Abstract
The aim of this study was to evaluate the impact of index case vaccination on SARS-CoV-2 transmission to household contacts. In our epidemiological cohort study (May 2022–November 2023), we surveyed registered index case vaccination status and test results for contacts (testing on day 0, and on day 7 for negative contacts) and calculated the secondary attack rate (SAR), i.e., newly infected contacts/susceptible included contacts. The association of the independent variable, index case COVID-19 vaccination (yes/no), with household contact infection was determined using the adjusted odds ratio (aOR) and its 95% confidence interval (CI). We recorded 181 index cases and 314 contacts, of whom 250 agreed to participate; 16 contacts were excluded upon testing positive on day 0. Of the 234 included contacts, 49.1% were women, and the mean (SD) age was 51.9 (19.8) years. The overall SAR of 37.2% (87/234) was lower in the contacts of both vaccinated index cases (34.9% vs. 63.2%; p = 0.014) and index cases with a previous SARS-CoV-2 infection history (27.0% vs. 46.3%; p = 0.002). Index case vaccination showed a protective effect against infection for their household contacts (aOR = 0.21; 95% CI: 0.07, 0.67). The household SAR was high when the Omicron variant circulated. Vaccinated index cases were less likely to transmit SARS-CoV-2 to their contacts.
1. Introduction
An ongoing global priority is reducing SARS-CoV-2 infection transmission and preventing severe COVID-19 [1]. Some studies suggest that most new SARS-CoV-2 infections develop in the home [2,3]. While observational epidemiological studies and systematic reviews have been conducted on the household secondary attack rate (SAR) [2,4], the results are very heterogeneous, and our understanding of household SARS-CoV-2 transmission remains incomplete [5].
Vaccination effectiveness (VE), defined as the attack rate in vaccinated individuals compared to the attack rate in unvaccinated individuals, is expressed as a percentage re-duction in infections in vaccinated individuals. While a public health priority is understanding VE as key to reducing household transmission, calculating VE presents several problems [1]. SARS-CoV-2 transmission requires three inputs: (i) an infected index case capable of transmitting the virus, (ii) a contact of the index case susceptible to infection (a possible secondary case), and (iii) a transmission event between the index case and the secondary case. Empirically separating VE estimates for index and secondary cases is complex because a previous infection may affect both the infectivity of an index case and the susceptibility of a contact to infection [6]. One approach to obtaining the corresponding VE estimates involves collecting information on infected index cases linked to their exposed contacts and studying the vaccination status of both cases and contacts along with other relevant transmission factors [7].
In terms of preventing hospital admissions and COVID-19 deaths, VE has been widely confirmed to be high [8,9]. However, in terms of preventing clinical and subclinical SARS-CoV-2 infection, VE has been notably low and, furthermore, is progressively decreasing [10,11], indicating that vaccinated individuals can acquire and transmit SARS-CoV-2.
Regarding SARS-CoV-2 transmission in households, while immune protection can be induced by previous infection or vaccination [6,10,11], infection is favored by factors such as exposure intensity (e.g., a shared bedroom), nonuse of non-pharmacological measures (e.g., face masks), and smoking [1,7,12,13].
Several studies suggest that SARS-CoV-2 transmission increases with the emergence of new variants [2,4]. This increase in transmission was especially evident with the emergence of the Omicron variant, with the possibility of new infections confirmed for vaccinated people and even in people with a history of previous infection [2,4]. The role of vaccines in reducing infection is especially interesting in households because of the multiple opportunities for transmission and high SAR [12].
There is evidence that despite not preventing infection in some people, influenza and COVID-19 vaccines can reduce severity and death [9,11]. It is also important to study the role of vaccinated people as a source of infection, taking into account the vaccination status of their contacts at home [5], for two reasons: first, to obtain updated estimates of COVID-19 transmission in households in a uniform period of circulation of a particular variant (e.g., Omicron) and its subvariants; second, to understand the transmission capacity of vaccinated people in a household given the high numbers of vaccinated people and the recommendation to stay at home once infected.
The hypothesis in our study was that vaccines, while they may not prevent infection of index cases at home, may have an effect in reducing infection transmission to household contacts. Given this hypothesis, the vaccination of index cases would play a relevant role in reducing household transmission and would support the recommendation to vaccinate contacts, especially if they belong to risk groups.
The aims of this study were, during a period when the SARS-CoV-2 Omicron variant was circulating, to estimate (1) household contact SAR; (2) the reduction in index case infectivity; (3) the reduction in household contact susceptibility to infection given VE in both index cases and contacts.
2. Materials and Methods
2.1. Study Design
We carried out an epidemiological cohort study of SARS-CoV-2 transmission of the household contacts of index cases in Catalonia and Navarre (Spain), between May 2022 and November 2023 (when the Omicron variant was circulating). In the 8 participating primary care centers, SARS-CoV-2 cases were identified and selected at the beginning of each week using rapid antigen testing (RAT) and/or real-time polymerase chain reaction (RT-PCR) testing.
2.2. Participants
Household contacts associated with COVID-19 cases were recruited in 8 primary healthcare centers (1 in Navarre and 7 in Catalonia). Primary care centers were selected in each epidemiological surveillance unit, according to convenience criteria, by public health officials attached to the corresponding epidemiological unit.
Patient inclusion criteria were as follows: cases positive for SARS-CoV-2 and household contacts who agreed to participate in the study and provided their oral consent (index cases and contacts, respectively). Excluded were individuals with severe and uncorrectable cognitive and visual disorders, and individuals with hearing disabilities that hindered their ability to complete interviews.
Index cases were defined as confirmed cases of SARS-CoV-2 in the previous 10 days in participating centers who had at least one household contact who agreed to participate. Household contacts were defined as contacts with the index case for at least 2 h in the period running from 2 days before index case diagnosis to confirmation.
2.3. Questionnaire Design
As the first step in designing the epidemiological questionnaires, a comprehensive literature review was conducted by the coordination committee [4]. The questionnaires were structured taking into account COVID-19 recommendations of the World Health Organization, European Centres for Disease Prevention and Control, and the Spanish Ministry of Health. The research team, composed of professionals with epidemiological and public health research experience, held a series of preliminary meetings to develop the questionnaires, including the different sections, questions, and number of included elements. Discussions focused on question relevance, consistency, completeness, and clarity, and questionnaire length. The final questionnaire versions were obtained after an iterative process of several revisions of the earlier drafts.
The final questionnaires contained the following sections: social and demographic data, comorbidities and risk factors, epidemiological information, and knowledge of COVID-19 and preventive measures. The questionnaires also included data on previous SARS-CoV-2 infection and COVID-19 vaccination status; these data were validated through electronic health record linkage with regional vaccination registers and epidemiological surveillance unit databases.
2.4. Data Collection
The questionnaires were administered to the index cases and their household contacts. To detect cases of secondary infection, contacts were followed up for 7 days from confirmation of the index case infection. All contacts took a RAT on day 0, and those who tested negative underwent RT-PCR testing at the end of follow-up (day 7), regardless of whether or not they were symptomatic.
Data were collected for cases and contacts as follows: demographic variables (age and sex); date of onset of first symptoms; specific symptoms; diagnostic tests (RAT, RT-PCR); exposure time to the index case; relationship with the index case (cohabitation with a partner, other); shared bedroom with the index case; vaccination history and dates; history of SARS-CoV-2 infection and dates; risk factors (comorbidities and smoking); and control actions following index case diagnosis (face mask use, hand washing, hydroalcoholic solution use, distancing, ventilation, and isolation).
Study variable data were collected in an initial face-to-face interview and a subsequent telephone interview. Vaccination history and COVID-19 data were verified from the medical records. Participants (both index cases and household contacts) who had been vaccinated in the previous 21 days and 7 days were considered vaccinated with a first dose and second dose, respectively. Due to the small number of single-dose index cases and contacts, VE was studied on the basis of participants having received at least 1 dose.
2.5. Sampling and Sample Size
In each of the participating primary care centers, the first confirmed cases that met the inclusion criteria were initially selected every 15 days. Subsequently, due to a reduced incidence of new cases, this criterion was expanded to the selection of cases every week with no limitation on number. The sample was composed of 234 household contacts. This sample size, which allowed us to estimate the SAR of household contacts with a precision (e) of ±6% for a 95% confidence interval (CI), was calculated according to the following formulas: n = Zα2 × p × (1 − p)/e2 and e = √ Zα2 × p × (1 − p)/n.
2.6. Statistical Analysis
The SAR, expressed as a percentage, was calculated as the number of infected contacts 7 days after symptom onset in the index case (numerator) divided by the number of included contacts (denominator). Index cases and infected contacts on day 0 were excluded from both the numerator and denominator. The dependent variable was SARS-CoV-2 infection in contacts (yes/no), the independent variable was household contact exposure to a vaccinated index case (yes/no), and the contact covariables were as follows: a previous history of SARS-CoV-2 infection (yes/no); vaccination (yes/no); smoking (yes/no); cohabitation with a partner (yes/no); shared bedroom with the index case (yes/no); and face mask use at home following index case diagnosis (yes/no).
Using a logistic regression model, the adjusted odds ratio (aOR) and the corresponding 95% CI were calculated to determine the association between contact exposure to the vaccinated index case (yes/no) and contact infection (yes/no). The variables studied in the multivariate logistic regression model were selected using the backward method, for a cut-off point of p < 0.2. The variables for household contacts and their interaction evaluated in the model were: exposure to the vaccinated index case, age group (years), sex, previous COVID-19, contact vaccination ≥ 1 dose, smoker, cohabitation with a partner, shared bedroom, face mask use, and number of household contacts.
Index case VE (in reducing transmission) and household contact VE (in reducing susceptibility) were both calculated as VE = (1 − aOR) × 100 with the corresponding 95% CI.
To detect a possible confounding effect of previous SARS-CoV-2 infection on the vaccination of index cases and contacts, we repeated the statistical analysis using a secondary variable with 4 categories: (0) nonvaccinated and no previous infection; (1) vaccinated and no previous infection; (2) nonvaccinated and previous infection; and (3) vaccinated and previous infection (Supplementary Tables S1 and S2). The aOR for each secondary variable category was calculated using the backward method and selecting the same variables as above (i.e., exposure to the vaccinated index case, age group (years), sex, previous COVID-19, contact vaccination ≥ 1 dose, smoker, and number of household contacts) for a cut-off point of p < 0.2 (Supplementary Tables S1 and S2).
Analyses were performed using EpiInfo 7.2.5 (Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA) and the SPSS v.24 statistical package (IBM, Armonk, New York, NY, USA).
2.7. Ethical Considerations
This study was approved by the Ethics Committee of the Arnau Vilanova University Hospital (code: CEIC-2464) and was conducted according to Declaration of Helsinki principles. All subjects included in the study received detailed information on the study aims and granted their consent to participate.
3. Results
Household contacts were studied for 181 index cases, with a mean (SD) age of 54.8 (19.1) years, 66.8% (121/181) of whom were women, and 97.8% (177/181) of whom had symptoms. In vaccination terms, 91.7% (166/181) had received at least one dose and 87.3% (158/181) at least two doses.
For the 181 index cases, 314 contacts were registered, of whom 250 agreed to participate. The exclusion of 16 contacts who tested positive to a RAT on day 0 left 234 contacts (Figure 1), with a mean (SD) age of 51.9 (19.8) years, 49.1% (115/234) of whom were women (Table 1). A high percentage—91.9% (215/234)—were contacts of vaccinated index cases, and, likewise, a high percentage had been vaccinated: 93.2% (218/234) with at least one dose and 87.2% (204/234) with at least two doses. Almost half—47.4% (111/234)—had a previous history of SARS-CoV-2 infection, 44.9% (105/234) were cohabiting with a partner, 38.5% (90/234) shared a bedroom with the index case, and 29.9% (70/234) were smokers. The overall SAR was 37.2% (87/234).
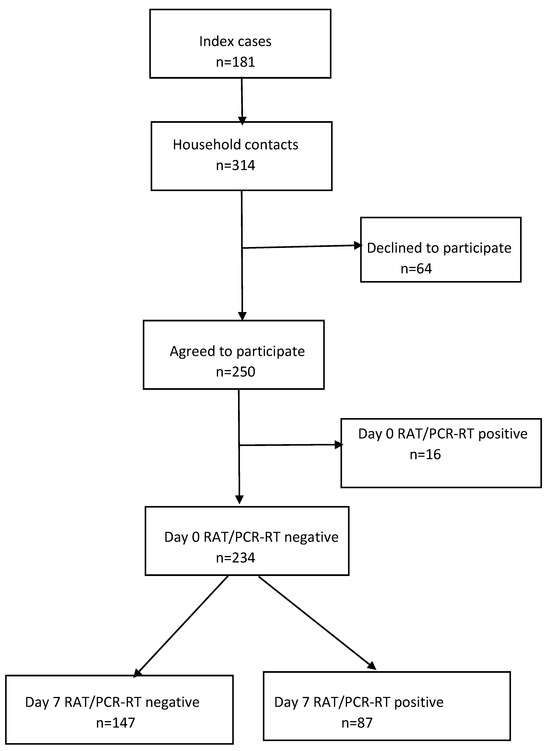
Figure 1.
Study flow diagram. RAT, rapid antigen test; RT-PCR, real-time polymerase chain reaction.

Table 1.
SARS-CoV-2 secondary attack rate (SAR) in household contacts.
Regarding factors associated with SARS-CoV-2 transmission to household contacts (Table 2), no statistically significant differences were observed in the SAR for men compared to women (37.0% vs. 37.4%; p = 0.947). The SAR was higher in participants as follows: aged ≥65 years compared to aged ≤17 years (65.3% vs. 18.0%; p < 0.001); with no previous history of SARS-CoV-2 infection (46.3% vs. 27.0%; p = 0.002); smokers (51.4% vs. 31.1%; p = 0.003); cohabiting with a partner (49.5% vs. 27.1%; p = 0.001); and sharing a bedroom with the index case (44.4% vs. 32.6%; p = 0.069). No statistically significant differences were observed in the SAR for vaccinated and unvaccinated contacts (37.6% vs. 31.2%; p = 0.611).

Table 2.
Factors associated with SARS-CoV-2 transmission to household contacts.
In the multivariate logistic regression model (Table 3), the infection risk was lower in household contacts exposed to vaccinated index cases (aOR = 0.21; 95% CI: 0.07, 0.67) and with a previous SARS-CoV-2 infection history (aOR = 0.43; 95% CI: 0.23, 0.81) and was higher in contacts aged ≥65 years (aOR = 3.34; 95% CI: 1.00, 11.18) and in contacts cohabiting with a partner (aOR = 2.34; 95% CI: 1.08, 5.09). No protective role was found for household contact vaccination (aOR = 0.95; 95% CI: 0.23, 3.80). As for the possible confounding effect of previous SARS-CoV-2 infection on the vaccination of index cases and contacts, similar results, with only small differences, were obtained for the statistical analysis repeated using the secondary variable with four categories based on combinations of vaccinated, nonvaccinated, previous infection, and no previous infection (Supplementary Tables S1 and S2).

Table 3.
Multivariate logistic regression of factors associated with SARS-CoV-2 transmission to household contacts.
4. Discussion
For the third year of the COVID-19 pandemic when the SARS-CoV-2 Omicron variant and its BA.4 and BA.5 subvariants were circulating, our main findings were that the household SAR was 37.2% and therefore, was among the highest transmission rates reported in meta-analyses [4,14], and that the SAR was lower in contacts exposed to vaccinated index cases. In terms of reducing the infection risk of household contacts, VE was 79% (95% CI: 33%, 93%) for vaccinated index cases but only 5% (95% CI: −280%, 67%) for vaccinated contacts. Other findings were that the SAR was much higher in participants aged ≥65 years and that a previous history of SARS-CoV-2 infection significantly reduced contact susceptibility.
The high SAR observed in our study is consistent with the increase in rates observed in the pandemic over time and with the emergence of new variants. López-Muñoz et al. [15], for their household contact study, estimated 58.2% SAR and 80.9% SAR for periods dominated by the Delta and Omicron variants, respectively. Madewell et al. [14], in an update of their systematic review of household SAR, estimated 42.7% SAR (95% CI: 35.4%, 50.4%) for periods dominated by the Omicron variant. While a direct comparison between variants is difficult, as vaccination levels and social restrictions varied, SAR estimates by Madewell et al. [14] for the Omicron (42.7%), Alpha (36.4%), and Delta (29.7%) variants were higher than the overall SAR of 18.9% previously reported for the earlier pandemic phase when the wild-type variant was prevalent. Transmission levels have been reported to be higher in households than in other community settings due to exposure intensity and multiple opportunities for transmission [9,16] and due to reduced use of protective measures such as face masks by vaccinated household contacts [15]. Most protocols still recommend home confinement for people infected with SARS-CoV-2 to reduce transmission in community settings, so studies of household transmission and control measures will continue to be a priority [16].
Various studies have indicated that vaccination, despite not preventing index case infection, may play a key role in reducing transmission to contacts [17] by reducing the viral load, symptoms, and even the number of transmission days in vaccinated individuals [18,19]. The high VE (79%) of index cases observed in our study points to a notable impact of vaccination in reducing household transmission, and underlines the especial importance of vaccinating people in contact with vulnerable populations, e.g., health workers, nursing home workers, individuals with frequent community contacts, and individuals cohabiting with elderly people and people at risk. Similar results have been observed in other studies that, using different methodologies, have estimated 40–80% reductions in household infection transmission [17,20,21].
In a U.K. study of secondary infection (defined as a positive SARS-CoV-2 test 2–14 days after a positive index case test), Harris et al. [5] compared risk for unvaccinated household contacts of infected persons who had been vaccinated at least once (ChAdOx1 nCoV-19 or BNT162b2) 21 days or more before testing positive with risk for unvaccinated household contacts of unvaccinated and infected persons; they reported that, overall, the transmission likelihood was around 40–50% lower in the households of vaccinated index cases and that results were similar for both vaccines. In a Dutch study, de Gier et al. [20] found that the household contact SAR was lower for fully vaccinated index cases than for unvaccinated index cases (11% vs. 31%), reporting an adjusted VE of 71% (95% CI: 63%, 77%). Eyre et al. [17], in their U.K. study, found that both the BNT162b2 and ChAdOx1 nCoV-19 vaccines were associated with reduced infection transmission from index cases that became infected despite vaccination (aOR = 0.32 and aOR = 0.48, respectively).
We found VE to be only 5% in terms of reducing infection susceptibility in contacts when the Omicron variant dominated; this corroborates the findings of other contact studies pointing to a comparative lack of VE for Omicron compared to the Delta and Alpha variants and pointing to a reduction in VE over time [21,22]. Some studies have reported superior vaccine protection in people with a history of previous SARS-CoV-2 infection (hybrid immunity). Suarez at al. [23] in France and Hall et al. [10] in the U.K. observed greater vaccine protection in previously infected individuals. In our study, greater vaccine protection was also observed in previously infected household contacts (aOR = 0.41; 95% CI: 0.07, 2.41), although this result was not statistically significant (Supplementary Table S2). Our finding is consistent with the results of a seroprevalence study conducted while the BA.4 and BA.5 Omicron subvariants were circulating: Castilla et al. [24] reported no change in COVID-19 risk in individuals who only had vaccine-induced antivirus spike (S) antibodies (suggesting that vaccines were ineffective in reducing infection susceptibility), whereas COVID-19 risk was significantly reduced in individuals with natural-infection-induced nucleocapsid (N) antibodies. The low VE observed for household contacts has also been associated with reduced use of nonpharmacological preventive measures by vaccinated individuals and higher exposure intensity and duration [16,25,26].
In our logistic regression model for both index case and contact vaccination, we found a statistically significant 57% effectiveness for previous infection; this rate is very similar to the 56% reported by Altarawaneh et al. [27] and the 51% reported by Suarez-Castillo et al. [23] and corroborates other findings of high protection due to infection-acquired immunity that could be increased with booster vaccination [6,10,28]. Regarding the effectiveness of booster doses, for a prospective cohort of healthcare workers, Hall et al. [10] found that, while infection-acquired immunity waned after 12 months in unvaccinated participants, it consistently remained above 90% in subsequently vaccinated participants, even if infected more than 18 months previously.
A result of our logistic regression model was that household contacts aged ≥65 years had a 3.3-fold greater risk of becoming infected compared to those aged ≤17 years, thereby corroborating the findings of various studies highlighting the greater infection risk of older household contacts [20,29,30], attributable to immunosenescence-related loss of protection from vaccines or previous infections [11,23,30,31].
Although our results were not statistically significant (probably due to the small number of subjects), we found that sharing a bedroom with an index case and smoking multiplied the infection risk 1.7-fold and 2.3-fold, respectively. Smoking, as has been confirmed for influenza, damages the respiratory immune system and increases general susceptibility to infection [13]. We found no evidence that face mask use by household contacts was effective in reducing infection. Note, however, that the role of face masks is difficult to establish because infection is transmitted before index case diagnosis, while contacts use face masks after index case diagnosis.
The main limitation of our study is that our sample was small and the statistical power to demonstrate VE in contacts was only 16%. Furthermore, the study’s capacity to demonstrate certain effects was constrained by the fact that over 90% of contacts had been vaccinated. The VE analysis was based on at least one dose, as most participants had received two or more doses (only under 5% of our index cases had received a single dose). While the index case vaccination effect on transmission could be confounded by a previous history SARS-CoV-2 infection, the effect was similar for index cases with and without this history (Supplementary Tables S1 and S2).
Another limitation of our study is that data on exposure, risk factors (smoking), and use of non-pharmacological measures were not directly observed, so may reflect social desirability bias (i.e., respondents may have answered questions to be viewed favorably by the interviewer). Previous SARS-CoV-2 infections and vaccinations may also have been incorrectly reported, although this information was crosschecked against electronic health records (regional vaccination registers and epidemiological surveillance unit databases) and so can be considered as validated. Another issue is that potential contacts may have been rendered more susceptible to infection by less frequent use of non-pharmacological protective measures by vaccinated people due to the security instilled by vaccination, as reported elsewhere [15]. Although we collected previous SARS-CoV-2 infection data from medical records, previous infections may have been underestimated due to undiagnosed and unrecorded cases [24], while lower viral loads and less-severe clinical presentation of infections in vaccinated individuals would also have been more difficult to detect [19,32]. Furthermore, there is the risk that some infections that may have occurred outside the home were falsely attributed to the studied index cases, although this risk was minimized by household contacts being interviewed by contact tracing experts. Finally, people from the households who agreed to participate may not be representative of the overall set of households, and the number of participants may have been too small to uncover certain associations.
The strengths of this research are its prospective design of a household contact study of confirmed COVID-19 cases in a period of Omicron predominance and the fact that recruitment was based on a contact study protocol that remained unchanged during the study period and was applied to the entire population. Furthermore, participant variables were collected before knowing test results and contacts were classified according to their test results.
5. Conclusions
Our study shows that in the third year of the COVID-19 pandemic, dominated by the Omicron variant, the household SAR was high. The notable effectiveness of the index case vaccination in reducing household transmission points to the importance of prioritizing vaccination of groups in contact with at-risk populations and with frequent community contacts. According to our study, index case vaccination plays a key role in reducing household transmission, thereby supporting the recommendation of vaccination to protect contacts, especially if they belong to at-risk groups. Our study confirms that in the Omicron pandemic phase, household contact vaccination did not prevent infection [33]. More studies are needed to assess important household SAR factors, such as cohabitant vaccination and face mask use.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines12030240/s1, Table S1: Household infection transmission effects of vaccination and previous SARS-CoV-2 infection history in index cases and contacts. Table S2: Multivariate logistic regression of factors associated with SARS-CoV-2 transmission to household contacts.
Author Contributions
P.G. contributed to the conceptualization, study design, figures, data collection, formal data analysis, data interpretation, and writing of the original draft. M.G.-C., M.A., N.F., N.B., I.P., M.C., J.F., M.G., D.T., I.M.-B., J.P., C.M. and I.S. contributed to the study design, data collection, access of the dataset, data analysis, and data interpretation, and critically reviewed the manuscript. P.P.-R., J.M., C.M.-A. and M.-R.S., contributed to data collection and data interpretation and critically reviewed the manuscript. All co-authors had access and verified the reported data used in the study. S.G., J.A.C., J.C. and Á.D. performed the literature search, contributed to data interpretation, wrote the manuscript, and critically reviewed the manuscript. All authors had full access to all the data in this study. The corresponding author had final responsibility for the decision to submit for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Instituto de Salud Carlos III (ISCIII) via project PI21/01883 (cofounded by the European Union); the Centre for Networked Biomedical Research—Epidemiology and Public Health (CIBERESP) via project ESP22PI01 and Agència de Gestió d’Ajuts Universitaris i de Recerca [2021/SGR00702].
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Arnau Vilanova University Hospital (code: CEIC-2464) and was conducted according to Declaration of Helsinki principles.
Informed Consent Statement
All subjects included in the study received detailed information on the study aims and granted their consent to participate. All data were fully anonymized before performing the analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
SARS-CoV-2 Transmission To Household Contacts Work Group: Factors associated with SARS-CoV-2 transmission to household contacts in Catalonia and Navarre and effectiveness of vaccines and non-pharmacological measures in reducing transmission (PI21/01883 and ESP22PI01). Work Group Members: Pere Godoy (PG), Manuel García-Cenoz (MGC), Miquel Alsedà (MA), Gloria Carmona (GC), Pere Plans (PP), Pilar Ciruela (PC), Nuria Follia (NF), Nuria Bes (NB), Núria Soriano (NS), Ignasi Parrón (IP), Caritat Planas (CP), Irene Barrabeig (IB), Mònica Carol (MC), Joaquim Ferras (JF), Montserrat Guillaumes (MG), David Palma (DP), Diana Toledo (DT), Iván Martínez-Baz (IMB), Carmen Muñoz-Almagro (CMA), Cristina Rius (CR), Glòria Perez (GP), Maria-Rosa Sala (MRS), Joan A Caylà, (JAC), Ángela Domínguez (AD), Jesús Castilla (JC), Sofia Godoy (SG), Jessica Pardo (JP), Carme Miret (CM), Mariona Vilar Pont (MVP), Aroa Illa Casarramona (AIC), Joaquim Solà Pou (JSP), Ivett Morales Arteaga (IMA), Blanca Manuel Marti (BMM), Javier Remón Piñol (JRP), Inmaculada Sanz (IS), Jose Abadin Barrantes (JAB), Alex Ortega Roca (AOR), Pablo Aldaz (PA), Cristina Burgui (CB), Alexandre Ortega Roca (AOR), Raquel Hurtado Portero (RHP), Victor Guadalupe (VG), Anna Vilalta (AV), and Montserrat Zayas (MZ).
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Factors associated with household transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2122240. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Burgui, C.; Casado, I.C.J. Transmission of SARS-CoV-2 infection and risk factors in a cohort of close contacts. Postgr Med. 2022, 134, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; García-cenoz, M.; Parron, I.; Carol, M.; Bes, N.; Soriano, N.; Guillaumes, M.; Plans, P.; Alseda, M.; Godoy, S.; et al. Factores asociados a la transmisión del SARS-CoV-2 en los domicilios: Una scoping review. Enf. Emerg. 2022, 21, 85–88. [Google Scholar]
- Harris, R.J.; Hall, J.A.; Zaidi, A.; Andrews, N.J.; Dunbar, J.K.; Dabrera, G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N. Engl. J. Med. 2021, 385, 759–760. [Google Scholar] [CrossRef]
- Arabi, M.; Al-Najjar, Y.; Sharma, O.; Kamal, I.; Javed, A.; Gohil, H.S.; Paul, P.; Al-Khalifa, A.M.; Laws, S.A.; Zakaria, D. Role of previous infection with SARS-CoV-2 in protecting against omicron reinfections and severe complications of COVID-19 compared to pre-omicron variants: A systematic review. BMC Infect. Dis. 2023, 23, 432. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Miqueleiz, A.; Egüés, N.; Casado, I.; Burgui, C.; Echeverría, A.; Navascués, A.; Fernández-Huerta, M.; Cenoz, M.G.; Trobajo-Sanmartín, C.; et al. Effect of COVID-19 vaccination on the SARS-CoV-2 transmission among social and household close contacts: A cohort study. J. Infect. Public. Health 2023, 16, 410–417. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: Test negative design study. BMJ 2021, 374, n1943. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Lopez-Bernal, J.; Panagiotopoulos, N.; Byers, C.; Vilaplana, T.G.; Boddington, N.; Zhang, X.S.; Charlett, A.; Elgohari, S.; Coughlan, L.; Whillock, R.; et al. Transmission dynamics of COVID-19 in household and community settings in the United Kingdom, January to March 2020. Euro. Surveill. 2022, 27, 2001551. [Google Scholar] [CrossRef]
- Godoy, P.; Castilla, J.; Mayoral, J.M.; Delgado-Rodriguez, M.; Martin, V.; Astray, J.; Soldevila, N.; Gonzalez-Candelas, F.; Castro, A.; Baricot, M.; et al. Smoking may increase the risk of hospitalization due to influenza. Eur. J. Public. Health 2016, 26, 882–887. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: An updated systematic review and meta-analysis. JAMA Netw. Open 2022, 5, E229317. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, I.; Torrella, A.; Pérez-Quílez, O.; Castillo-Zuza, A.; Martró, E.; Bordoy, A.E.; Saludes, V.; Blanco, I.; Soldevila, L.; Estrada, O.; et al. SARS-CoV-2 secondary attack rates in vaccinated and unvaccinated household contacts during replacement of Delta with Omicron variant, Spain. Emerg. Infect. Dis. 2022, 28, 1999–2008. [Google Scholar] [CrossRef]
- Thompson, H.A.; Mousa, A.; Dighe, A.; Fu, H.; Arnedo-Pena, A.; Barrett, P.; Bellido-Blasco, J.; Bi, Q.; Caputi, A.; Chaw, L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: A systematic review and meta-analysis. Clin. Infect. Dis. 2021, 73, E754–E764. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E. Effect of COVID-19 vaccination on transmission of Alpha and Delta variants. N. Engl. J. Med. 2022, 386, 744–756. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Lyngse, F.P.; Mølbak, K.; Denwood, M.; Christiansen, L.E.; Møller, C.H.; Rasmussen, M.; Cohen, A.S.; Stegger, M.; Fonager, J.; Sieber, R.N.; et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta variant of concern. Nat. Commun. 2022, 13, 3764. [Google Scholar] [CrossRef] [PubMed]
- de Gier, B.; Andeweg, S.; Joosten, R.; Ter Schegget, R.; Smorenburg, N.; van de Kassteele, J.; Hahné, S.J.; van den Hof, S.; de Melker, H.E.; Knol, M.J. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro. Surveill. 2021, 26, 7–13. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Monge, S.; Rojas-Benedicto, A.; Olmedo, C.; Mazagatos, C.; Sierra, M.J.; Limia, A.; Martín-Merino, E.; Larrauri, A.; Hernán, M.A.; Moreno, D.; et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: A nationwide cohort study. Lancet Infect. Dis. 2022, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Castillo, S.; Khaoua, H.; Courtejoie, N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro. Surveill. 2022, 27, 2200250. [Google Scholar] [CrossRef]
- Castilla, J.; Lecea, Ó.; Salas, C.M.; Quílez, D.; Miqueleiz, A.; Trobajo-Sanmartín, C.; Navascués, A.; Martínez-Baz, I.; Casado, I.; Burgui, C.; et al. Seroprevalence of antibodies against SARS-CoV-2 and risk of COVID-19 in Navarre, Spain, May to July 2022. Euro. Surveill. 2022, 27, 2200619. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, M.; Richiardi, L.; Alladio, F.; Staffilano, E.; Longo, B.; Venuti, F.; Ghisetti, V.; Burdino, E.; Bonora, S.; Vineis, P.; et al. Determinants of SARS-CoV-2 contagiousness in household contacts of symptomatic adult index cases. Front. Mircrobiol. 2022, 13, 829393. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Chiew, C.J.; Pang, D.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: A national cohort study. Lancet Infect. Dis. 2023, 23, 799–805. [Google Scholar] [CrossRef]
- Ellingson, K.D.; Hollister, J.; Porter, C.J.; Khan, S.M.; Feldstein, L.R.; Naleway, A.L.; Gaglani, M.; Caban-Martinez, A.J.; Tyner, H.L.; Lowe, A.A.; et al. Risk factors for reinfection with SARS-CoV-2 Omicron variant among previously infected frontline workers. Emerg. Infect. Dis. 2023, 29, 599–604. [Google Scholar] [CrossRef]
- Miyahara, R.; Tamura, K.; Kato, T.; Nakazaki, M.; Otani, K.; Ko, Y.K.; Kamigaki, T.; Arima, Y.; Tani, H.; Oishi, K.; et al. SARS-CoV-2 variants and age-dependent infection rates among household and nonhousehold contacts. Emerg. Infect. Dis. 2023, 29, 1648–1650. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.; Amlag, J.O.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.G.; Linde, L.; Ali, A.R.; DeSantis, A.; Shi, M.; Adam, C.; Armstrong, B.; Armstrong, B.; Asbell, M.; Auche, S.; et al. COVID-19 Incidence and mortality among unvaccinated and vaccinated persons aged ≥ 12 years by receipt of bivalent booster doses and time since vaccination—24 U.S. jurisdictions, October 3, 2021–December 24, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 145–152. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).