Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure
Abstract
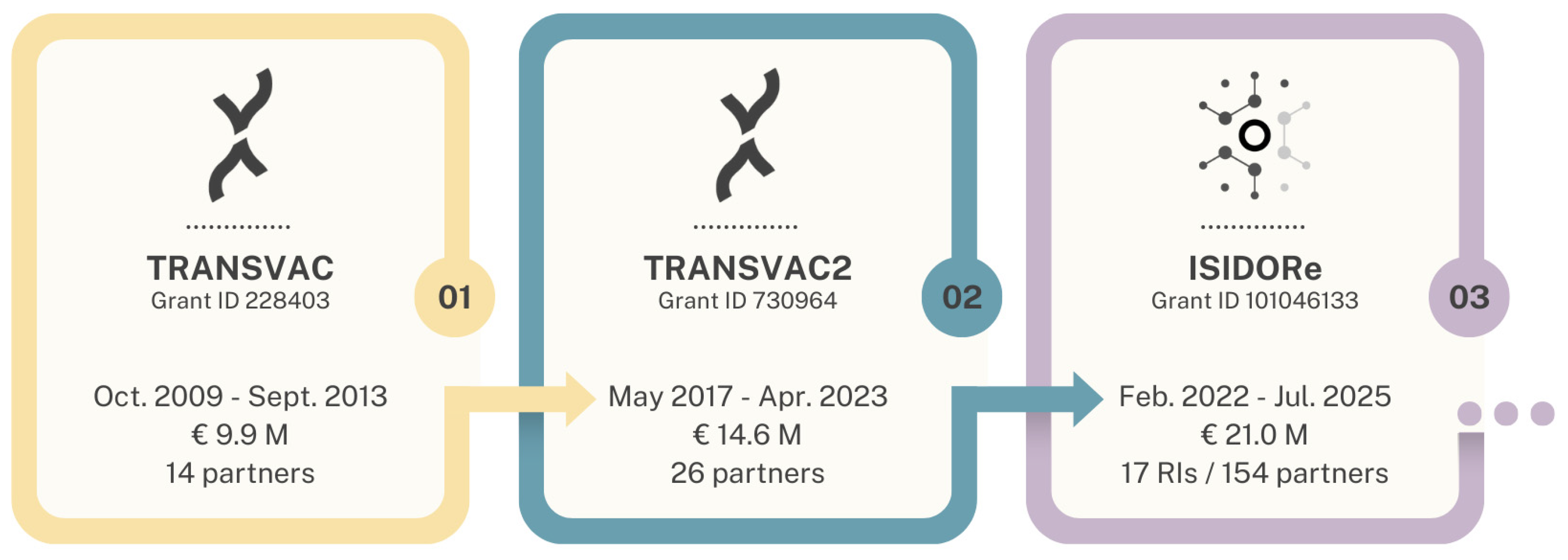
:1. Introduction
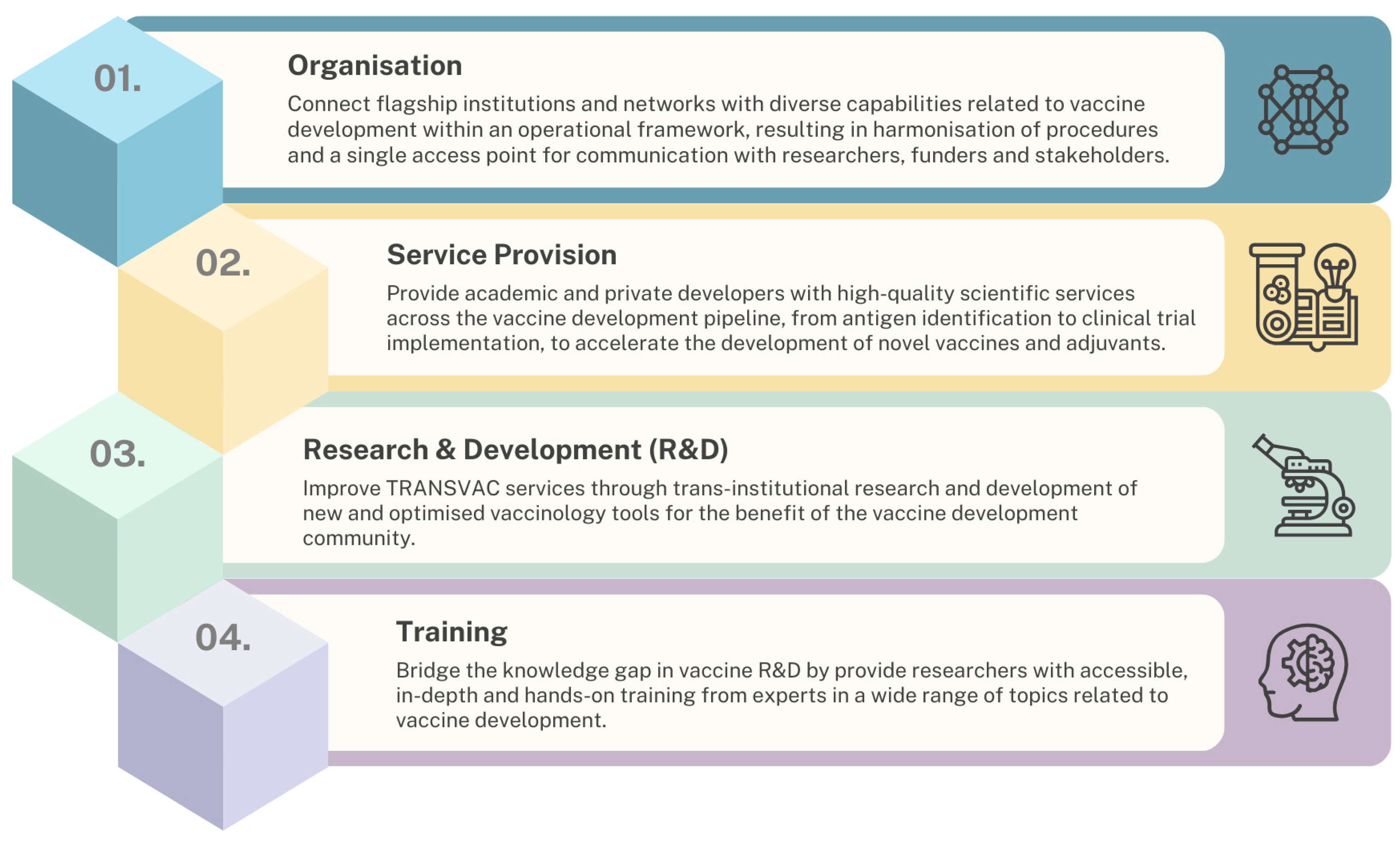
2. Summary and Analysis of TRANSVAC Activities
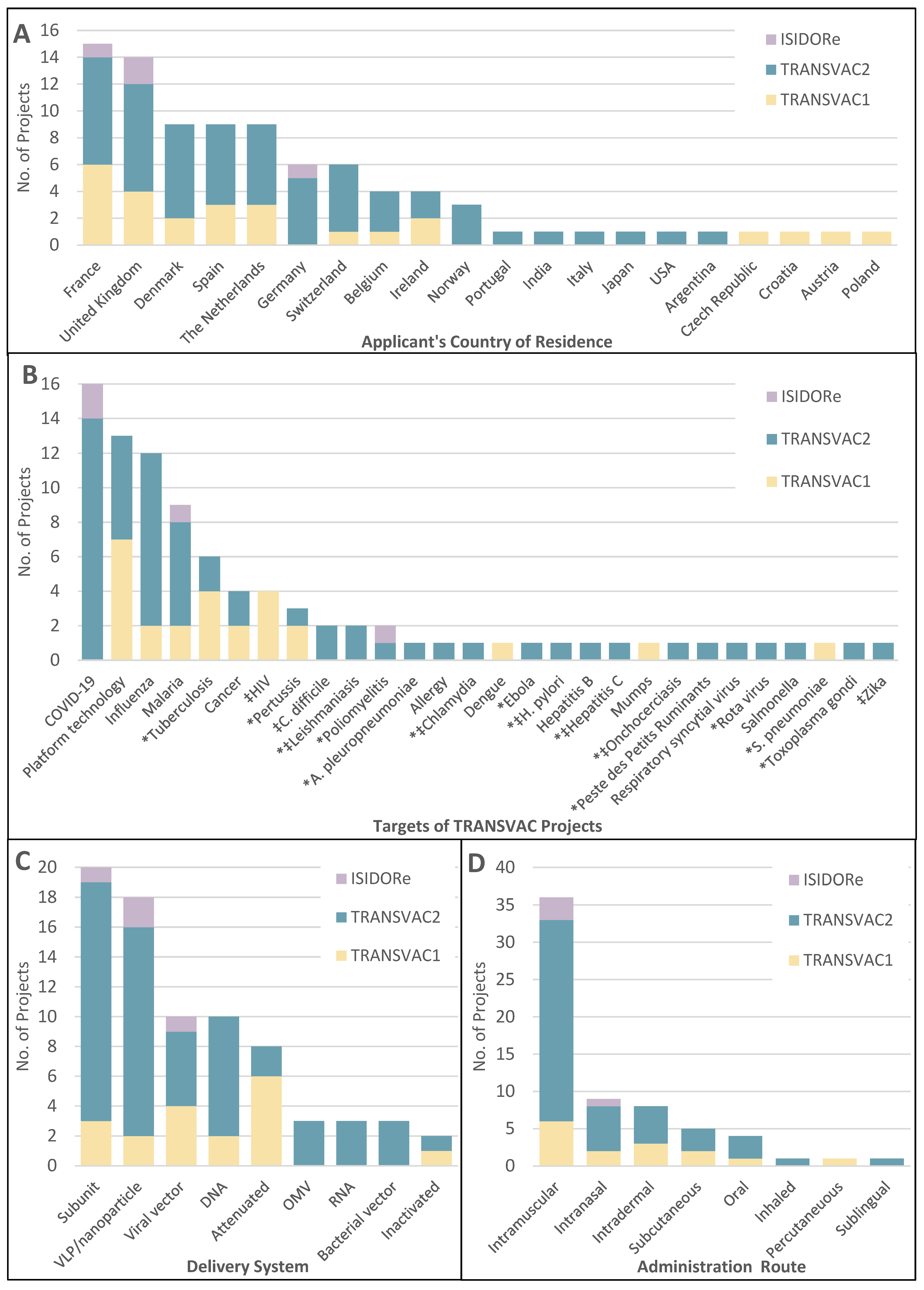
2.1. Overview of TRANSVAC Services for Vaccine Candidates
2.1.1. Therapeutic Vaccines
2.1.2. Interaction with the Vaccine Industry
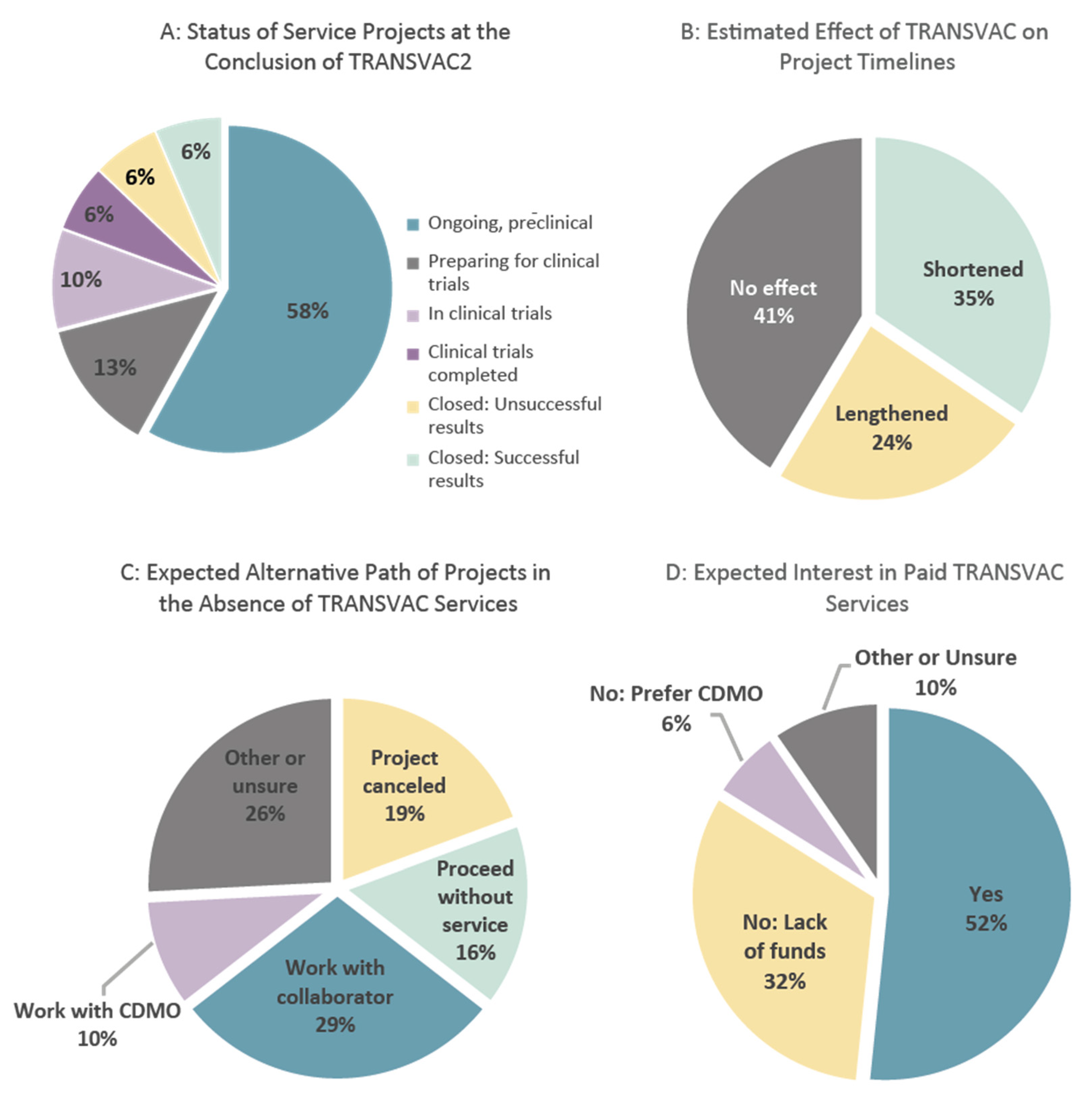
2.1.3. Developer Feedback
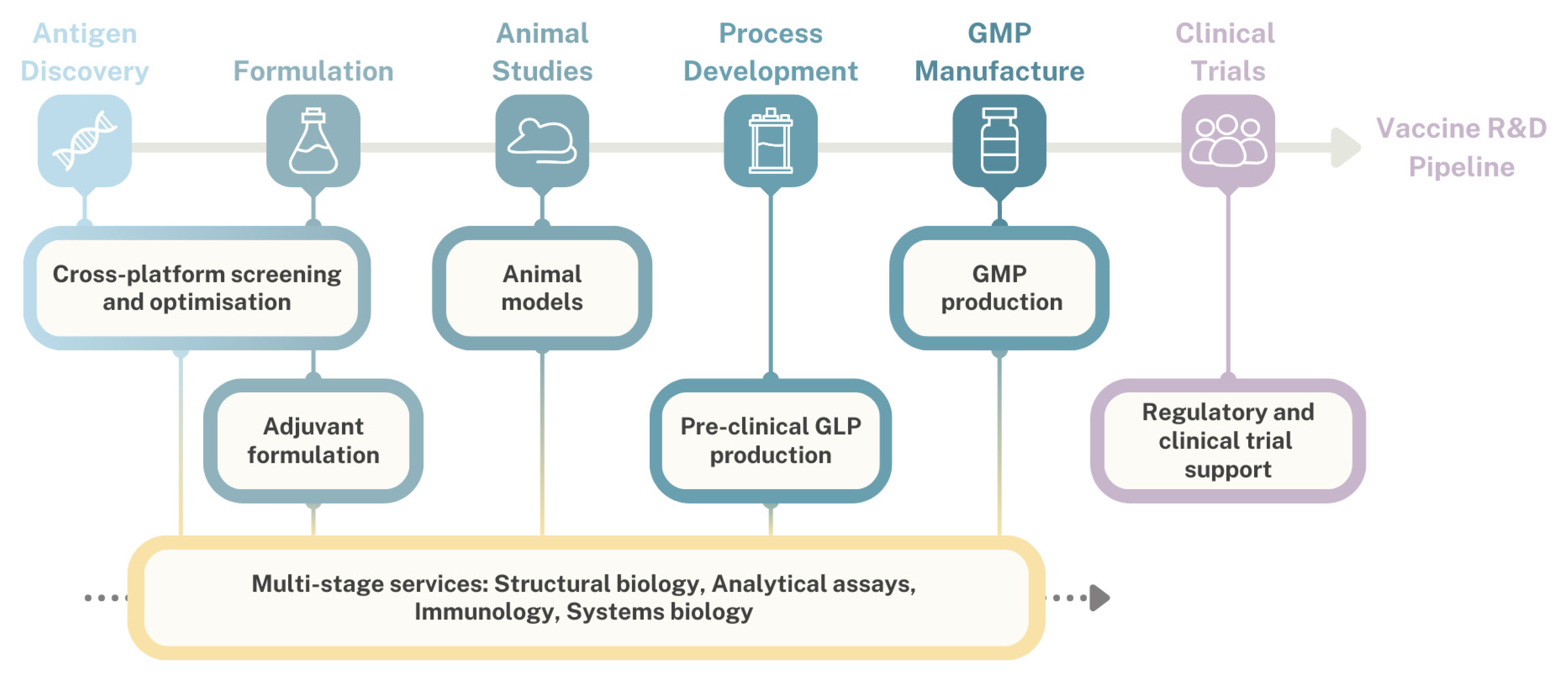
2.2. Development of TRANSVAC Services to Support Vaccine R&D
2.2.1. Antigen Characterization and Selection
2.2.2. Adjuvants
2.2.3. Immunization Routes
2.2.4. Vaccine Manufacturing Support
2.3. Trainings in Vaccinology
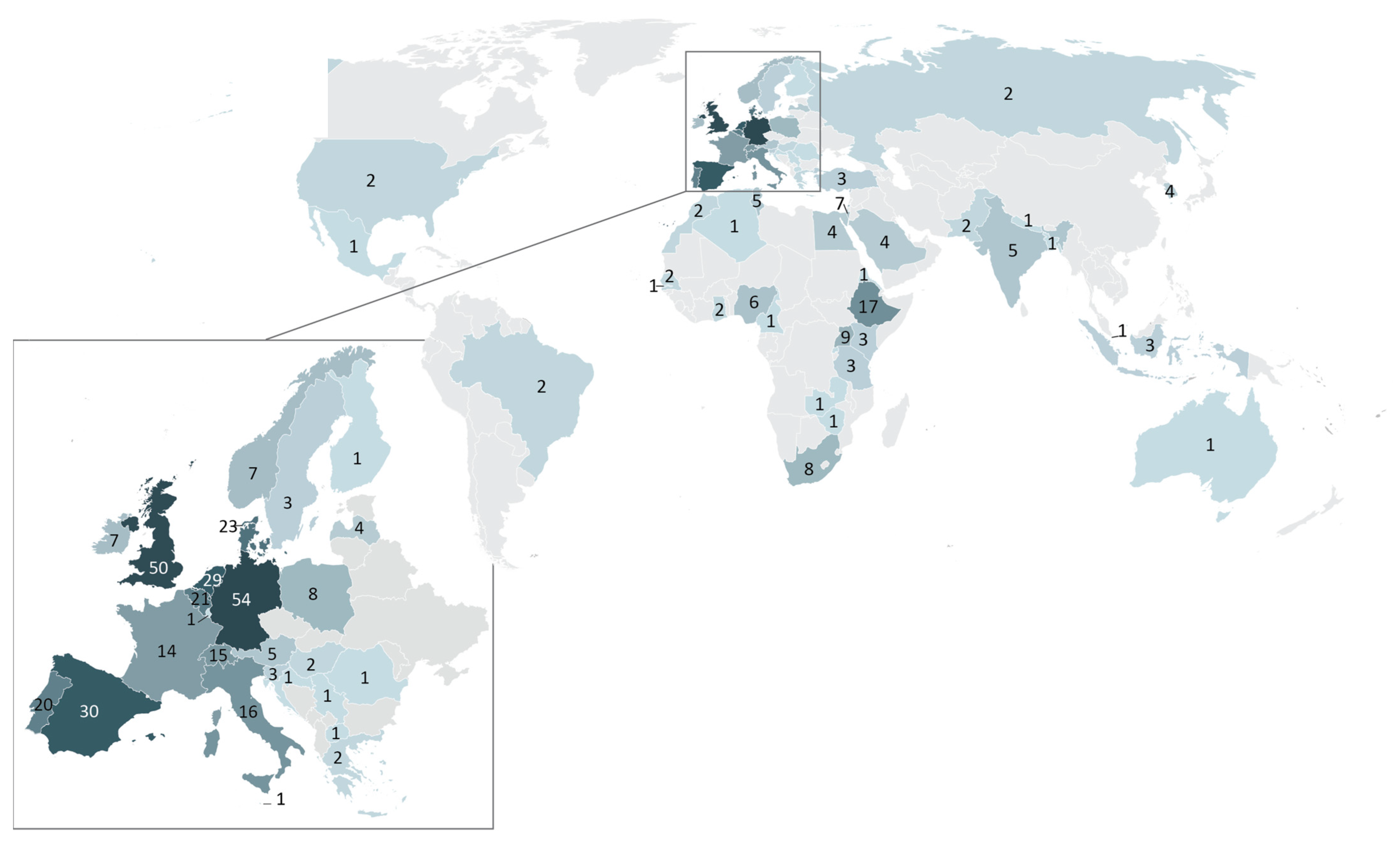
2.3.1. TRANSVAC Trainee Demographics
2.3.2. Outlook for TRANSVAC Training Courses
3. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geels, M.J.; Thøgersen, R.L.; Guzman, C.A.; Ho, M.M.; Verreck, F.; Collin, N.; Robertson, J.S.; McConkey, S.J.; Kaufmann, S.H.E.; Leroy, O. TRANSVAC Research Infrastructure–Results and Lessons Learned from the European Network of Vaccine Research and Development. Vaccine 2015, 33, 5481–5487. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, S.; Depraetere, H.; Slezak, M.; Christensen, D.; Stockhofe, N.; Beloeil, L. A Gaps-and-Needs Analysis of Vaccine R&D in Europe: Recommendations to Improve the Research Infrastructure. Biologicals 2022, 76, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Meslé, M.M.I.; Brown, J.; Mook, P.; Katz, M.A.; Hagan, J.; Pastore, R.; Benka, B.; Redlberger-Fritz, M.; Bossuyt, N.; Stouten, V.; et al. Estimated Number of Lives Directly Saved by COVID-19 Vaccination Programmes in the WHO European Region from December, 2020, to March, 2023: A Retrospective Surveillance Study. Lancet Respir. Med. 2024, 12, 714–727. [Google Scholar] [CrossRef] [PubMed]
- European Network of Vaccine Development and Research|TRANSVAC|Project|Fact Sheet|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/228403 (accessed on 15 October 2024).
- Vaccines Europe Pipeline Review 2023. Available online: https://www.vaccineseurope.eu/wp-content/uploads/2023/11/VaccinesEurope-PipelineReview2023.pdf (accessed on 15 October 2024).
- Vaccines Licensed for Use in the United States|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (accessed on 3 December 2024).
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges, and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Collin, N.; Dubois, P.M. The Vaccine Formulation Laboratory: A Platform for Access to Adjuvants. Vaccine 2011, 29 (Suppl. S1), A37–A39. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Christensen, D.; Perrie, Y. Applying Microfluidics for the Production of the Cationic Liposome-Based Vaccine Adjuvant CAF09b. Pharmaceutics 2020, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzmán, C.A. Bis-(3′,5′)-Cyclic Dimeric Adenosine Monophosphate: Strong Th1/Th2/Th17 Promoting Mucosal Adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, D.; Weissmann, S.F.; Ebensen, T.; Nitsche-Gloy, U.; Franz, H.B.G.; Guzmán, C.A. Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines. Pharmaceutics 2021, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Zariri, A.; Pupo, E.; Van Riet, E.; Van Putten, J.P.M.; Van Der Ley, P. Modulating Endotoxin Activity by Combinatorial Bioengineering of Meningococcal Lipopolysaccharide. Sci. Rep. 2016, 6, 36575. [Google Scholar] [CrossRef]
- López-Serrano, S.; Mahmmod, Y.S.; Christensen, D.; Ebensen, T.; Guzmán, C.A.; Rodríguez, F.; Segalés, J.; Aragón, V. Immune Responses Following Neonatal Vaccination with Conserved F4 Fragment of VtaA Proteins from Virulent Glaesserella Parasuis Adjuvanted with CAF®01 or CDA. Vaccine X 2023, 14, 100330. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, S.; Cordoba, L.; Pérez-Maillo, M.; Pleguezuelos, P.; Remarque, E.J.; Ebensen, T.; Guzmán, C.A.; Christensen, D.; Segalés, J.; Darji, A. Immune Responses to Pandemic H1N1 Influenza Virus Infection in Pigs Vaccinated with a Conserved Hemagglutinin HA1 Peptide Adjuvanted with CAF®01 or CDA/AGalCerMPEG. Vaccines 2021, 9, 751. [Google Scholar] [CrossRef]
- Su, J.; Brunner, L.; Ates Oz, E.; Sacherl, J.; Frank, G.; Kerth, H.A.; Thiele, F.; Wiegand, M.; Mogler, C.; Aguilar, J.C.; et al. Activation of CD4 T Cells during Prime Immunization Determines the Success of a Therapeutic Hepatitis B Vaccine in HBV-Carrier Mouse Models. J. Hepatol. 2023, 78, 717–730. [Google Scholar] [CrossRef]
- Ma, B.; Tao, M.; Li, Z.; Zheng, Q.; Wu, H.; Chen, P. Mucosal Vaccines for Viral Diseases: Status and Prospects. Virology 2024, 593, 110026. [Google Scholar] [CrossRef] [PubMed]
- Katsande, P.M.; Fernández-Bastit, L.; Ferreira, W.T.; Vergara-Alert, J.; Hess, M.; Lloyd-Jones, K.; Hong, H.A.; Segales, J.; Cutting, S.M. Heterologous Systemic Prime-Intranasal Boosting Using a Spore SARS-CoV-2 Vaccine Confers Mucosal Immunity and Cross-Reactive Antibodies in Mice as Well as Protection in Hamsters. Vaccines 2022, 10, 1900. [Google Scholar] [CrossRef]
- Sisteré-Oró, M.; López-Serrano, S.; Veljkovic, V.; Pina-Pedrero, S.; Vergara-Alert, J.; Córdoba, L.; Pérez-Maillo, M.; Pleguezuelos, P.; Vidal, E.; Segalés, J.; et al. DNA Vaccine Based on Conserved HA-Peptides Induces Strong Immune Response and Rapidly Clears Influenza Virus Infection from Vaccinated Pigs. PLoS ONE 2019, 14, e0222201. [Google Scholar] [CrossRef]
- Vierboom, M.P.M.; Dijkman, K.; Sombroek, C.C.; Hofman, S.O.; Boot, C.; Vervenne, R.A.W.; Haanstra, K.G.; van der Sande, M.; van Emst, L.; Domínguez-Andrés, J.; et al. Stronger Induction of Trained Immunity by Mucosal BCG or MTBVAC Vaccination Compared to Standard Intradermal Vaccination. Cell Rep. Med. 2021, 2, 100185. [Google Scholar] [CrossRef] [PubMed]
- Stockhofe, N.; Ślęzak, M. Workshop on Mucosal Vaccines and Aerosol Use in Vaccine Development. 2023. Available online: https://cordis.europa.eu/project/id/730964/results (accessed on 15 November 2024).
- iPROVE. A Strategic European Roadmap for the Vaccines of Tomorrow: A Joint Stakeholder Reflection; iPROVE: Barcelona, Spain, 2016. [Google Scholar]
- Kaur, M.; Coppeta, L.; Olesen, O.F. Vaccine Hesitancy among Healthcare Workers in Europe: A Systematic Review. Vaccines 2023, 11, 1657. [Google Scholar] [CrossRef]
- Directorate-General for Health and Food Safety. State of Vaccine Confidence in the EU; Directorate-General for Health and Food Safety: Luxembourg, 2022. [Google Scholar]
- VACCELERATE–European Corona Vaccine Trial Accelerator Platform|VACCELERATE|Project|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/101037867 (accessed on 20 September 2024).
- Regulation–1394/2007–EN–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32007R1394 (accessed on 29 April 2024).
- Jungbluth, S.; Martin, W.; Slezak, M.; Depraetere, H.; Guzman, C.A.; Ussi, A.; Morrow, D.; Van Heuverswyn, F.; Arnouts, S.; Carrondo, M.J.T.; et al. Potential Business Model for a European Vaccine R&D Infrastructure and Its Estimated Socio-Economic Impact. F1000Res 2023, 12, 1401. [Google Scholar] [CrossRef]
- Africa CDC. Partnerships for African Vaccine Manufacturing (PAVM) Framework for Action; Africa CDC: Addis Ababa, Ethiopia, 2022. [Google Scholar]
| Institution | Country | TV1 | TV2 | ISIDORe | Primary Roles |
|---|---|---|---|---|---|
| Biomedical Primate Research Center (BPRC) | The Netherlands | X | X | X | Immunogenicity studies in NHPs |
| Department of Health (DH)/Medicines and Healthcare Products Regulatory Agency (MHRA) | United Kingdom | X | X | X | Optimization of immunoassays incl. Luminex and ELISpot |
| European Vaccine Initiative (EVI) | Germany | X | X | X | Coordination, regulatory advice |
| Helmholtz-Zentrum für Infektionsforschung (HZI) | Germany | X | X | X | Murine immunogenicity studies; development and formulation of mucosal adjuvants |
| Stichting Wageningen Research (SWR) | The Netherlands | X | X | X | Immunogenicity studies in ferrets and pigs |
| University of Oxford | United Kingdom | X | X | X | Antigen expression and production in viral vaccine vectors |
| London School of Hygiene and Tropical Medicine (LSHTM) | United Kingdom | X | X | Immunoassays incl. Luminex, ELISpot | |
| Public Health England (PHE)/UK Health Security Agency | United Kingdom | X | X | Immunogenicity studies in ferrets | |
| Vaccine Formulation Laboratory (VFL), University of Lausanne (UNIL) | Switzerland | X | X | Adjuvant formulation and characterization, training | |
| Commissariat à l’énergie atomique (CEA) | France | X | X | NHP studies, immune analysis via mass and flow cytometry | |
| European Advanced Translational Research Infrastructure in Medicine (EATRIS) | The Netherlands | X | X | Regulatory advice and resources | |
| European Clinical Research Infrastructure Network (ECRIN) | France | X | X | Clinical trial support | |
| Fraunhofer-Gesellschaft (Fraunhofer) | Germany | X | X | Antigen expression and characterization | |
| GenIbet Biopharmaceuticals | Portugal | X | X | GMP production | |
| Institut de Recerca i Tecnologia Agroalimentaries (IRTA) | Spain | X | X | Immunogenicity studies (hamsters, mice, ferrets, small ruminants, and swine) | |
| Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE) | France | X | X | Immunogenicity studies in rabbits and pigs | |
| Instituto de Biologia Experimental e Tecnológica (iBET) | Portugal | X | X | Development and production of GLP material | |
| Instruct-ERIC | United Kingdom | X | X | Structural biology incl. cryo-EM | |
| Leiden University | The Netherlands | X | X | Metabolomics | |
| Leiden University Medical Center (LUMC) | The Netherlands | X | X | Multiplex transcriptome profiling | |
| Statens Serum Institut (SSI) | Denmark | X | X | Development and formulation of liposomal adjuvants; murine immunogenicity studies; protein expression/ GLP production | |
| Universita degli Studi di Siena (UNISI) | Italy | X | X | Flow cytometry and sequencing assays and analysis | |
| Vaccine Formulation Institute (VFI) (formerly VFL) | Switzerland | X | X | Adjuvant formulation and characterization, training | |
| LIONEX Diagnostics and Therapeutics | Germany | X | Antigen expression and purification | ||
| Max Planck Institute for Infection Biology (MPIIB) | Germany | X | Functional genomics | ||
| Serum Life Science Europe (SLS Europe, formerly Vakzine Projekt Management) | Germany | X | Clinical trial support and project evaluation | ||
| Tuberculosis Vaccine Initiative (TBVI) | The Netherlands | X | Coordination | ||
| University of Regensburg | Germany | X | Genomic analysis | ||
| BIOASTER | France | X | -omics (metabolomics, proteomics, RNA-Seq) | ||
| Eidgenoessische Technische Hochschule Zuerich (ETHZ) | Switzerland | X | Systems biology modeling | ||
| Intravacc | The Netherlands | X | Proteomics; development and formulation of LPS adjuvants | ||
| Sclavo Vaccines Association (SVA) | Italy | X | Impact analysis |
| Course | Organizer | 1st Round | 2nd Round | 3rd Round | Total Applications | Selected Trainees |
|---|---|---|---|---|---|---|
| Clinical Vaccine Development and Biomanufacturing | University of Oxford | October 2018 | September 2021 | October 2022 | 58 | 33 |
| Human and Veterinary Vaccinology | University of Oxford | November 2018 | October 2021 | 39 | 29 | |
| Adjuvants and Vaccine Formulations | VFI | March 2018 | March 2022 | April 2023 | 155 | 40 |
| Validity and Translational Aspects of Animal Models in Vaccine Research | SWR | March 2022 | October 2022 | 50 | 47 | |
| Statistics for Vaccine Evaluation Program | BPRC | June 2019 | March 2022 | March 2023 | 40 | 20 |
| Mass Cytometry | CEA | September 2019 | April 2023 | 29 | 24 | |
| Flow Cytometry | CEA | September 2019 | Cancelled | 17 | 12 | |
| In Vivo Imaging | CEA | March 2020 | April 2023 | 14 | 13 | |
| Process Development of Cell Culture Viral Vaccines | VFI/ Merck | September 2019 | September 2021 | 41 | 23 | |
| Application of SPR Technologies in Vaccine Development and Manufacturing | Fraunhofer | October 2019 | November 2021 | 27 | 18 | |
| Process Development and Scale-Up | Fraunhofer | October 2019 | October 2021 | 49 | 27 | |
| Requirements for GMP Production | Fraunhofer | October 2019 | November 2021 | 33 | 24 | |
| Systems Biology of Vaccinology | UNISI | June 2020 | February 2023 | 56 | 36 | |
| Regulatory Aspects of Vaccine Development | EATRIS | November 2020 | March 2022 | March 2023 | 110 | 80 |
| Total | 718 | 426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, W.; Luís, C.; Jungbluth, S.; Slezak, M.; Verreck, F.A.W.; Spiegel, H.; Guzman, C.A.; Roldão, A.; Carrondo, M.J.T.; Van der Ley, P.; et al. Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure. Vaccines 2024, 12, 1446. https://doi.org/10.3390/vaccines12121446
Martin W, Luís C, Jungbluth S, Slezak M, Verreck FAW, Spiegel H, Guzman CA, Roldão A, Carrondo MJT, Van der Ley P, et al. Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure. Vaccines. 2024; 12(12):1446. https://doi.org/10.3390/vaccines12121446
Chicago/Turabian StyleMartin, William, Catarina Luís, Stefan Jungbluth, Monika Slezak, Frank A. W. Verreck, Holger Spiegel, Carlos A. Guzman, António Roldão, Manuel J. T. Carrondo, Peter Van der Ley, and et al. 2024. "Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure" Vaccines 12, no. 12: 1446. https://doi.org/10.3390/vaccines12121446
APA StyleMartin, W., Luís, C., Jungbluth, S., Slezak, M., Verreck, F. A. W., Spiegel, H., Guzman, C. A., Roldão, A., Carrondo, M. J. T., Van der Ley, P., Segalés, J., Dockrell, H. M., Ho, M. M., Pedersen, G. K., Lawrenz, M., & Olesen, O. F. (2024). Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure. Vaccines, 12(12), 1446. https://doi.org/10.3390/vaccines12121446







