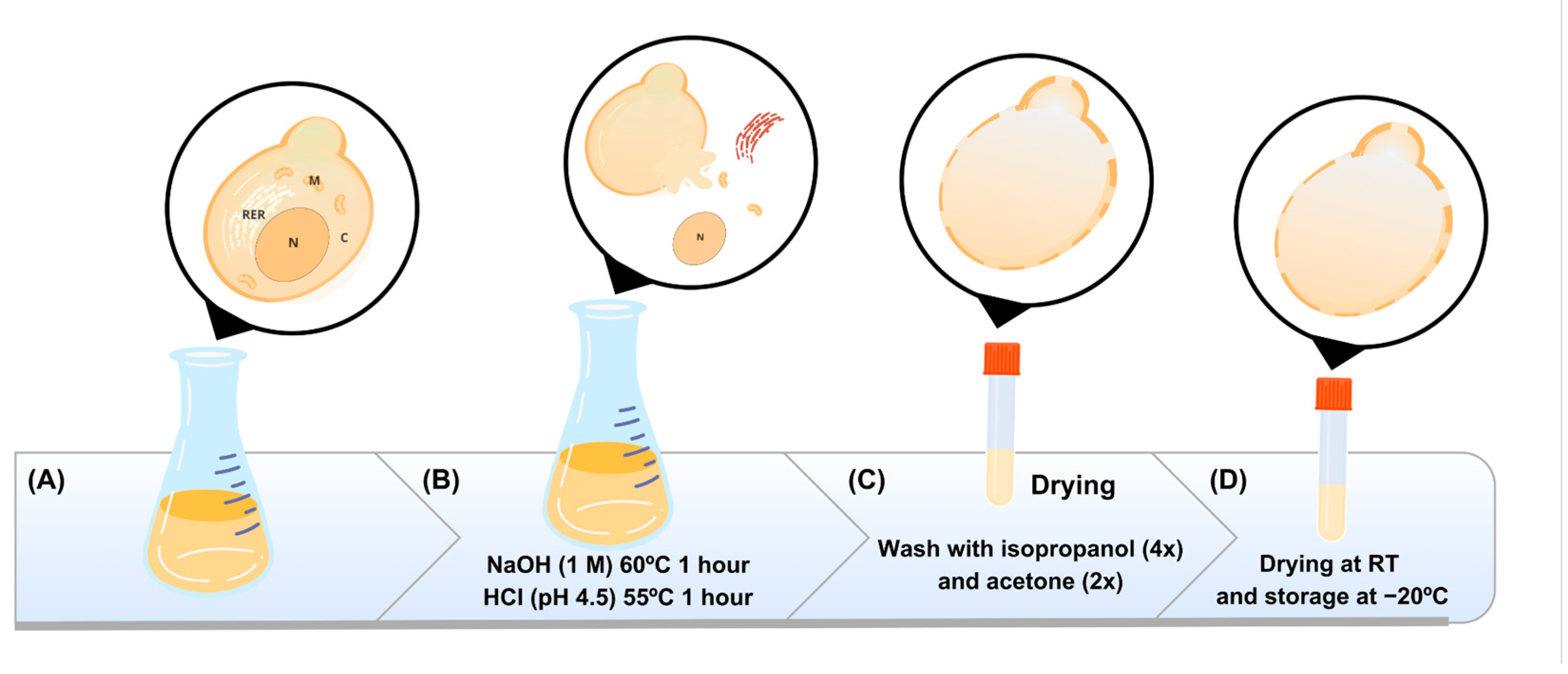
2.3. Obtaining the Capsules
2.4. Internalization of Plasmids into Capsules
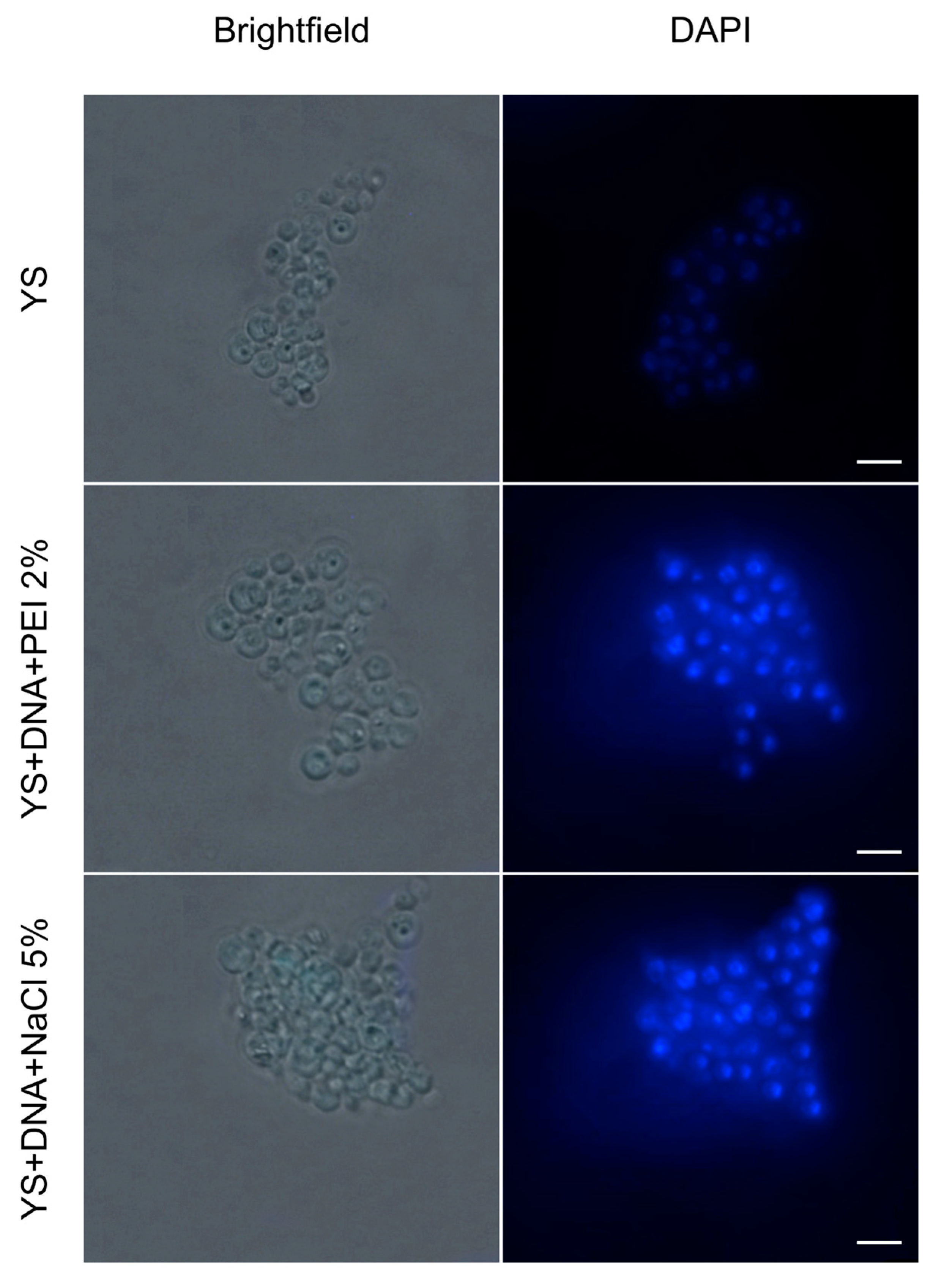
2.5. Fluorescence Microscopy
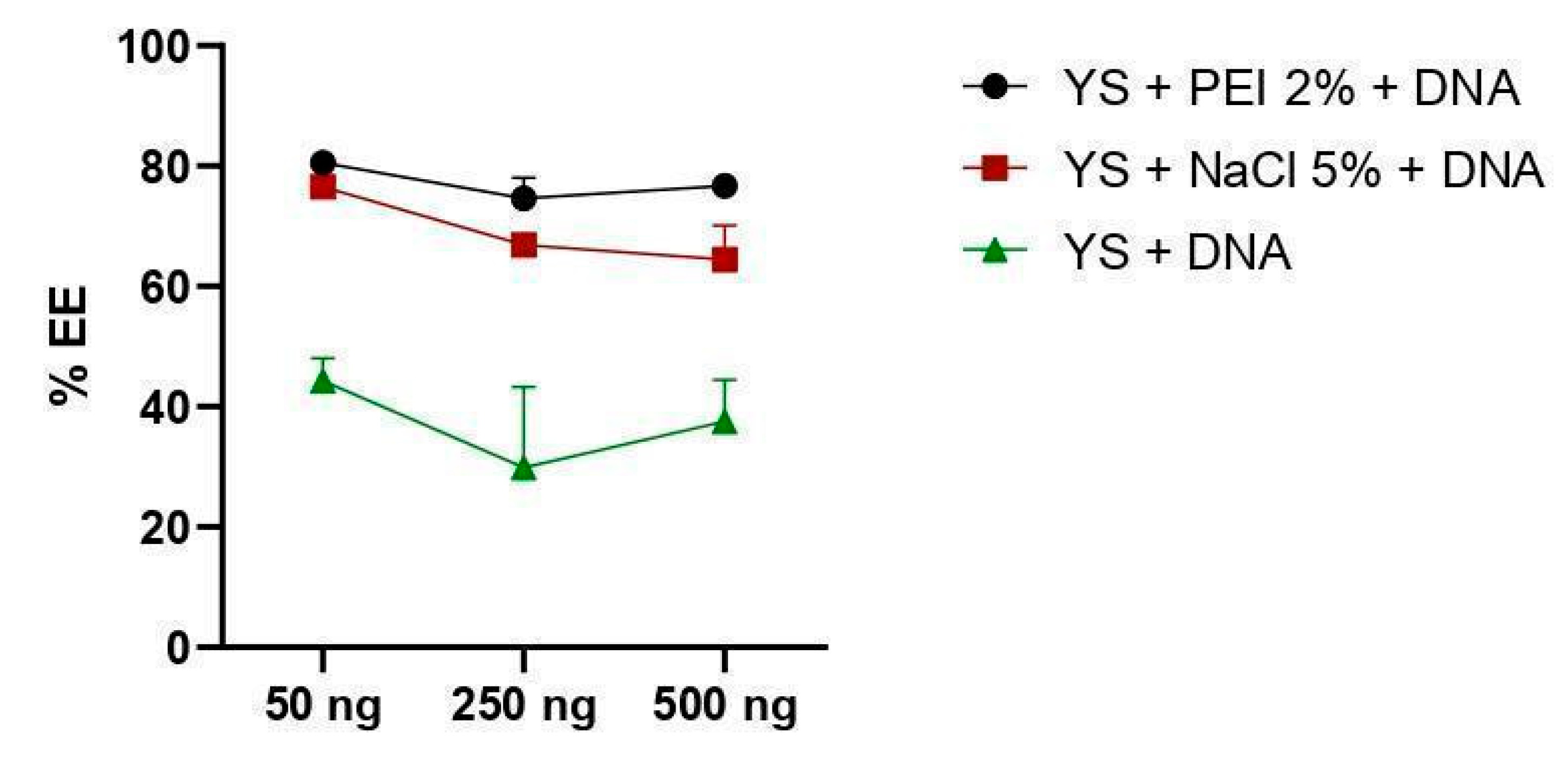
2.6. Encapsulation Efficiency (EE%)
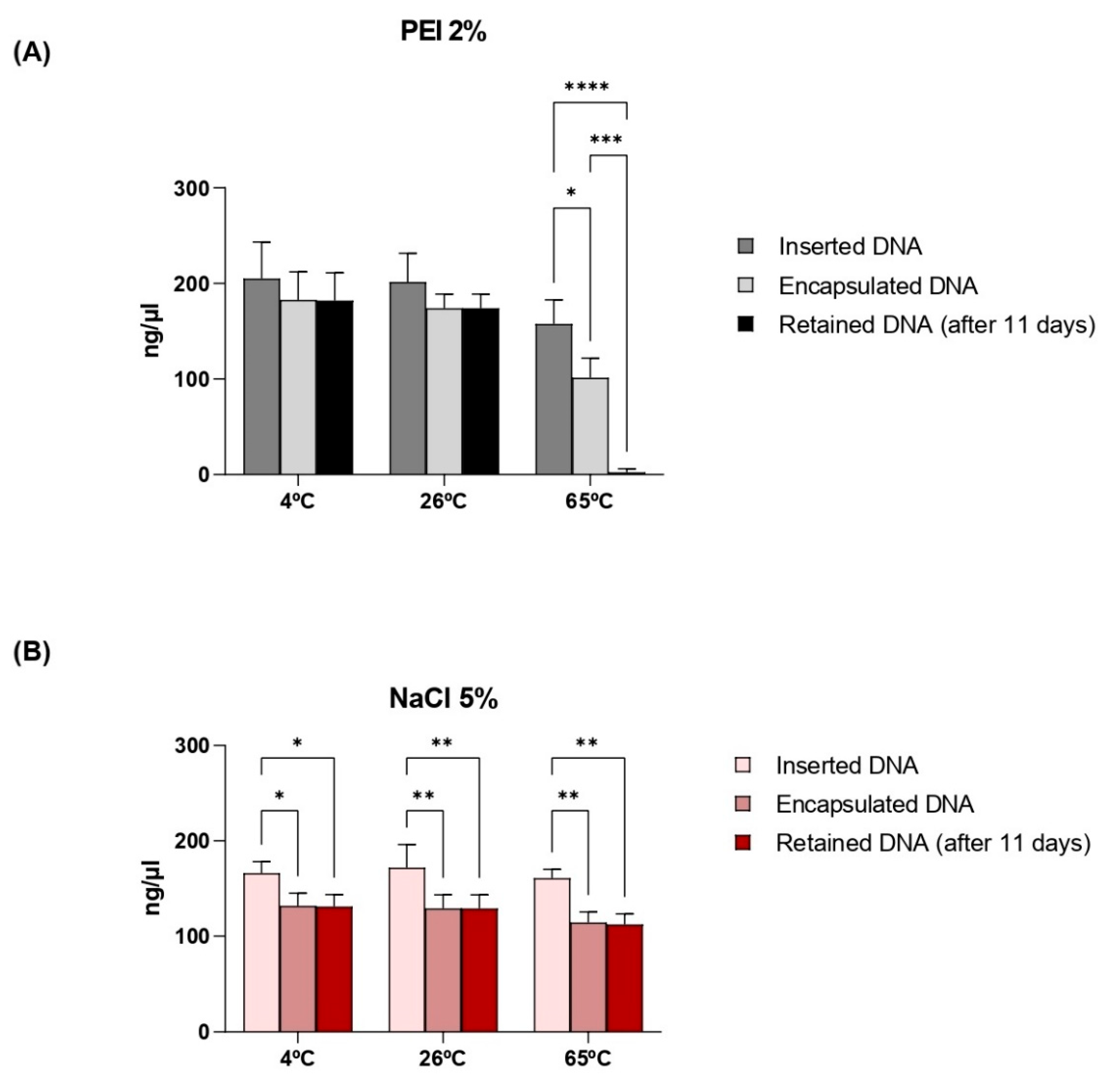
2.7. Thermal Stability Test
2.8. Phagocytosis Analysis
2.9. Cytotoxicity Assessment
2.10. Zeta Potential (ζ) Measurements
2.11. DNAse Assay
2.12. Statistical Analysis
3. Results
3.1. Obtaining β-Glucan Capsules
3.2. DNA Encapsulation Capacity by Yeast Capsules
3.3. Internalization of Plasmid DNA
3.4. Thermostability of Capsules and Retention of Plasmid DNA
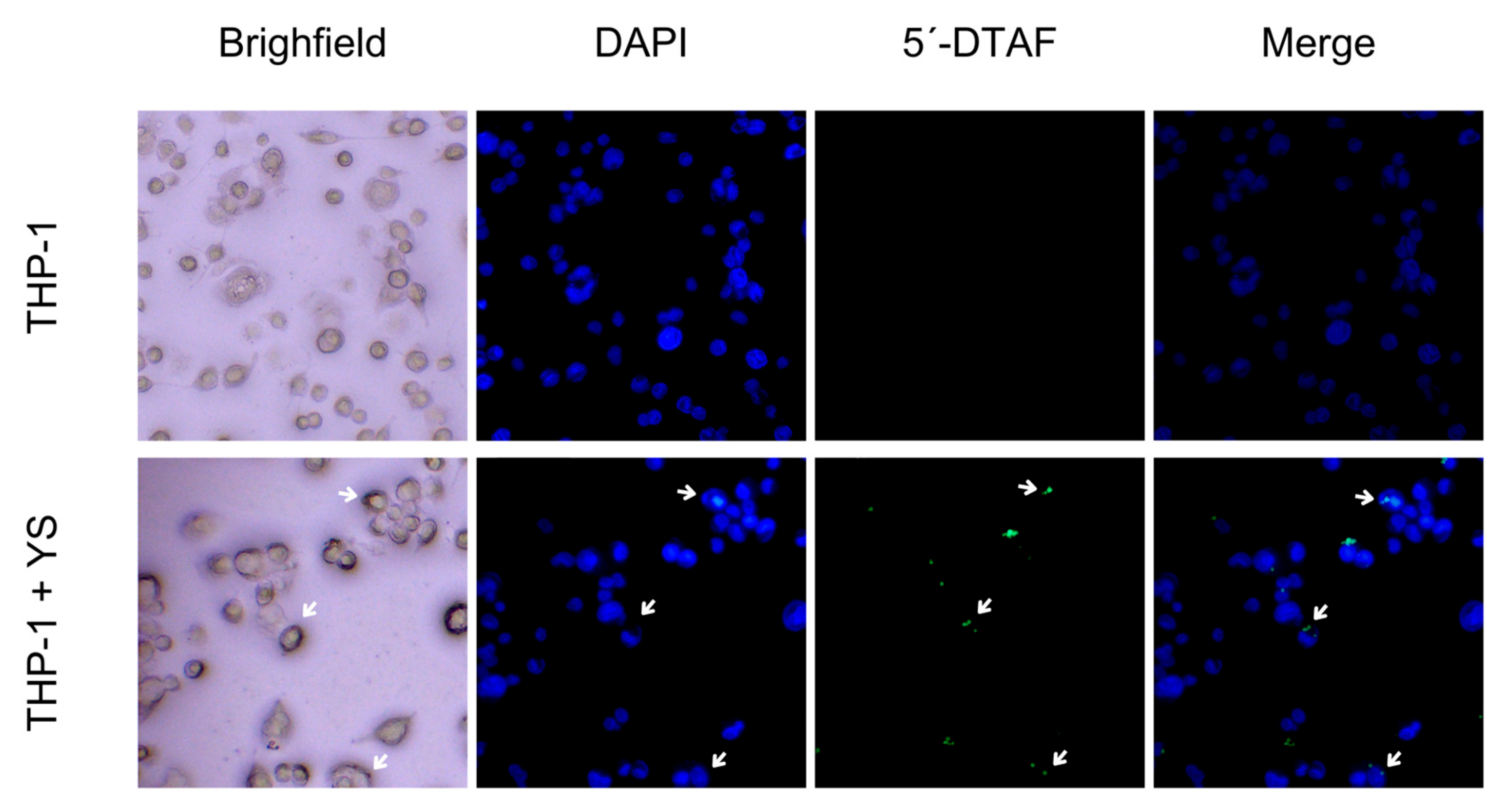
3.5. Phagocytosis of Capsules by Macrophages
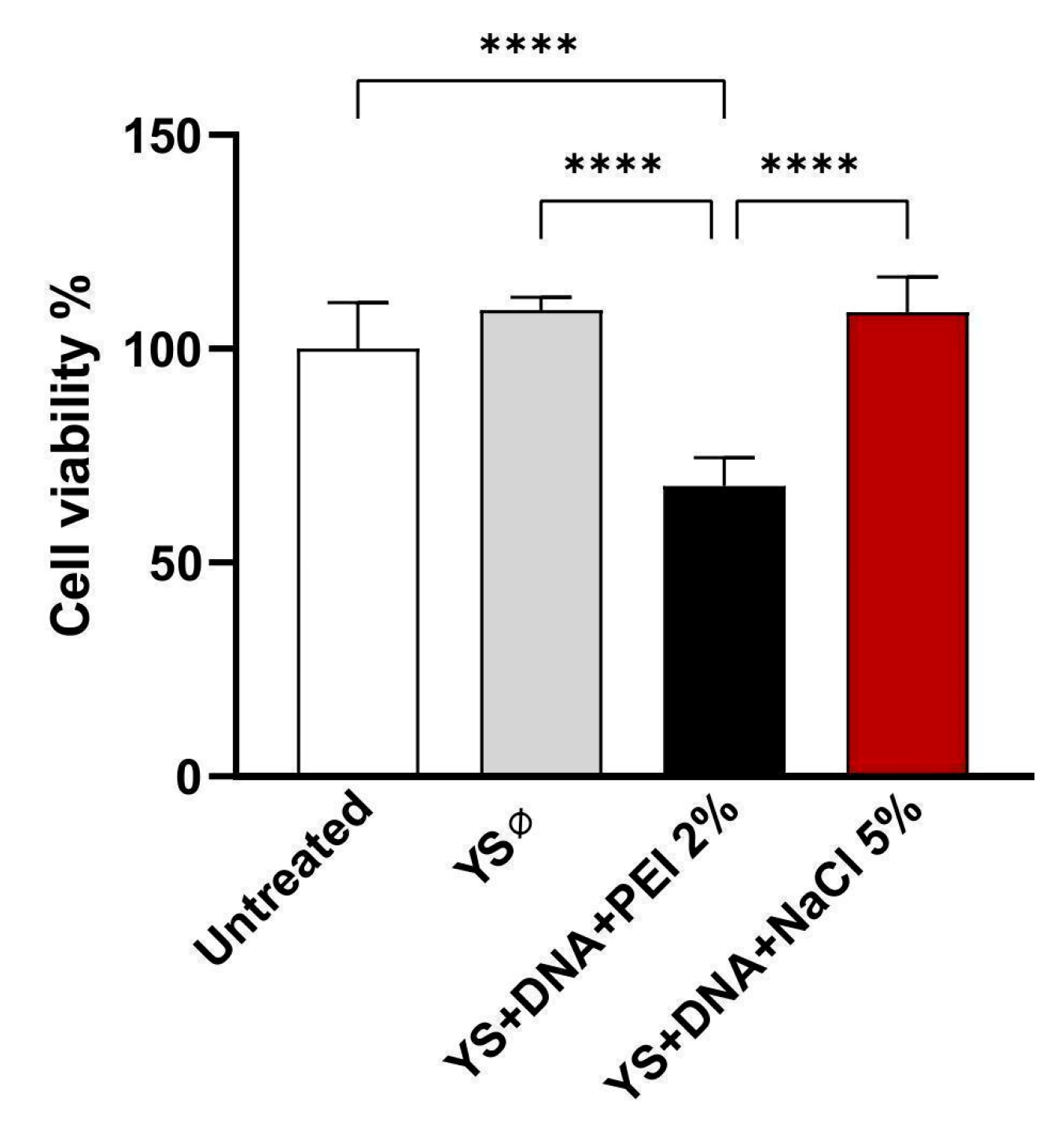
3.6. Induction of Cytotoxicity
3.7. Zeta Potential (ζ) Analyses
3.8. Evaluation of Enzymatic Protection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. Publisher Correction: A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel Approaches for Vaccine Development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, F. A Comprehensive Comparison of DNA and RNA Vaccines. Adv. Drug Deliv. Rev. 2024, 210, 115340. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Jin, S.; Xu, J.; Wang, P.C.; Liang, X.-J.; Zhang, X. Engineered Biomaterials for Development of Nucleic Acid Vaccines. Biomater. Res. 2015, 19, 5. [Google Scholar] [CrossRef]
- Shah, M.A.A.; Ali, Z.; Ahmad, R.; Qadri, I.; Fatima, K.; He, N. DNA Mediated Vaccines Delivery Through Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 41–53. [Google Scholar] [CrossRef]
- Kisakova, L.A.; Apartsin, E.K.; Nizolenko, L.F.; Karpenko, L.I. Dendrimer-Mediated Delivery of DNA and RNA Vaccines. Pharmaceutics 2023, 15, 1106. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of mRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, B.; Ruiz, E.F.; Zhang, F. Advances in the Polymeric Delivery of Nucleic Acid Vaccines. Theranostics 2022, 12, 4081–4109. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a Promising Delivery Platform for DNA and RNA Vaccines. FEMS Yeast Res. 2021, 21, foab018. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, L.; Li, K.; Lou, B.; Liu, Y.; Liu, Z. Yeast as Carrier for Drug Delivery and Vaccine Construction. J. Control. Release 2022, 346, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, G. Drug Delivery Product and Methods. U.S. Patent 7,740,861, 22 June 2010. [Google Scholar]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA Delivery. Bioconjugate Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, Preparation, Modification, and Bioactivities of β-Glucan and Mannan from Yeast Cell Wall: A Review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. Β-glucan as a New Tool in Vaccine Development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef] [PubMed]
- Silva De Macêdo, L.; Sousa De Pinho, S.; Duarte Silva, A.J.; De Moura, I.A.; Flores Espinoza, B.C.; Viana Invenção, M.D.C.; Silva Novis, P.V.; Turiah Machado Da Gama, M.A.; Do Nascimento Carvalho, M.; Rosa Sales Leal, L.; et al. Understanding Yeast Shells: Structure, Properties and Applications. ADMET DMPK 2024, 12, 299–317. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J. Yeast Capsules for Targeted Delivery: The Future of Nanotherapy? Nanomedicine 2017, 12, 955–957. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, K.; Liu, M.; Zhang, X.; Ding, J.; Dong, Y.; Liang, Z.; Li, J.; Zhang, J. Targeted Delivery of Cisplatin-Derived Nanoprecursors via a Biomimetic Yeast Microcapsule for Tumor Therapy by the Oral Route. Theranostics 2019, 9, 6568–6586. [Google Scholar] [CrossRef]
- Austriaco, N. Yeast Oral Vaccines against Infectious Diseases. Front. Microbiol. 2023, 14, 1150412. [Google Scholar] [CrossRef]
- Alexander, E. Yeasts in Nanotechnology-Enabled Oral Vaccine and Gene Delivery. Bioengineered 2021, 12, 8325–8335. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Allais, L.; Cuvelier, C.A. Recent Advances in Oral Vaccine Development: Yeast-Derived β-Glucan Particles. Hum. Vaccines Immunother. 2014, 10, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shang, S.; Qi, M.; Xiang, Y.; Wang, L.; Wang, X.; Lin, T.; Hao, D.; Chen, J.; Liu, J.; et al. Yeast Glucan Particles: An Express Train for Oral Targeted Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 253, 127131. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-Derived β-Glucan in Cancer: Novel Uses of a Traditional Therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.-M.; Daneshmandi, S.; Cassel, T.A.; Uddin, M.B.; Sledziona, J.; Thompson, P.T.; Lin, P.; Higashi, R.M.; Lane, A.N. Polarization and β-Glucan Reprogram Immunomodulatory Metabolism in Human Macrophages and Ex Vivo in Human Lung Cancer Tissues. J. Immunol. 2022, 209, 1674–1690. [Google Scholar] [CrossRef]
- Bazan, S.B.; Geginat, G.; Breinig, T.; Schmitt, M.J.; Breinig, F. Uptake of Various Yeast Genera by Antigen-Presenting Cells and Influence of Subcellular Antigen Localization on the Activation of Ovalbumin-Specific CD8 T Lymphocytes. Vaccine 2011, 29, 8165–8173. [Google Scholar] [CrossRef]
- Brown, G.D. Innate Antifungal Immunity: The Key Role of Phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, Z.; Xia, X.; Zhang, Y.; Chen, Y.; Wang, B.; Li, J.; Li, G.; Yang, G.; Cao, G.; et al. Yeast Shells Encapsulating Adjuvant AS04 as an Antigen Delivery System for a Novel Vaccine against Toxoplasma gondii. ACS Appl. Mater. Interfaces 2021, 13, 40415–40428. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of Methods Used for Assessing the Viability and Vitality of Yeast Cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses Following Vaccination with Antigen-Loaded β-Glucan Particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Microencapsulation of Curcumin in Cells of Saccharomyces Cerevisiae. Food Chem. 2011, 125, 892–902. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Pen, G.; Long, S.; Yang, C. Yeast-Cell-Based Microencapsulation of Chlorogenic Acid as a Water-Soluble Antioxidant. J. Food Eng. 2007, 80, 1060–1067. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally Delivered siRNA Targeting Macrophage Map4k4 Suppresses Systemic Inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bazan, S.B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Heat Treatment Improves Antigen-Specific T Cell Activation after Protein Delivery by Several but Not All Yeast Genera. Vaccine 2014, 32, 2591–2598. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, S.; Xu, K.; Tang, Q.; Li, W.; Zheng, W.; Shi, H.; Su, K.; Liu, Y.; Hong, Z. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Adv. Sci. 2022, 9, 2201496. [Google Scholar] [CrossRef]
- Shao, Q.; Fan, Y.; Yang, L.; Qin Gao, Y. From Protein Denaturant to Protectant: Comparative Molecular Dynamics Study of Alcohol/Protein Interactions. J. Chem. Phys. 2012, 136, 115101. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Muszyński, A.; Widanage, M.C.D.; Chen, A.; Azadi, P.; Wang, P.; Mentink-Vigier, F.; Wang, T. Molecular Architecture of Fungal Cell Walls Revealed by Solid-State NMR. Nat. Commun. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Yang, E.H.; Suh, H.; Kim, S.H.; Chung, D.S.; Choi, K.; Yang, C.W.; Park, J. Investigation of the Factors Influencing the Release Rates of Cyclosporin A-Loaded Micro- and Nanoparticles Prepared by High-Pressure Homogenizer. J. Control. Release 2002, 84, 115–123. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of Poly(Lactic-Co-Glycolic Acid) Microspheres: Effect of Copolymer Composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Clancy, C.; Erasmus, J.; Feldmann, H.; Hawman, D.W. An Intramuscular DNA Vaccine for SARS-CoV-2 Decreases Viral Lung Load but Not Lung Pathology in Syrian Hamsters. Microorganisms 2021, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, J.; Sun, B. Polysaccharide Dual Coating of Yeast Capsules for Stabilization of Anthocyanins. Food Chem. 2021, 357, 129652. [Google Scholar] [CrossRef] [PubMed]
- Gavryushov, S. Electrostatics of B-DNA in NaCl and CaCl 2 Solutions: Ion Size, Interionic Correlation, and Solvent Dielectric Saturation Effects. J. Phys. Chem. B 2008, 112, 8955–8965. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Song, L.; Guan, J.; Sun, C.; Zhou, D.; Zhu, B. Encapsulation of Antarctic Krill Oil in Yeast Cell Microcarriers: Evaluation of Oxidative Stability and in Vitro Release. Food Chem. 2021, 338, 128089. [Google Scholar] [CrossRef]
- Hong, Z.; Yu, M.; Chen, Z.; Guo, W.; Wang, J.; Feng, Y.; Kong, X. Specifically Targeted Delivery of Protein To Phagocytic Macrophages. Int. J. Nanomed. 2015, 10, 1743–1757. [Google Scholar] [CrossRef][Green Version]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and Development of Polymers for Gene Delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Silveira, N.; Longuinho, M.M.; Leitão, S.G.; Silva, R.S.F.; Lourenço, M.C.; Silva, P.E.A.; Pinto, M.D.C.F.R.; Abraçado, L.G.; Finotelli, P.V. Synthesis and Characterization of the Antitubercular Phenazine Lapazine and Development of PLGA and PCL Nanoparticles for Its Entrapment. Mater. Sci. Eng. C 2016, 58, 458–466. [Google Scholar] [CrossRef]
- Booysen, L.L.I.J.; Kalombo, L.; Brooks, E.; Hansen, R.; Gilliland, J.; Gruppo, V.; Lungenhofer, P.; Semete-Makokotlela, B.; Swai, H.S.; Kotze, A.F.; et al. In Vivo/in Vitro Pharmacokinetic and Pharmacodynamic Study of Spray-Dried Poly-(Dl-Lactic-Co-Glycolic) Acid Nanoparticles Encapsulating Rifampicin and Isoniazid. Int. J. Pharm. 2013, 444, 10–17. [Google Scholar] [CrossRef]
- Do, D.P.; Pai, S.B.; Rizvi, S.A.A.; D’Souza, M.J. Development of Sulforaphane-Encapsulated Microspheres for Cancer Epigenetic Therapy. Int. J. Pharm. 2010, 386, 114–121. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Golniya, Z.; Daniels, A.U. Bioactive Glass Nanoparticles with Negative Zeta Potential. Ceram. Int. 2011, 37, 2311–2316. [Google Scholar] [CrossRef]
- Pan, Y.; Li, X.; Kang, T.; Meng, H.; Chen, Z.; Yang, L.; Wu, Y.; Wei, Y.; Gou, M. Efficient Delivery of Antigen to DCs Using Yeast-Derived Microparticles. Sci. Rep. 2015, 5, 10687. [Google Scholar] [CrossRef] [PubMed]
- Bos, H.; De Souza, W. Phagocytosis of Yeast: A Method for Concurrent Quantification of Binding and Internalization Using Differential Interference Contrast Microscopy. J. Immunol. Methods 2000, 238, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.; Vaz, C.; Carvalho-Pereira, J.; Pais, C.; Sampaio, P. A New Method for Yeast Phagocytosis Analysis by Flow Cytometry. J. Microbiol. Methods 2014, 101, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liang, X.; Ren, X.; Liu, Z.; Liu, Y.; Wang, J.; Wang, J.; Zhang, L.-M.; Deng, D.Y.B.; Quan, D. An Efficient Nonviral Gene-Delivery Vector Based on Hyperbranched Cationic Glycogen Derivatives. Int. J. Nanomed. 2014, 9, 419–435. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Xu, B.; Mai, K.; Deng, Y.; Zhang, L.-M. Dendronized Chitosan Derivative as a Biocompatible Gene Delivery Carrier. Biomacromolecules 2011, 12, 642–649. [Google Scholar] [CrossRef]
- Farahpour, A.; Ramezanian, N.; Gholami, L.; Askarian, S.; Banisadr, A.; Kazemi Oskuee, R. Synthesis and Characterization of Polyethyleneimine-Terminated Poly(β-Amino Esters) Conjugated with Pullulan for Gene Delivery. Pharm. Dev. Technol. 2022, 27, 606–614. [Google Scholar] [CrossRef]
- Jin, J.W.; Rong, M.Z.; Zhang, M.Q.; Wong, W. Preparation of a Water Soluble Aminated Β-1, 3-D -glucan for Gene Carrier: The in Vitro Study of the Anti-inflammatory Activity and Transfection Efficiency. J. Biomed. Mater. Res. 2021, 109, 2506–2515. [Google Scholar] [CrossRef]
- Nguyen, J.; Reul, R.; Betz, T.; Dayyoub, E.; Schmehl, T.; Gessler, T.; Bakowsky, U.; Seeger, W.; Kissel, T. Nanocomposites of Lung Surfactant and Biodegradable Cationic Nanoparticles Improve Transfection Efficiency to Lung Cells. J. Control. Release 2009, 140, 47–54. [Google Scholar] [CrossRef]



| Sample | ζ (mV) |
|---|---|
| Empty capsule PEI 2% | 12.8 ± 0.73 |
| Capsule pVAX1 PEI 2% | 10.17 ± 0.17 |
| Empty capsule NaCl 5% | −4.23 ± 0.14 |
| Capsule pVAX1 NaCl 5% | −3.18 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, S.S.d.; Invenção, M.d.C.V.; Silva, A.J.D.; Macêdo, L.S.d.; Espinoza, B.C.F.; Leal, L.R.S.; da Gama, M.A.T.M.; Moura, I.A.d.; Silva, M.E.d.S.; Souza, D.V.S.d.; et al. Pichia pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines. Vaccines 2024, 12, 1428. https://doi.org/10.3390/vaccines12121428
Pinho SSd, Invenção MdCV, Silva AJD, Macêdo LSd, Espinoza BCF, Leal LRS, da Gama MATM, Moura IAd, Silva MEdS, Souza DVSd, et al. Pichia pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines. Vaccines. 2024; 12(12):1428. https://doi.org/10.3390/vaccines12121428
Chicago/Turabian StylePinho, Samara Sousa de, Maria da Conceição Viana Invenção, Anna Jéssica Duarte Silva, Larissa Silva de Macêdo, Benigno Cristofer Flores Espinoza, Lígia Rosa Sales Leal, Marco Antonio Turiah Machado da Gama, Ingrid Andrêssa de Moura, Micaela Evellin dos Santos Silva, Débora Vitória Santos de Souza, and et al. 2024. "Pichia pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines" Vaccines 12, no. 12: 1428. https://doi.org/10.3390/vaccines12121428
APA StylePinho, S. S. d., Invenção, M. d. C. V., Silva, A. J. D., Macêdo, L. S. d., Espinoza, B. C. F., Leal, L. R. S., da Gama, M. A. T. M., Moura, I. A. d., Silva, M. E. d. S., Souza, D. V. S. d., Lara, M. L., Alves, J. N. S. A., & Freitas, A. C. d. (2024). Pichia pastoris-Derived β-Glucan Capsules as a Delivery System for DNA Vaccines. Vaccines, 12(12), 1428. https://doi.org/10.3390/vaccines12121428










