Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023
Abstract
1. Introduction
2. Materials and Methods
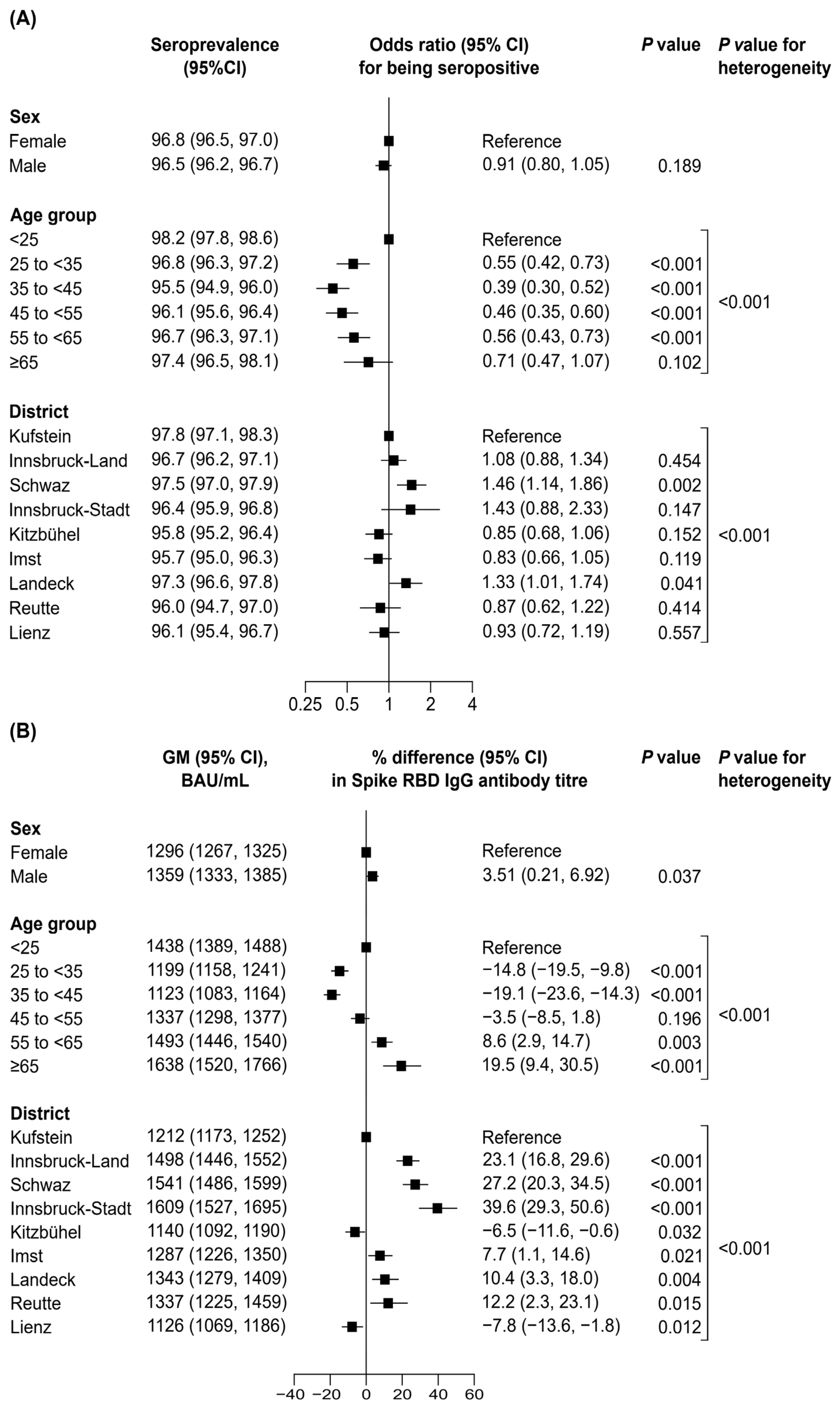
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef]
- Seekircher, L.; Bánki, Z.; Kimpel, J.; Rössler, A.; Schäfer, H.; Falkensammer, B.; Bante, D.; Forer, L.; Schönherr, S.; Harthaller, T.; et al. Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: An open-label, observational phase 4 trial. Lancet Microbe 2023, 4, e612–e621. [Google Scholar] [CrossRef]
- Siller, A.; Seekircher, L.; Wachter, G.A.; Astl, M.; Tschiderer, L.; Pfeifer, B.; Gaber, M.; Schennach, H.; Willeit, P. Seroprevalence, Waning and Correlates of Anti-SARS-CoV-2 IgG Antibodies in Tyrol, Austria: Large-Scale Study of 35,193 Blood Donors Conducted between June 2020 and September 2021. Viruses 2022, 14, 568. [Google Scholar] [CrossRef]
- Seekircher, L.; Siller, A.; Astl, M.; Tschiderer, L.; Wachter, G.A.; Pfeifer, B.; Huber, A.; Gaber, M.; Schennach, H.; Willeit, P. Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies in Tyrol, Austria: Updated Analysis Involving 22,607 Blood Donors Covering the Period October 2021 to April 2022. Viruses 2022, 14, 1877. [Google Scholar] [CrossRef]
- Karl, T.; Schuster, A.; Stangassinger, L.M.; Stiboller, T.; Cadamuro, J.; Oostingh, G.J. Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study. Vaccines 2023, 11, 1615. [Google Scholar] [CrossRef]
- Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH. Gesundheit für Mensch, Tier & Pflanze: Coronavirus. Available online: https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus (accessed on 17 September 2022).
- Abbott. SARS-CoV-2 Immunoassays. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2.html (accessed on 29 February 2024).
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval Estimation for a Binomial Proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Borena, W.; Bánki, Z.; Bates, K.; Winner, H.; Riepler, L.; Rössler, A.; Pipperger, L.; Theurl, I.; Falkensammer, B.; Ulmer, H.; et al. Persistence of immunity to SARS-CoV-2 over time in the ski resort Ischgl. EBioMedicine 2021, 70, 103534. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.-K.; Breyer-Kohansal, R.; Hartl, S.; Kundi, M.; Weseslindtner, L.; Stiasny, K.; Puchhammer-Stöckl, E.; Schrott, A.; Födinger, M.; Binder, M.; et al. Low SARS-CoV-2 seroprevalence in the Austrian capital after an early governmental lockdown. Sci. Rep. 2021, 11, 10158. [Google Scholar] [CrossRef] [PubMed]
- Harthaller, T.; Borena, W.; Bante, D.; Schäfer, H.; Strallhofer, O.; Zöggeler, T.; Hochmuth, E.; Hoch, L.; Rössler, A.; von Laer, D.; et al. High Prevalence of Undocumented SARS-CoV-2 Infections in the Pediatric Population of the Tyrolean District of Schwaz. Viruses 2022, 14, 2294. [Google Scholar] [CrossRef] [PubMed]
- Kerbl, R.; Strenger, V.; Bernar, B.; Zurl, C.; Simma, B. SARS-CoV-2-Seroprävalenz in Österreich. Die Situation vor der Omikronwelle. Monatsschrift Kinderheilkd. 2022, 170, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Ladage, D.; Höglinger, Y.; Ladage, D.; Adler, C.; Yalcin, I.; Harzer, O.; Braun, R.J. SARS-CoV-2-Specific Antibody Prevalence and Symptoms in a Local Austrian Population. Front. Med. 2021, 8, 632942. [Google Scholar] [CrossRef]
- Nunhofer, V.; Weidner, L.; Hoeggerl, A.D.; Zimmermann, G.; Badstuber, N.; Grabmer, C.; Jungbauer, C.; Lindlbauer, N.; Held, N.; Pascariuc, M.; et al. Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection. Viruses 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, H.; Braun, R.J.; Saha, T.; Harzer, O.; Schneider, M.; Ladage, D. Longitudinal monitoring of SARS-CoV-2 spike protein-specific antibody responses in Lower Austria. PLoS ONE 2022, 17, e0271382. [Google Scholar] [CrossRef] [PubMed]
- Szépfalusi, Z.; Schmidthaler, K.; Sieber, J.; Kopanja, S.; Götzinger, F.; Schoof, A.; Hoz, J.; Willinger, B.; Makristathis, A.; Weseslindtner, L.; et al. Lessons from low seroprevalence of SARS-CoV-2 antibodies in schoolchildren: A cross-sectional study. Pediatr. Allergy Immunol. 2021, 32, 762–770. [Google Scholar] [CrossRef]
- Willeit, P.; Kimpel, J.; Winner, H.; Harthaller, T.; Schäfer, H.; Bante, D.; Falkensammer, B.; Rössler, A.; Riepler, L.; Ower, C.; et al. Seroprevalence of SARS-CoV-2 infection in the Tyrolean district of Schwaz at the time of the rapid mass vaccination in March 2021 following B.1.351-variant outbreak. Front. Public Health 2022, 10, 989337. [Google Scholar] [CrossRef] [PubMed]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.-Y.; et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell 2022, 185, 1539–1548.e5. [Google Scholar] [CrossRef]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Zhang, S.; Kong, Y.; Shen, Z.; Zhang, J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: A systematic review and analysis. J. Med. Virol. 2022, 94, 5790–5801. [Google Scholar] [CrossRef] [PubMed]
- Solastie, A.; Nieminen, T.; Ekström, N.; Nohynek, H.; Lehtonen, L.; Palmu, A.A.; Melin, M. Changes in SARS-CoV-2 seroprevalence and population immunity in Finland, 2020–2022. Emerg. Microbes Infect. 2023, 12, 2222849. [Google Scholar] [CrossRef]
- Zaballa, M.-E.; Perez-Saez, J.; de Mestral, C.; Pullen, N.; Lamour, J.; Turelli, P.; Raclot, C.; Baysson, H.; Pennacchio, F.; Villers, J.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in Geneva, Switzerland: A population-based study. Lancet Reg. Health Eur. 2022, 24, 100547. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Weygaerde, Y.V.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef]
- Majdoubi, A.; Michalski, C.; O’connell, S.E.; Dada, S.; Narpala, S.; Gelinas, J.; Mehta, D.; Cheung, C.; Winkler, D.F.; Basappa, M.; et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight 2021, 6, e146316. [Google Scholar] [CrossRef] [PubMed]
- Asamoah-Boaheng, M.; Grunau, B.; Haig, S.; Karim, M.E.; Kirkham, T.; Lavoie, P.M.; Sediqi, S.; Drews, S.J.; O’Brien, S.F.; Barakauskas, V.; et al. Eleven-month SARS-CoV-2 binding antibody decay, and associated factors, among mRNA vaccinees: Implications for booster vaccination. Access Microbiol. 2023, 5. [Google Scholar] [CrossRef] [PubMed]
- Bešević, J.; Lacey, B.; Callen, H.; Omiyale, W.; Conroy, M.; Feng, Q.; Crook, D.W.; Doherty, N.; Ebner, D.; Eyre, D.W.; et al. Persistence of SARS-CoV-2 antibodies over 18 months following infection: UK Biobank COVID-19 Serology Study. J. Epidemiol. Community Health 2023, 78, 105–108. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Infectious Diseases. Why hybrid immunity is so triggering. Lancet Infect. Dis. 2022, 22, 1649. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]

| Characteristics | No. (%) or Median [IQR] |
|---|---|
| Age in years at baseline | 45.4 [31.1–55.4] |
| Age groups at baseline | |
| <25 | 4125 (14.3%) |
| 25 to <35 | 4951 (17.2%) |
| 35 to <45 | 5063 (17.6%) |
| 45 to <55 | 7128 (24.8%) |
| 55 to <65 | 6401 (22.3%) |
| ≥65 | 1100 (3.8%) |
| Sex | |
| Female | 12,440 (43.2%) |
| Male | 16,328 (56.8%) |
| Residence district at baseline | |
| Kufstein | 5719 (19.9%) |
| Innsbruck-Land | 5150 (17.9%) |
| Schwaz | 4040 (14.0%) |
| Innsbruck-Stadt | 1659 (5.8%) |
| Kitzbühel | 3400 (11.8%) |
| Imst | 2822 (9.8%) |
| Landeck | 2296 (8.0%) |
| Reutte | 1047 (3.6%) |
| Lienz | 2635 (9.2%) |
| Availability of antibody measurements 1 | |
| Spike RBD IgG antibodies | 37,065 (100%) |
| Nucleocapsid IgG antibodies | 12,645 (34.1%) |
| Spike RBD IgG Antibodies | Nucleocapsid IgG Antibodies | |||||
|---|---|---|---|---|---|---|
| Year | Month | N | % Seropositive (95% CI) | GM (95% CI) in BAU/mL 1 | N | % Seropositive (95% CI) |
| 2022 | May | 3314 | 96.3 (95.6–96.9) | 1400 (1333–1471) | 0 | - |
| June | 3776 | 95.1 (94.4–95.7) | 1248 (1190–1309) | 0 | - | |
| July | 4615 | 95.7 (95.0–96.2) | 1093 (1047–1142) | 0 | - | |
| August | 3884 | 96.2 (95.5–96.7) | 1137 (1087–1190) | 0 | - | |
| September | 4142 | 96.6 (96.0–97.1) | 1153 (1105–1204) | 3641 | 36.5 (35.0–38.1) | |
| October | 3421 | 97.3 (96.6–97.8) | 1339 (1278–1403) | 3394 | 39.6 (38.0–41.3) | |
| November | 3399 | 97.4 (96.8–97.9) | 1590 (1516–1668) | 3382 | 41.0 (39.4–42.7) | |
| December | 2247 | 97.4 (96.7–98.0) | 1821 (1717–1932) | 2228 | 39.2 (37.2–41.2) | |
| 2023 | January | 4432 | 96.8 (96.3–97.3) | 1510 (1448–1574) | 0 | - |
| February | 3221 | 97.7 (97.1–98.2) | 1410 (1346–1477) | 0 | - | |
| March | 614 | 97.9 (96.4–98.8) | 1559 (1405–1729) | 0 | - | |
| N | Seropositive, N (%) 1 | GM (95% CI), BAU/mL 2 | |
|---|---|---|---|
| All | 8457 | 8232 (97.3) | 1537 (1491–1584) |
| Prior SARS-CoV-2 infection 3 | |||
| Uninfected | 1764 | 1724 (97.73) | 1067 (996–1143) |
| Latest infection before 2022 | 1425 | 1402 (98.39) | 1019 (952–1091) |
| Latest infection in 2022 | 5268 | 5106 (96.92) | 1946 (1875–2020) |
| SARS-CoV-2 vaccination status | |||
| Unvaccinated | 918 | 696 (75.82) | 142 (127–159) |
| Vaccinated without booster 4 | 1433 | 1433 (100.00) | 1063 (1007–1121) |
| Vaccinated with booster 4 | 6106 | 6104 (99.97) | 2196 (2133–2261) |
| Latest vaccination before 2022 | 5129 | 5127 (99.96) | 1687 (1634–1742) |
| Latest vaccination in 2022 | 2410 | 2409 (99.96) | 2507 (2398–2621) |
| SARS-CoV-2 infection and vaccination status | |||
| Unvaccinated + uninfected | 45 | 7 (15.56) | 31 (9–108) |
| Unvaccinated + infected | 873 | 689 (78.92) | 144 (129–161) |
| Vaccinated without booster 4 + uninfected | 128 | 128 (100.00) | 514 (400–662) |
| Vaccinated with booster 4 + uninfected | 1591 | 1589 (99.87) | 1150 (1072–1232) |
| Vaccinated without booster 4 + infected | 1305 | 1304 (99.92) | 1148 (1091–1208) |
| Vaccinated with booster 4 + infected | 4515 | 4515 (100.00) | 2758 (2683–2835) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siller, A.; Seekircher, L.; Astl, M.; Tschiderer, L.; Wachter, G.A.; Penz, J.; Pfeifer, B.; Huber, A.; Gaber, M.; Schennach, H.; et al. Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023. Vaccines 2024, 12, 284. https://doi.org/10.3390/vaccines12030284
Siller A, Seekircher L, Astl M, Tschiderer L, Wachter GA, Penz J, Pfeifer B, Huber A, Gaber M, Schennach H, et al. Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023. Vaccines. 2024; 12(3):284. https://doi.org/10.3390/vaccines12030284
Chicago/Turabian StyleSiller, Anita, Lisa Seekircher, Manfred Astl, Lena Tschiderer, Gregor A. Wachter, Julia Penz, Bernhard Pfeifer, Andreas Huber, Manfred Gaber, Harald Schennach, and et al. 2024. "Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023" Vaccines 12, no. 3: 284. https://doi.org/10.3390/vaccines12030284
APA StyleSiller, A., Seekircher, L., Astl, M., Tschiderer, L., Wachter, G. A., Penz, J., Pfeifer, B., Huber, A., Gaber, M., Schennach, H., & Willeit, P. (2024). Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023. Vaccines, 12(3), 284. https://doi.org/10.3390/vaccines12030284






