Vaccination Coverage at Birth in Brazil: Spatial and Temporal Trends in the Impact of COVID-19 on Uptake of BCG and Hepatitis B Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Source of Information
2.3. Spatial Variation in Temporal Trends—SVTT
2.4. Time Series Analysis
2.5. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donalisio, M.R.; Boing, A.C.; Sato, A.P.S.; Martinez, E.Z.; Xavier, M.O.; de Almeida, R.L.F.; Moreira, R.d.S.; Queiroz, R.C.d.S.; Matijasevich, A. Vacinação contra poliomielite no Brasil de 2011 a 2021: Sucessos, reveses e desafios futuros. Ciência Saúde Coletiva 2023, 28, 337. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Immunization Vision and Strategy, 2006–2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Brasil Ministério da Saúde. Programa Nacional de Imunizações: 50 anos. Secretaria de Vigilância em Saúde, Departamento de Imunizações e Doenças Transmissíveis; Ministério da Saúde: Brasília, Brazil, 2023. Available online: https://www.gov.br/saude (accessed on 23 October 2024).
- Domingues, C.M.A.S.; Maranhão, A.G.K.; Teixeira, A.M.; Fantinato, F.F.S.; Domingues, R.A. 46 anos do Programa Nacional de Imunizações: Uma história repleta de conquistas e desafios a serem superados. Cad. de Saúde Pública 2020, 36 (Suppl. 2), e00222919. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.P.S. What is the importance of vaccine hesitancy in the drop of vaccination coverage in Brazil? Rev. Saúde Pública 2018, 52, 96. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.P.S. Pandemia e coberturas vacinais: Desafios para o retorno às escolas. Rev. Saúde Pública 2020, 54, 115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Sirilert, S.; Tongsong, T. Hepatitis B Virus Infection in Pregnancy: Immunological Response, Natural Course and Pregnancy Outcomes. J. Clin. Med. 2021, 10, 2926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hepatitis B Vaccines: WHO Position Paper, July 2017–Recommendations. Vaccine 2019, 37, 223–225. [Google Scholar] [CrossRef]
- Santoli, J.M.; Lindley, M.C.; DeSilva, M.B.; Kharbanda, E.O.; Daley, M.F.; Galloway, L.; Gee, J.; Glover, M.; Herring, B.; Kang, Y.; et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration—United States. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Bramer, C.A. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care Improvement Registry, May 2016–May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 630–631. [Google Scholar] [CrossRef]
- Procianoy, G.S.; Rossini Junior, F.; Lied, A.F.; Jung, L.F.; Souza, M.C. Impacto da pandemia do COVID-19 na vacinação de crianças de até um ano de idade: Um estudo ecológico. Cienc. Saude Coletiva 2022, 27, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.; Vieira, S.C.F.; Barreto, I.D.C.; de Gois-Santos, V.T.; Celestino, A.O.; Domingues, C.; Cuevas, L.E.; Gurgel, R.Q. Effects of the COVID-19 pandemic on routine pediatric vaccination in Brazil. Expert Rev. Vaccines 2021, 20, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Skirrow, H.; Bedford, H. Routine vaccination during covid-19 pandemic response. BMJ 2020, 369, m2392. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.I.; Tessier, E.; White, J.M.; Woodruff, M.; Knowles, C.; Bates, C.; Parry, J.; Walker, J.L.; Scott, J.A.; Smeeth, L.; et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Eurosurveillance 2020, 25, 2000848. [Google Scholar] [CrossRef] [PubMed]
- Sangeda, R.Z.; James, D.; Mariki, H.; Mbwambo, M.E.; Mwenesi, M.E.; Nyaki, H.; Tinuga, F.; Manyanga, D.P. Childhood vaccination trends during 2019 to 2022 in Tanzania and the impact of the COVID-19 pandemic. Hum. Vaccines Immunother. 2024, 20, 2356342. [Google Scholar] [CrossRef]
- Skirrow, H.; Lewis, C.; Haque, H.; Choundary-Salter, L.; Foley, K.; Whittaker, E.; Costelloe, C.; Bedford, H.; Saxena, S. The impact of the COVID-19 pandemic on UK parents’ attitudes towards routine childhood vaccines: A mixed-methods study. PLoS ONE 2024, 19, e0306484. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Implementing the Immunization Agenda 2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Brasil Ministério da Saúde; Secretaria de Vigilância em Saúde e Ambiente; Departamento de Imunização e Doenças Imunopreveníveis. Estratégia de Multivacinação para Atualização da Caderneta de Vacinação da Criança e do Adolescente [recurso eletrônico]/Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente, Departamento de Imunização e Doenças Imunopreveníveis, 1ª ed. rev.; Ministério da Saúde: Brasília, Brazil, 2023; 25p. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/estrategia_multivacinacao_atualizacao_caderneta_criancaeadolescente.pdf (accessed on 27 November 2024).
- Brasil Ministério da Saúde. Queda na Proteção. Em 2021, Cobertura da Vacina BCG em Bebês foi a Menor em uma Década; Ministério da Saúde: Brasília, Brazil, 2023. Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/2023/fevereiro/em-2021-cobertura-da-vacina-bcg-em-bebes-foi-a-menor-em-uma-decada (accessed on 27 November 2024).
- Brasil Ministério da Saúde. Alerta. Percentual de Bebês Vacinados Contra Hepatite B é um dos Menores da História; Ministério da Saúde: Brasília, Brazil, 2023. Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/2023/fevereiro/percentual-de-bebes-vacinados-contra-hepatite-b-e-um-dos-menores-da-historia (accessed on 28 November 2024).
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Kleinbaum, D.G.; Kupper, L.L.; Morgenstern, H. Epidemiologic Research: Principles and Quantitative Methods, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística. Áreas Territoriais. 2022. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/estrutura-territorial/15761-areas-dos-municipios.html#:~:text=Trata%2Dse%20do%20reprocessamento%20anual,do%20recebimento%20das%20atualiza%C3%A7%C3%B5es%20territoriais (accessed on 24 October 2024).
- Kulldorff, M.; Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 1995, 14, 799–810. [Google Scholar] [CrossRef]
- Moraga, P.; Kulldorff, M. Detection of spatial variations in temporal trends with a quadratic function. Stat. Methods Med. Res. 2016, 25, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. SaTScan User Guide V9.4. SaTScan TM User Guid Version 94. 2015. Available online: https://studylib.net/doc/18657818/satscan-user-guide (accessed on 25 October 2024).
- Jaisankar, R.; Kesavan, J. A study on spatial variations in temporal trends of dengue incidences in Tamil Nadu, India. Int. J. Sci. Technol. Res. 2019, 8, 788–792. [Google Scholar]
- Han, J.; Zhu, L.; Kulldorff, M.; Hostovich, S.; Stinchcomb, D.G.; Tatalovich, Z.; Lewis, D.R.; Feuer, E.J. Using Gini coefficient to determining optimal cluster reporting sizes for spatial scan statistics. Int. J. Health Geogr. 2016, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, R.; Cleveland, W.; McRee, J.E. Seasonal-trend decomposition procedure based on LOESS. J. Off. Stat. 1990, 6, 3–73. [Google Scholar]
- Hyndman, R.; Bergmeir, C.; Caceres, G.; Chhay, L.; Kuroptev, K.; O’Hara-Wild, M.; Petropoulos, F.; Razbash, S.; Wang, E.; Yasmeen, F.; et al. Package ‘Forecast’. 2020. Available online: https://cran.r-project.org/web/packages/forecast/forecast.pdf (accessed on 18 October 2024).
- Antunes, J.L.F.; Cardoso, M.R.A. Uso da análise de séries temporais em estudos epidemiológicos. Epidemiol. Serv. Saúde 2015, 24, 565–576. Available online: http://www.scielo.br/pdf/ress/v24n3/2237-9622-ress-24-03-00565.pdf (accessed on 17 October 2024). [CrossRef]
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12174032 (accessed on 18 October 2024). [CrossRef]
- Berra, T.Z.; Alves, Y.M.; Popolin, M.A.; da Costa, F.B.P.; Tavares, R.B.V.; Tártaro, A.F.; Moura, H.S.D.; Ferezin, L.P.; de Campos, M.C.T.; Ribeiro, N.M.; et al. The COVID-19 pandemic in Brazil: Space-time approach of cases, deaths, and vaccination coverage (February 2020–April 2024). BMC Infect. Dis. 2024, 24, 704. [Google Scholar] [CrossRef]
- de Cantuária Tauil, M.; Sato, A.P.; Waldman, E.A. Factors associated with incomplete or delayed vaccination across countries: A systematic review. Vaccine 2016, 34, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; De Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, A.R.; Jones, N.S. The state of vaccine confidence 2016: Global insights through a 67-country survey. eBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Césare, N.; Mota, T.F.; Lopes, F.F.; Lima, A.C.M.; Luzardo, R.; Quintanilha, L.F.; Andrade, B.B.; Queiroz, A.T.; Fukutani, K.F. Longitudinal profiling of the vaccination coverage in Brazil reveals a recent change in the patterns hallmarked by differential reduction across regions. Int. J. Infect. Dis. 2020, 98, 275–280. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Immunization Coverage. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 25 October 2024).
- Souto, E.P.; Fernandez, M.V.; Rosário, C.A.; Petra, P.C.; Matta, G.C. Hesitação vacinal infantil e COVID-19: Uma análise a partir da percepção dos profissionais de saúde. Cad. De Saúde Pública 2024, 40, e00061523. [Google Scholar] [CrossRef]
- Moore, D.C.B.C.; Nehab, M.F.; Camacho, K.G.; Reis, A.T.; Junqueira-Marinho, M.d.F.; Abramov, D.M.; de Azevedo, Z.M.A.; de Menezes, L.A.; Salú, M.d.S.; Figueiredo, C.E.d.S.; et al. Low COVID-19 vaccine hesitancy in Brazil. Vaccine 2021, 39, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Dhamania, M.; Gaur, K. Impact of COVID-19 pandemic on routine immunization services in a tertiary care hospital of Rajasthan, India. Clin. Exp. Vaccine Res. 2023, 12, 313. [Google Scholar] [PubMed]
- Cruz, A. A queda da imunização no Brasil. Rev. Consens. 2017, 7, 20–29. [Google Scholar]
- Silveira, M.M.; Conrad, N.L.; Leivas Leite, F.P. Effect of COVID-19 on vaccination coverage in Brazil. J. Med. Microbiol. 2021, 70, 001466. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.H.; Ramos, A.C.V.; Yamamura, M.; Weiller, T.H.; Crispim, J.d.A.; Cartagena-Ramos, D.; Fuentealba-Torres, M.; dos Santos, D.T.; Palha, P.F.; Arcêncio, R.A. Áreas com queda da cobertura vacinal para BCG, poliomielite e tríplice viral no Brasil (2006–2016): Mapas da heterogeneidade regional. Cad. De Saúde Pública 2020, 36, e00015619. [Google Scholar] [CrossRef]
- Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). Butantan já Produz Vacina Contra Hepatite B. 2023. Available online: https://revistapesquisa.fapesp.br/butantan-ja-produz-vacina-contra-hepatite-b/ (accessed on 29 October 2024).
- Butanta. Portal Butanta. Vacinas. 2024. Available online: https://butantan.gov.br/soros-e-vacinas/vacinas (accessed on 29 October 2024).
- Alexandre, K.V.F.; Martins, R.M.B.; Souza, M.M.D.; Rodrigues, I.M.X.; Teles, S.A. Brazilian hepatitis B vaccine: A six-year follow-up in adolescents. Memórias Inst. Oswaldo Cruz 2012, 107, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, Y.d.S. Fatores que Interferem nos Índices de Coberturas Vacinais: Uma Revisão Integrativa. 2021. Available online: https://ri.ucsal.br/items/5ca52888-4d79-4860-bf0d-86ec425ec3b3 (accessed on 29 October 2024).
- Anderson, K.-A.M.; Creanza, N. Internal and external factors affecting vaccination coverage: Modeling the interactions between vaccine hesitancy, accessibility, and mandates. PLoS Glob. Public Health 2023, 3, e0001186. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, A.; Carrasco-Escobar, G.; Richardson, R.; Benmarhnia, T. Essential childhood immunization in 43 low- and middle-income countries: Analysis of spatial trends and socioeconomic inequalities in vaccine coverage. PLoS Med. 2023, 20, e1004166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brasil Ministério da Saúde. Municípios Começam a Aplicar Estratégias Para Aumentar Coberturas Vacinais; Ministério da Saúde: Brasília, Brazil, 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/2024/junho/municipios-comecam-a-aplicar-estrategias-para-aumentar-coberturas-vacinais#:~:text=Munic%C3%ADpios%20come%C3%A7am%20a%20aplicar%20estrat%C3%A9gias%20para%20aumentar%20coberturas%20vacinais,-Microplanejamento%20ensina%20os&text=O%20Minist%C3%A9rio%20da%20Sa%C3%BAde%20concluiu,para%20aumentar%20as%20coberturas%20vacinais (accessed on 30 November 2024).
- Vulnerabilidade Social no Brasil: Como Anda o Amparo a População? Oxfam Brasil. 2021. Available online: https://www.oxfam.org.br/blog/vulnerabilidade-social-no-brasil-como-anda-o-amparo-a-populacao/ (accessed on 30 November 2024).
- Organização Pan-Americana da Saúde. Princípios Norteadores Para as Atividades de Imunização Durante a Pandemia do Vírus COVID-19. Orientação Provisória, 26 de Março de 2020. 2020. Available online: https://iris.paho.org/handle/10665.2/51989 (accessed on 1 December 2024).
- Sato, A.P.S. National Immunization Program: Computerized System as a tool for new challenges. Rev. De Saúde Pública 2015, 49, 39. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.d.L.; Neto, M.; Souza-Santos, R. Heterogeneidade espaço-temporal dos indicadores de imunização da vacina tríplice viral em crianças no Brasil. Rev. Panam. De Salud Pública 2024, 48, e34. [Google Scholar] [CrossRef]
- Dias, L.C. Movimento antivacinas: Uma séria ameaça à saúde global. J. Da Unicamp 2020. Available online: https://unicamp.br/unicamp/ju/artigos/luiz-carlos-dias/movimento-antivacinas-uma-seria-ameaca-saude-global/ (accessed on 27 November 2024).
- Da Conceição Ramos, A.C.L.; Pacheco, B.d.A.B.; Sousa, J.E.A.; Petrilli, J.D.; Costa, G.N.d.O. Cobertura vacinal e o movimento antivacina: O impacto na saúde pública no Brasil. Rev. Baiana De Saúde Pública 2023, 47, 210–226. [Google Scholar] [CrossRef]
- Rochel de Camargo, K., Jr. Here we go again: The reemergence of anti-vaccine activism on the Internet. Cad. Saúde Pública 2020, 36, e00037620. [Google Scholar] [CrossRef] [PubMed]


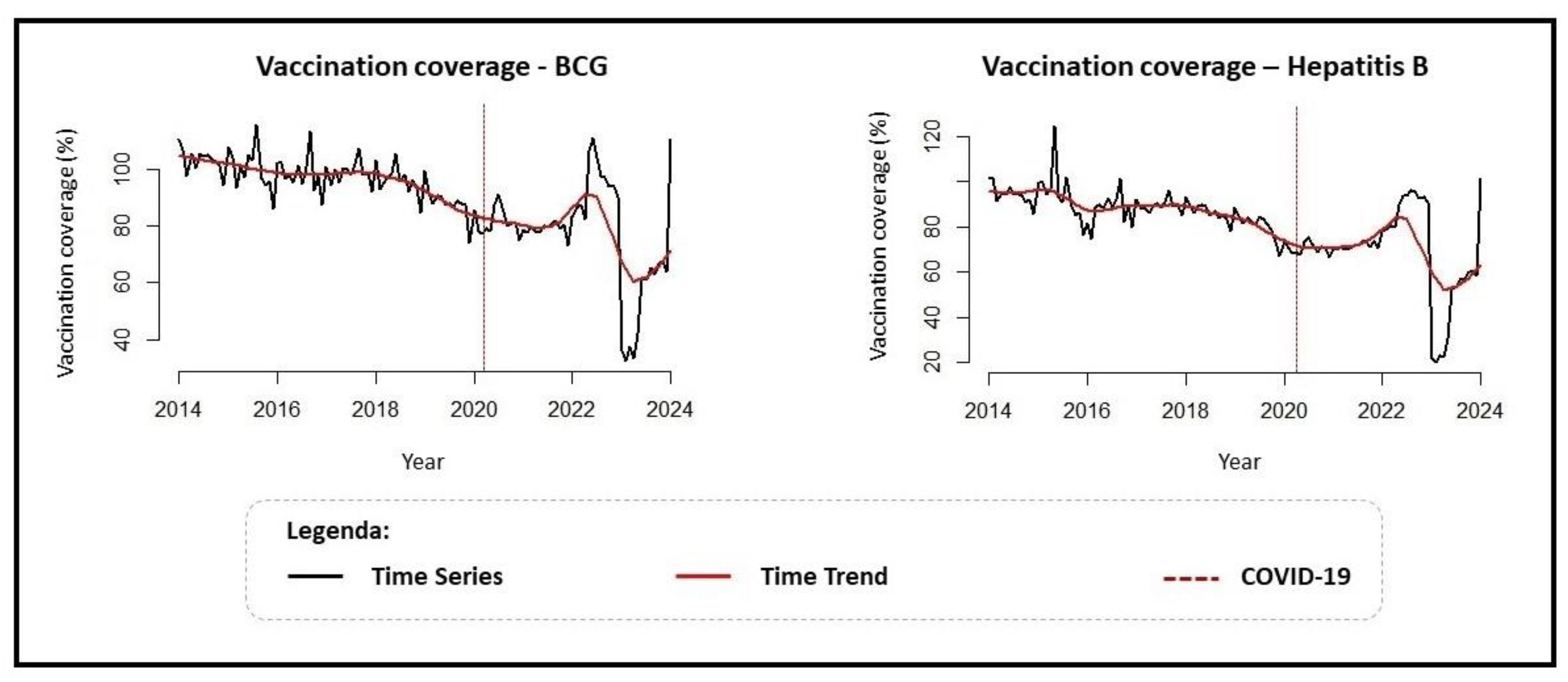
| TimeSeries Analysis (Prais–Winsten) | |||
| Immunizer | Coefficient (95% CI) | Trend | MPC (95% CI) |
| BCG | −0.001 (−0.002; <−0.000) | Descending | −0.43% (−0.66; −0.19) |
| Hepatitis B | −0.002 (−0.003; <−0.000) | Descending | −0.49% (−0.81; −0.16) |
| Interrupted Time Series Intervention: COVID-19 (Cutoff Point: Feb 2020) | |||
| Immunizer | Coefficient (95% CI) | Trend | MPC (95% CI) |
| BCG | −0.033 (−0.116; 0.049) | Stationary | - |
| Hepatitis B | −0.026 (−0.140; 0.086) | Stationary | - |
| Interrupted Time Series Post-Intervention: Post-COVID-19 | |||
| Immunizer | Coefficient (95% CI) | Trend | MPC (95% CI) |
| BCG | −0.03 (−0.006; <−0.000) | Descending | −0.81% (−23.60; −0.09) |
| Hepatitis B | −0.004 (−0.008; 0.001) | Stationary | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, Y.M.; Berra, T.Z.; Tavares, R.B.V.; Zini, N.; Ferreira, Q.R.; Andrade, L.K.d.A.; Tártaro, A.F.; Pelodan, M.E.P.; Vigato, B.F.; Silveira, B.K.; et al. Vaccination Coverage at Birth in Brazil: Spatial and Temporal Trends in the Impact of COVID-19 on Uptake of BCG and Hepatitis B Vaccines. Vaccines 2024, 12, 1434. https://doi.org/10.3390/vaccines12121434
Alves YM, Berra TZ, Tavares RBV, Zini N, Ferreira QR, Andrade LKdA, Tártaro AF, Pelodan MEP, Vigato BF, Silveira BK, et al. Vaccination Coverage at Birth in Brazil: Spatial and Temporal Trends in the Impact of COVID-19 on Uptake of BCG and Hepatitis B Vaccines. Vaccines. 2024; 12(12):1434. https://doi.org/10.3390/vaccines12121434
Chicago/Turabian StyleAlves, Yan Mathias, Thaís Zamboni Berra, Reginaldo Bazon Vaz Tavares, Nathalia Zini, Quézia Rosa Ferreira, Licia Kellen de Almeida Andrade, Ariela Fehr Tártaro, Maria Eduarda Pagano Pelodan, Beatriz Fornaziero Vigato, Beatriz Kuroda Silveira, and et al. 2024. "Vaccination Coverage at Birth in Brazil: Spatial and Temporal Trends in the Impact of COVID-19 on Uptake of BCG and Hepatitis B Vaccines" Vaccines 12, no. 12: 1434. https://doi.org/10.3390/vaccines12121434
APA StyleAlves, Y. M., Berra, T. Z., Tavares, R. B. V., Zini, N., Ferreira, Q. R., Andrade, L. K. d. A., Tártaro, A. F., Pelodan, M. E. P., Vigato, B. F., Silveira, B. K., Assumpção, A. L. B. N. M., Popolin, M. A. P., Curvo, P. A., Protti-Zanatta, S., Serrano-Gallardo, M. D. P., Arcêncio, R. A., Palha, P. F., & Ballestero, J. G. d. A. (2024). Vaccination Coverage at Birth in Brazil: Spatial and Temporal Trends in the Impact of COVID-19 on Uptake of BCG and Hepatitis B Vaccines. Vaccines, 12(12), 1434. https://doi.org/10.3390/vaccines12121434





