Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statements
2.2. Immunization and Sample Collection
2.3. Antibody Measurement
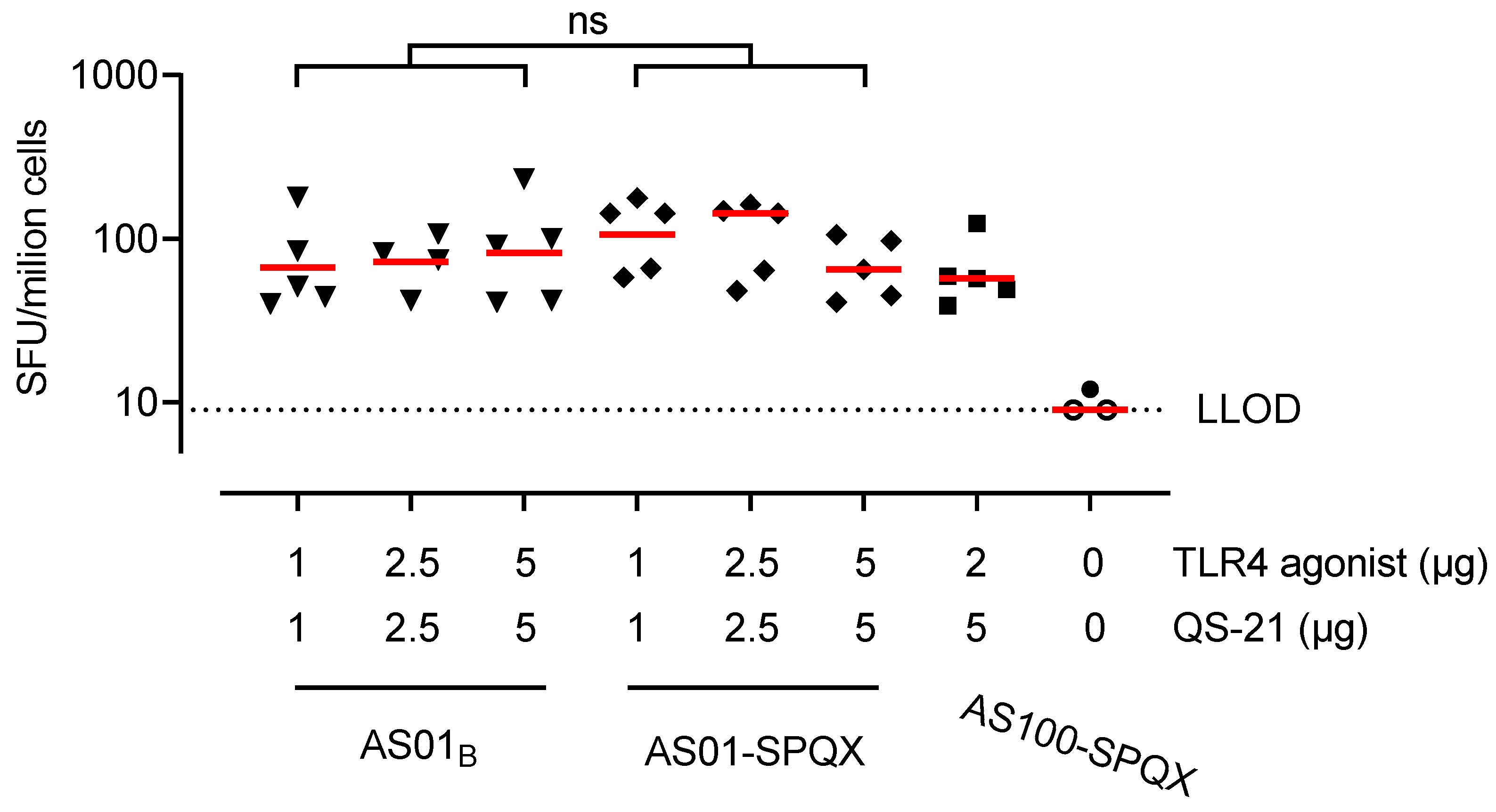
2.4. Measurement of T Cell Responses
2.5. QS-21 Liposome Formulations
| Formulation Name | Source of QS-21 | QS-21 μg/mL | TLR4 Agonist | TRL4 Agonist μg/mL |
|---|---|---|---|---|
| AS01 | Bark extract from GSK | 100 | MPLA | 100 |
| AS01-SPQX | Cell culture from SaponiQx | 100 | PHAD® | 100 |
| AS100-SPQX | Cell culture from SaponiQx | 100 | PHAD® | 40 |
2.6. Statistical Analysis
3. Results
3.1. AS01 Formulated with cpcQS-21 or beQS-21 Elicited Similar Humoral Immune Responses in a Prime-Boost Model of HMPV Vaccination

3.2. HMPV Vaccine Formulations Containing cpcQS-21 or beQS-21 Induced HMPV-Specific T Cell-Mediated Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Mohakud, N.K.; Pena, L.; Kumar, S. Human metapneumovirus: Review of an important respiratory pathogen. Int. J. Infect. Dis. 2014, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Deloria-Knoll, M.; Madhi, S.A.; Cohen, C.; Ali, A.; Basnet, S.; Bassat, Q.; Brooks, W.A.; Chittaganpitch, M.; et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob Health 2021, 9, e33–e43. [Google Scholar] [CrossRef]
- Edwards, K.M.; Zhu, Y.; Griffin, M.R.; Weinberg, G.A.; Hall, C.B.; Szilagyi, P.G.; Staat, M.A.; Iwane, M.; Prill, M.M.; Williams, J.V.; et al. Burden of human metapneumovirus infection in young children. N. Engl. J. Med. 2013, 368, 633–643. [Google Scholar] [CrossRef]
- Englund, J.A.; Boeckh, M.; Kuypers, J.; Nichols, W.G.; Hackman, R.C.; Morrow, R.A.; Fredricks, D.N.; Corey, L. Brief communication: Fatal human metapneumovirus infection in stem-cell transplant recipients. Ann. Intern. Med. 2006, 144, 344–349. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Pulendran, B.; SArunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- den Brok, M.A.-O.; Büll, C.; Wassink, M.; de Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef]
- Ragupathi, G.; Gardner Jr Fau-Livingston, P.O.; Livingston Po Fau-Gin, D.Y.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert. Rev. Vaccines 2011, 10, 463–470. [Google Scholar] [CrossRef]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar] [CrossRef]
- Lv, X.; Martin, J.; Hoover, H.; Joshi, B.; Wilkens, M.; Ullisch, D.A.; Leibold, T.; Juchum, J.S.; Revadkar, S.; Kalinovska, B.; et al. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 2024, 27, 109006. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Syed, Y.Y. Recombinant Zoster Vaccine (Shingrix(®)): A Review in Herpes Zoster. Drugs Aging 2018, 35, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.A.-O.X. RTS, S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccin. Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.A.-O.; Ison, M.A.-O.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 78, 202–209. [Google Scholar] [CrossRef]
- Bakkers, M.J.G.; Ritschel, T.; Tiemessen, M.; Dijkman, J.; Zuffianò, A.A.; Yu, X.; van Overveld, D.; Le, L.; Voorzaat, R.; van Haaren, M.M.; et al. Efficacious human metapneumovirus vaccine based on AI-guided engineering of a closed prefusion trimer. Nat. Commun. 2024, 15, 6270. [Google Scholar] [CrossRef]
- Karron, R.A.; San Mateo, J.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Evaluation of a Live Attenuated Human Metapneumovirus Vaccine in Adults and Children. J. Pediatr. Infect. Dis. Soc. 2018, 7, 86–89. [Google Scholar] [CrossRef]
- Cseke, G.; Wright, D.W.; Tollefson, S.J.; Johnson, J.E.; Crowe, J.E., Jr.; Williams, J.V. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J. Virol. 2007, 81, 698–707. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Kolli, D.; Bataki, E.L.; Spetch, L.; Guerrero-Plata, A.; Jewell, A.M.; Piedra, P.A.; Milligan, G.N.; Garofalo, R.P.; Casola, A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J. Virol. 2008, 82, 8560–8569. [Google Scholar] [CrossRef]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef]
- Miranda-Katz, M.; Erickson, J.J.; Lan, J.; Ecker, A.; Zhang, Y.; Joyce, S.; Williams, J.V. Novel HLA-B7-restricted human metapneumovirus epitopes enhance viral clearance in mice and are recognized by human CD8+ T cells. Sci. Rep. 2021, 11, 20769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Sig. Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.Y.; Barnier-Quer, C.; Heuking, S.; Sommandas, V.; Brunner, L.; Vd Werff, N.; Dubois, P.; Friede, M.; Kocken, C.; Collin, N.; et al. Down selecting adjuvanted vaccine formulations: A comparative method for harmonized evaluation. BMC Immunol. 2018, 19, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Gan, F.; Chen, X.; Deng, K.; Crowe, S.A.; Hudson, G.A.; Belcher, M.S.; Schmidt, M.; Astolfi, M.C.T.; et al. Complete biosynthesis of QS-21 in engineered yeast. Nature 2024, 629, 937–944. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Tan, D.S.; Gin, D.Y. Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis. Acc. Chem. Res. 2016, 49, 1741–1756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swart, M.; Allen, J.; Reed, B.; Izquierdo Gil, A.; Verspuij, J.; Schmit-Tillemans, S.; Chakkumkal, A.; Findeis, M.; Hafner, A.V.; Harjivan, C.; et al. Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate. Vaccines 2024, 12, 1435. https://doi.org/10.3390/vaccines12121435
Swart M, Allen J, Reed B, Izquierdo Gil A, Verspuij J, Schmit-Tillemans S, Chakkumkal A, Findeis M, Hafner AV, Harjivan C, et al. Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate. Vaccines. 2024; 12(12):1435. https://doi.org/10.3390/vaccines12121435
Chicago/Turabian StyleSwart, Maarten, Jessica Allen, Brendan Reed, Ana Izquierdo Gil, Johan Verspuij, Sonja Schmit-Tillemans, Anish Chakkumkal, Mark Findeis, Angela V. Hafner, Chandresh Harjivan, and et al. 2024. "Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate" Vaccines 12, no. 12: 1435. https://doi.org/10.3390/vaccines12121435
APA StyleSwart, M., Allen, J., Reed, B., Izquierdo Gil, A., Verspuij, J., Schmit-Tillemans, S., Chakkumkal, A., Findeis, M., Hafner, A. V., Harjivan, C., Kurnat, R., Kuipers, H., Zahn, R., & Brandenburg, B. (2024). Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate. Vaccines, 12(12), 1435. https://doi.org/10.3390/vaccines12121435







