Cardiac Function Evaluation after SARS-CoV-2 mRNA Vaccination in Children and Adolescents: A Prospective Speckle-Tracking Echocardiography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
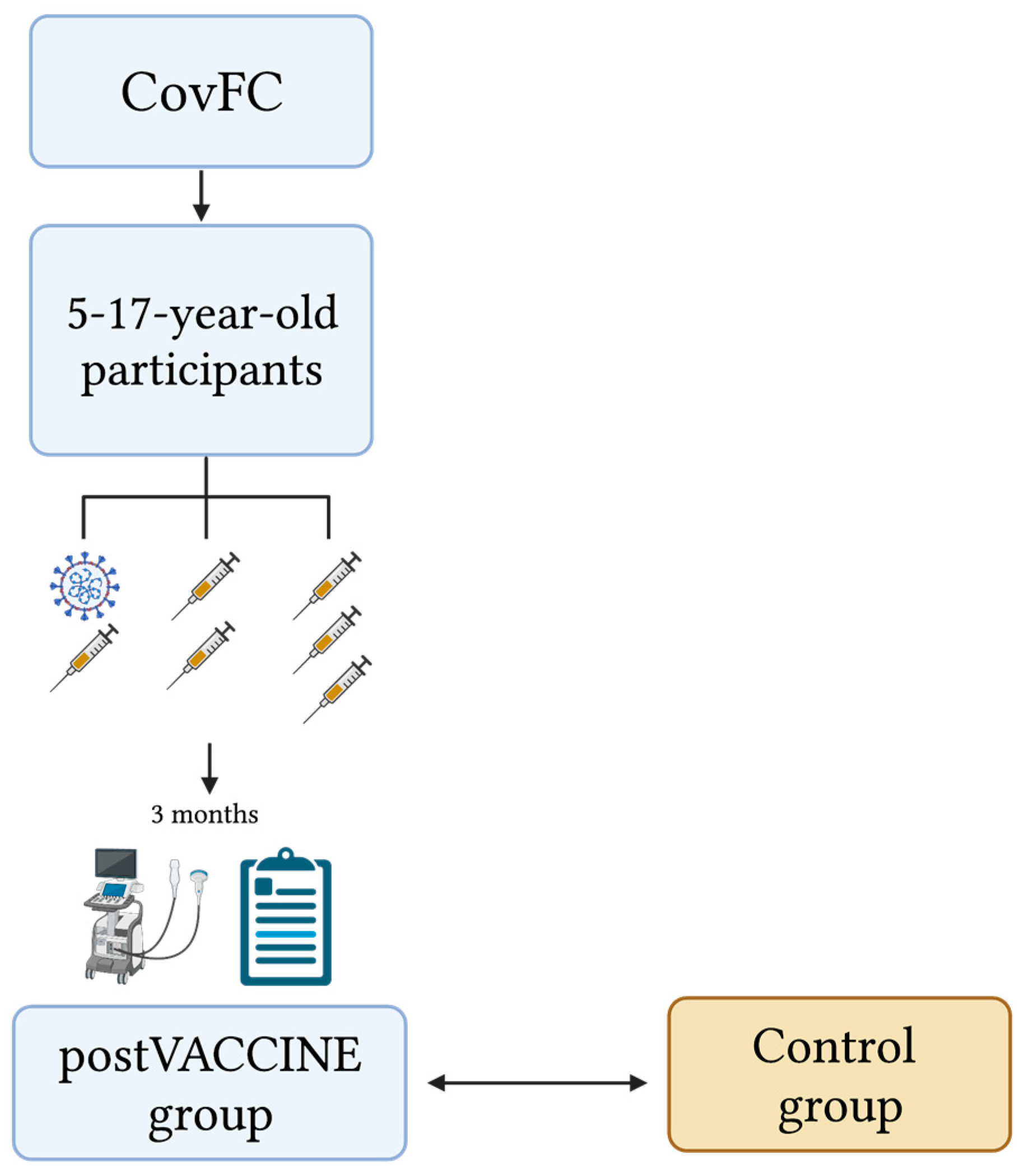
2.2. Study Design, Study Population, and Definitions
2.3. Control Group
2.4. Cardiac Evaluation
2.5. Reproducibility
2.6. Statistical Analysis
3. Results
3.1. Study Population Characteristics
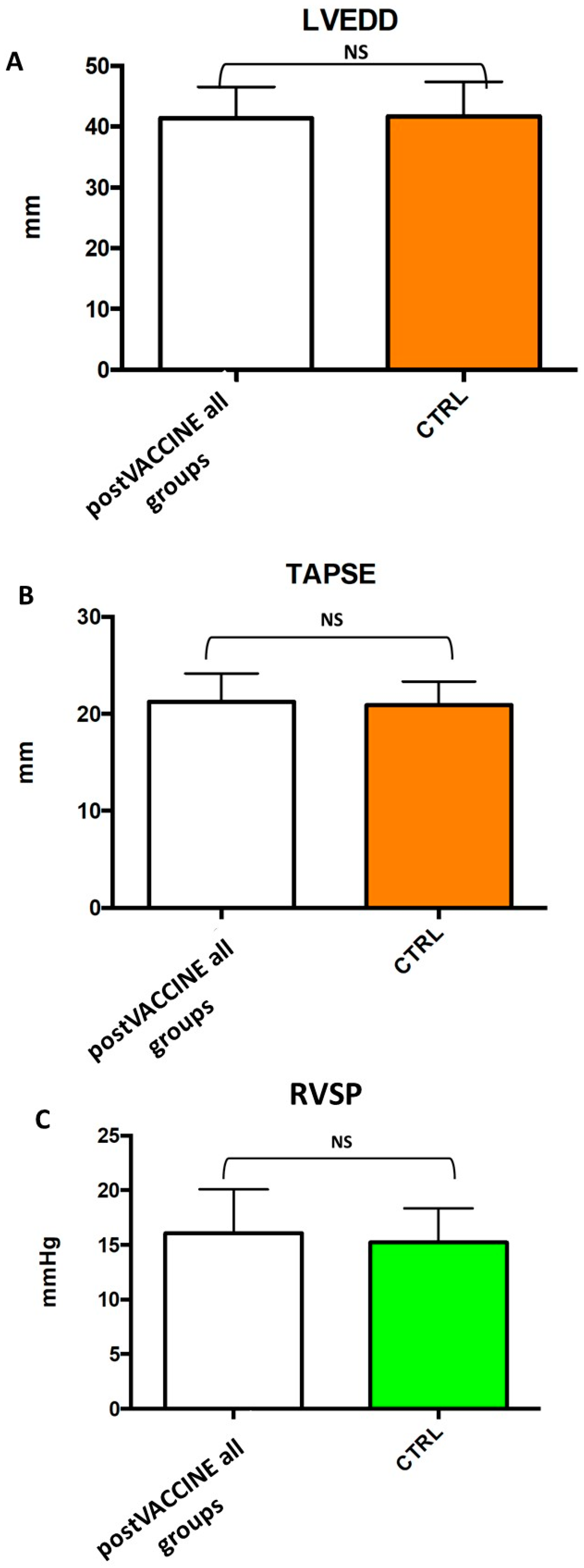
3.2. Standard Echocardiographic Measurements
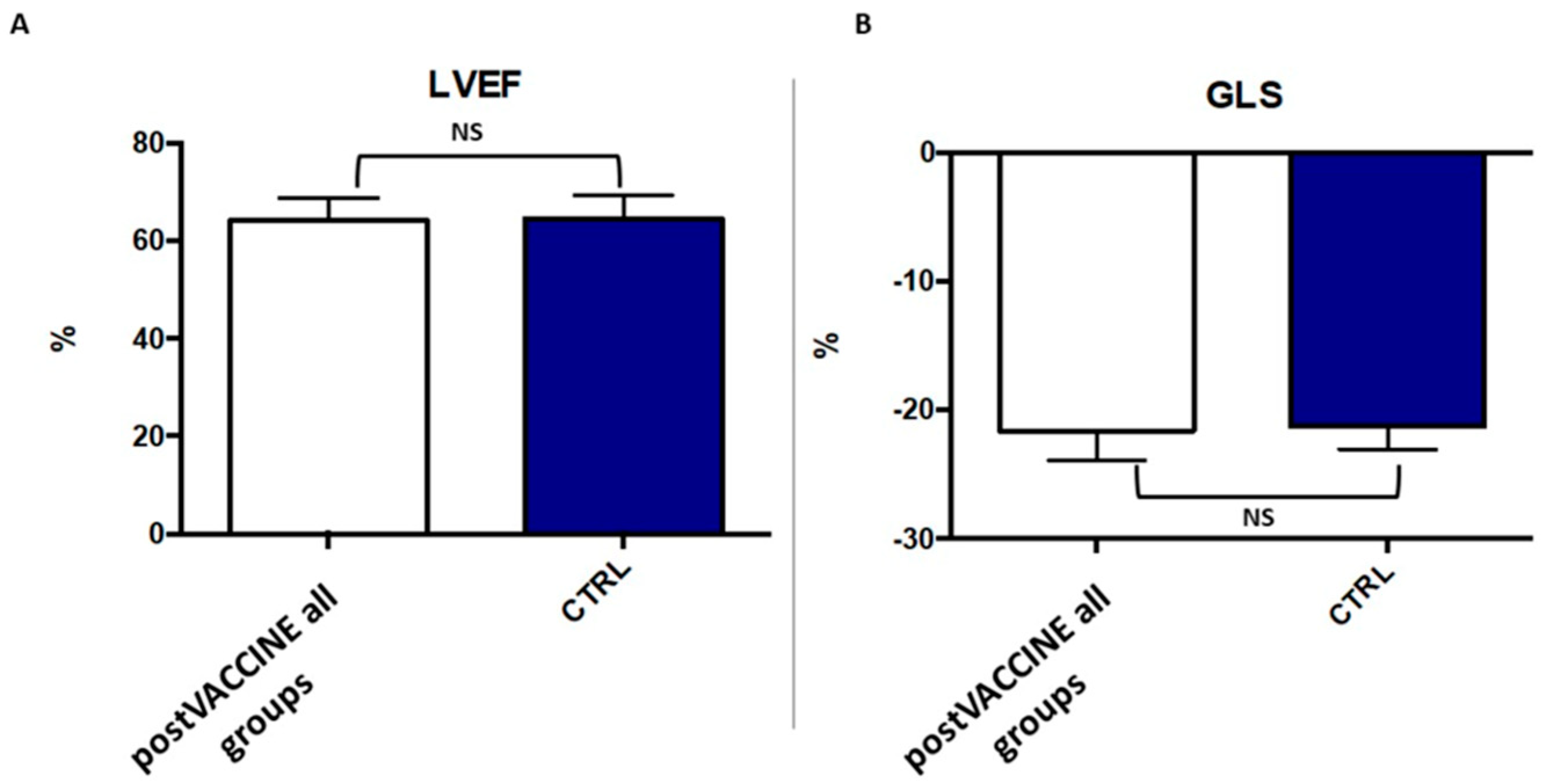
3.3. Left Ventricular Longitudinal Strain
3.4. Reproducibility Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA—J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef] [PubMed]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Pellegrino, R.; Chiappini, E.; Licari, A.; Galli, L.; Marseglia, G.L. Prevalence and clinical presentation of long COVID in children: A systematic review. Eur. J. Pediatr. 2022, 181, 3995–4009. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Recher, M.; Hubert, H.; Javouhey, E.; Fléchelles, O.; Leteurtre, S.; Angoulvant, F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 2022, 327, 281–283. [Google Scholar] [CrossRef]
- Jain, E.; Donowitz, J.R.; Aarons, E.; Marshall, B.C.; Miller, M.P. Multisystem Inflammatory Syndrome in Children after SARS-CoV-2 Vaccination. Emerg. Infect. Dis. 2022, 28, 990–993. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Tran, V.T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms of patients with long covid: Target trial emulation based on ComPaRe e-cohort. BMJ Med. 2023, 2, e000229. [Google Scholar] [CrossRef]
- Larson, H.J. The Vaccine-Hesitant Moment. Reply. N. Engl. J. Med. 2022, 387, 1050–1051. [Google Scholar] [CrossRef]
- Ten Threats to Global Health in 2019, WHO. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 26 March 2023).
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nature reviews. Cardiology 2022, 19, 75–77. [Google Scholar] [CrossRef]
- Report Italiano Vaccini Anti-COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 12 March 2023).
- Machingaidze, S.; Wiysonge, C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021, 27, 1338–1339. [Google Scholar] [CrossRef]
- Wang, Q.; Xiu, S.; Yang, L.; Han, Y.; Cui, T.; Shi, N.; Liu, M.; Yi, Y.; Liu, C.; Wang, X.; et al. Changes in Parental Attitudes Toward COVID-19 Vaccination and Routine Childhood Vaccination During the COVID-19 Pandemic: Repeated Cross-sectional Survey Study. JMIR Public Health Surveill. 2022, 8, e33235. [Google Scholar] [CrossRef]
- Aw, J.; Seng, J.J.B.; Seah, S.S.Y.; Low, L.L. COVID-19 Vaccine Hesitancy-A Scoping Review of Literature in High-Income Countries. Vaccines 2021, 9, 900. [Google Scholar] [CrossRef]
- Suran, M. Why Parents Still Hesitate to Vaccinate Their Children Against COVID-19. JAMA 2022, 327, 23–25. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Sirico, D.; Di Chiara, C.; Costenaro, P.; Bonfante, F.; Cozzani, S.; Plebani, M.; Reffo, E.; Castaldi, B.; Donà, D.; Da Dalt, L.; et al. Left ventricular longitudinal strain alterations in asymptomatic or mildly symptomatic paediatric patients with SARS-CoV-2 infection. European heart journal. Cardiovasc. Imaging 2022, 23, 1083–1089. [Google Scholar] [CrossRef]
- Wassenaar, J.W.; Clark, D.E.; Dixon, D.D.; George-Durrett, K.; Parikh, A.; Li, D.L.; Baker, M.T.; Gupta, D.K.; Hughes, S.G.; Soslow, J.H.; et al. Reduced Circumferential Strain in Athletes with Prior COVID-19. Radiol. Cardiothorac. Imaging 2022, 4, e210310. [Google Scholar] [CrossRef]
- Sabatino, J.; Di Chiara, C.; Di Candia, A.; Sirico, D.; Donà, D.; Fumanelli, J.; Basso, A.; Pogacnik, P.; Cuppini, E.; Romano, L.R.; et al. Mid- and Long-Term Atrio-Ventricular Functional Changes in Children after Recovery from COVID-19. J. Clin. Med. 2022, 12, 186. [Google Scholar] [CrossRef]
- Liu, K.; Yu, J.; Song, G. Global Myocardial Strain in Multisystem Inflammatory Syndrome in Children, Kawasaki Disease, and Healthy Children: A Network Meta-Analysis. Front. Pediatr. 2022, 10, 848306. [Google Scholar] [CrossRef]
- Piccinelli, E.; Bautista-Rodriguez, C.; Herberg, J.; Kang, H.; Krupickova, S.; Altamar, I.B.; Moscatelli, S.; Sabatino, S.; Josen, M.; Paredes, J.; et al. Segmental and global longitudinal strain differences between Kawasaki disease and multi-system inflammatory syndrome in children. Cardiol. Young 2022, 1–7. [Google Scholar] [CrossRef]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 951314. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Di Chiara, C.; Cantarutti, A.; Costenaro, P.; Donà, D.; Bonfante, F.; Cosma, C.; Ferrarese, M.; Cozzani, S.; Petrara, M.R.; Carmona, F.; et al. Long-term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection. JAMA Netw. Open 2022, 5, e2221616. [Google Scholar] [CrossRef]
- Bonfante, F.; Costenaro, P.; Cantarutti, A.; Di Chiara, C.; Bortolami, A.; Petrara, M.R.; Carmona, F.; Pagliari, M.; Cosma, C.; Cozzani, S.; et al. Mild SARS-CoV-2 Infections and Neutralizing Antibody Titers. Pediatrics 2021, 148, e2021052173. [Google Scholar] [CrossRef]
- Chen, C.; Nadeau, S.; Yared, M.; Voinov, P.; Ning, X.; Roemer, C.; Stadler, T. CoV-Spectrum: Analysis of globally shared SARS-CoV-2 data to Identify and Characterize New Variants. Bioinformatics 2022, 38, 1735–1737. [Google Scholar] [CrossRef]
- Clinical Management. Available online: https://apps.who.int/iris/bitstream/handle/10665/338871/WHO-2019-nCoV-clinical-web_annex-2021.1-eng.pdf (accessed on 15 January 2022).
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio Medica Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Lai, W.W.; Cohen, M.S.; Geva, T.; Mertens, L. Echocardiography in Pediatric and Congenital Heart Disease; Wiley-Blackwell: West Sussex, UK, 2009; pp. 765–785. [Google Scholar]
- Kuehn, B.M. Myocarditis adverse event less common after COVID-19 vaccine booster. JAMA 2022, 327, 1324. [Google Scholar] [CrossRef]
- Julious, S.A. Sample sizes for clinical trials with Normal data. Stat. Med. 2004, 23, 1921–1986. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 vaccine and myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef]
- Cordero, A.; Cazorla, D.; Escribano, D.; Quintanilla, M.A.; López-Ayala, J.M.; Berbel, P.P.; Bertomeu-González, V. Myocarditis after RNA-based vaccines for coronavirus. Int. J. Cardiol. 2022, 353, 131–134. [Google Scholar] [CrossRef]
- Bellos, I.; Karageorgiou, V.; Viskin, D. Myocarditis following mRNA COVID-19 vaccination: A pooled analysis. Vaccine 2022, 40, 1768–1774. [Google Scholar] [CrossRef]
- Yasuhara, J.; Masuda, K.; Aikawa, T.; Shirasu, T.; Takagi, H.; Lee, S.; Kuno, T. Myopericarditis After COVID-19 mRNA Vaccination Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 42–52. [Google Scholar] [CrossRef]
- Sirico, D.; Basso, A.; Sabatino, J.; Reffo, E.; Cavaliere, A.; Biffanti, R.; Cerutti, A.; Castaldi, B.; Zulian, F.; Da Dalt, L.; et al. Evolution of echocardiographic and cardiac magnetic resonance imaging abnormalities during follow-up in patients with multisystem inflammatory syndrome in children. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1066–1074. [Google Scholar] [CrossRef]
- First COVID-19 Vaccine Approved for Children Aged 12 to 15 in EU|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty#:~:text=Comirnaty%20received%20a%20conditional%20marketing,authorisation%20on%2010%20October%202022 (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva il Vaccino BioNTech/Pfizer. Comunicato AIFA n. 620 22 Dicembre 2020. Available online: https://www.aifa.gov.it/documents/20142/0/Comunicato%20AIFA%20n.%20620%20-%20Autorizzato%20il%20vaccino%20BioNTech%20Pfizer.%20Sul%20sito%20AIFA%20risposte%20alle%20domande%20pi%C3%B9%20frequenti(1).pdf (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva la Dose Booster con il Vaccino Comirnaty per la Fascia di età a partire dai 16 anni. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=84077&parte=1%20&serie=null (accessed on 4 February 2022).
- First COVID-19 Vaccine Approved for Children Aged 12 to 15 in EU|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva l’utilizzo del vaccino Comirnaty per la fascia di età 12–15 anni. Comunicato AIFA n. 647 31 Maggio 2021. Available online: https://www.aifa.gov.it/documents/20142/1289678/Comunicato_stampa_AIFA_n.647.pdf (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva la dose Booster con il vaccino Comirnaty per la fascia di età 12–15 anni. Comunicato AIFA n. 681 5 Gennaio 2022. Available online: https://www.aifa.gov.it/documents/20142/1618244/Comunicato_AIFA_N.681.pdf (accessed on 4 February 2022).
- COVID-19 Vaccine Spikevax Approved for Children Aged 12 to 17 in EU|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-spikevax-approved-children-aged-12-17-eu (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva l’utilizzo del Vaccino Spikevax (Moderna) per la fascia di età 12–17 anni. Comunicato AIFA n. 656 28 Luglio 2021. Available online: https://www.aifa.gov.it/documents/20142/1289678/Comunicato_AIFA_656.pdf (accessed on 4 February 2022).
- Comirnaty COVID-19 Vaccine: EMA Recommends Approval for Children Aged 5 to 11|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11 (accessed on 4 February 2022).
- La Commissione Tecnico Scientifica di AIFA (CTS) Approva il Vaccino Comirnaty per la fascia di età 5–11 anni. Comunicato AIFA n. 674 1 Dicembre 2021. Available online: https://www.aifa.gov.it/documents/20142/1289678/Comunicato_AIFA_674.pdf (accessed on 4 February 2022).

| CTRL (n = 39) | post-VACCINE (All Groups) (n = 39) | post-VACCINE+ COVID-19 (n = 7) | p Value between CTRL (n = 39) and post-VACCINE (n = 39) Groups | |
|---|---|---|---|---|
| Age (years) | 12.7 ± 3.1 | 12.6 ± 2.6 | 12.7 ± 3.2 | 0.937 |
| Female. n (%) | 24 (61%) | 24 (61%) | 7 (100%) | 0.817 |
| BSA (m2) | 1.41 ± 0.27 | 1.36 ± 0.24 | 1.27 ± 0.27 | 0.319 |
| LVEDD | 41.7 ± 5.7 | 41.6 ± 5.2 | 39.4 ± 3.3 | 0.940 |
| LVEDD Z score | −0.7 ± 1.0 | −0.5 ± 1.0 | −0.8 ± 0.7 | 0.467 |
| LVESD (mm) | 26.1 ± 4.2 | 26.1 ± 3.5 | 24.6 ± 3.5 | 0.999 |
| LVESD Z score | −0.4 ± 1.0 | −0.3 ± 0.9 | −0.6 ± 0.9 | 0.645 |
| IVSd (mm) | 7.3 ± 1.3 | 7.5 ± 1.6 | 7.5 ± 1.2 | 0.624 |
| IVSd Z score | +0.3 ± 0.8 | +0.4 ± 0.9 | +0.6 ± 0.4 | 0.695 |
| PWd (mm) | 6.9 ± 1.1 | 7.0 ± 1.4 | 7.1 ± 1.4 | 0.626 |
| PWd Z score | +0.5 ± 0.6 | +0.6 ± 0.9 | +0.9 ± 0.9 | 0.780 |
| RVSP (mmHg) | 15.2 ± 3.1 | 16.1 ± 4.0 | 14.3 ± 1.1 | 0.394 |
| TAPSE (mm) | 21.0 ± 2.4 | 21.3 ± 2.9 | 21.5 ± 2.8 | 0.575 |
| LVEF (%) | 64.4 ± 4.7 | 64.1 ± 4.7 | 62.4 ± 3.1 | 0.732 |
| GLS (%) | −21.2 ± 1.8 | −21.7 ± 2.3 | −19.9 ± 1.1 | 0.338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatino, J.; Di Chiara, C.; Lauretta, D.; Fumanelli, J.; D’Ascoli, G.L.; Donà, D.; Cozzani, S.; Oletto, A.; Giaquinto, C.; Di Salvo, G. Cardiac Function Evaluation after SARS-CoV-2 mRNA Vaccination in Children and Adolescents: A Prospective Speckle-Tracking Echocardiography Study. Vaccines 2023, 11, 1348. https://doi.org/10.3390/vaccines11081348
Sabatino J, Di Chiara C, Lauretta D, Fumanelli J, D’Ascoli GL, Donà D, Cozzani S, Oletto A, Giaquinto C, Di Salvo G. Cardiac Function Evaluation after SARS-CoV-2 mRNA Vaccination in Children and Adolescents: A Prospective Speckle-Tracking Echocardiography Study. Vaccines. 2023; 11(8):1348. https://doi.org/10.3390/vaccines11081348
Chicago/Turabian StyleSabatino, Jolanda, Costanza Di Chiara, Daria Lauretta, Jennifer Fumanelli, Greta Luana D’Ascoli, Daniele Donà, Sandra Cozzani, Andrea Oletto, Carlo Giaquinto, and Giovanni Di Salvo. 2023. "Cardiac Function Evaluation after SARS-CoV-2 mRNA Vaccination in Children and Adolescents: A Prospective Speckle-Tracking Echocardiography Study" Vaccines 11, no. 8: 1348. https://doi.org/10.3390/vaccines11081348
APA StyleSabatino, J., Di Chiara, C., Lauretta, D., Fumanelli, J., D’Ascoli, G. L., Donà, D., Cozzani, S., Oletto, A., Giaquinto, C., & Di Salvo, G. (2023). Cardiac Function Evaluation after SARS-CoV-2 mRNA Vaccination in Children and Adolescents: A Prospective Speckle-Tracking Echocardiography Study. Vaccines, 11(8), 1348. https://doi.org/10.3390/vaccines11081348








