Protective Efficacy of a Candidate Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Virus Isolation
2.2. Sequencing Analysis
2.3. Immunofluorescence Assay (IFA)
2.4. Animals and Experimental Design
2.5. Serological Testing
2.6. Macroscopic and Histopathological Lesions
2.7. Assessment of Viremia and Viral Loads in Tissues
2.8. Statistical Analysis
3. Results
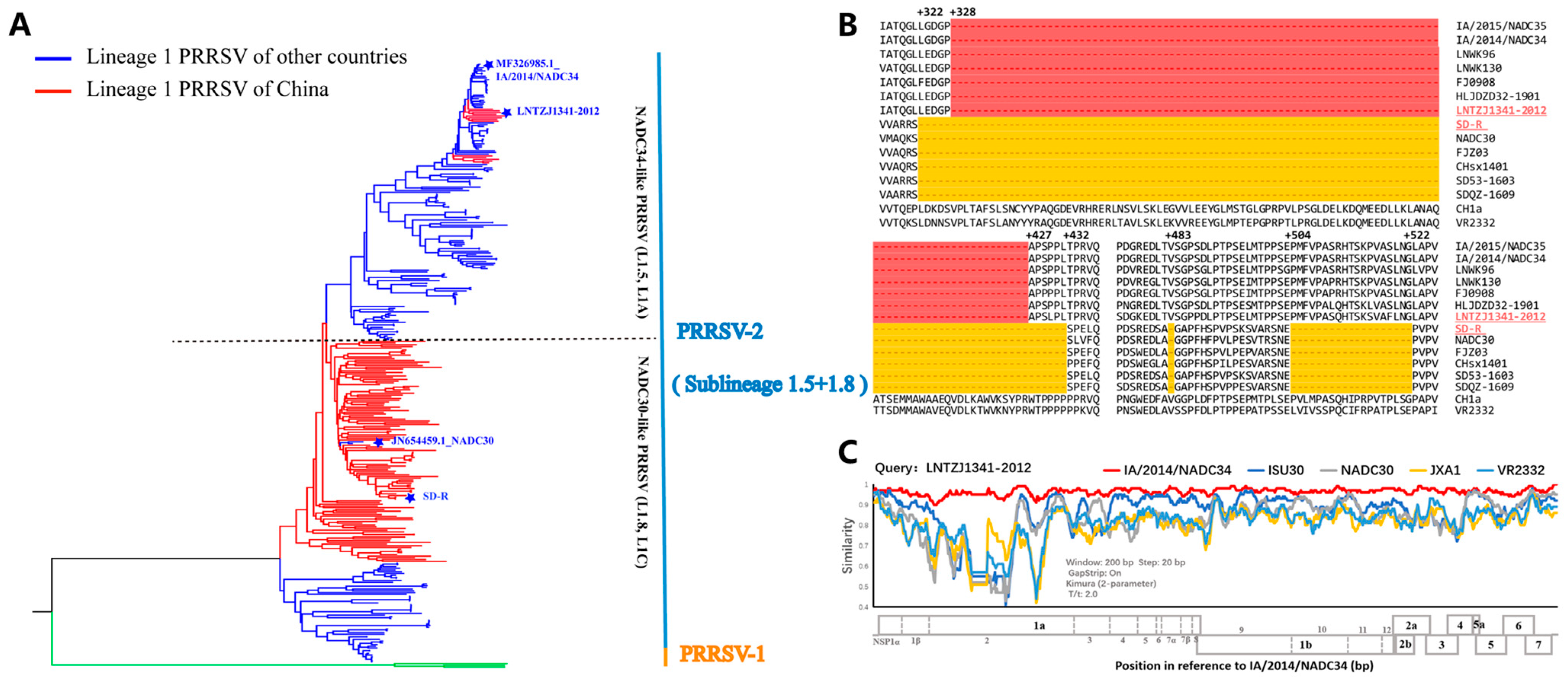
3.1. Genomic Characteristics of the Vaccine Candidate Strain SD-R and NADC34-like PRRSV LNTZJ1341-2012
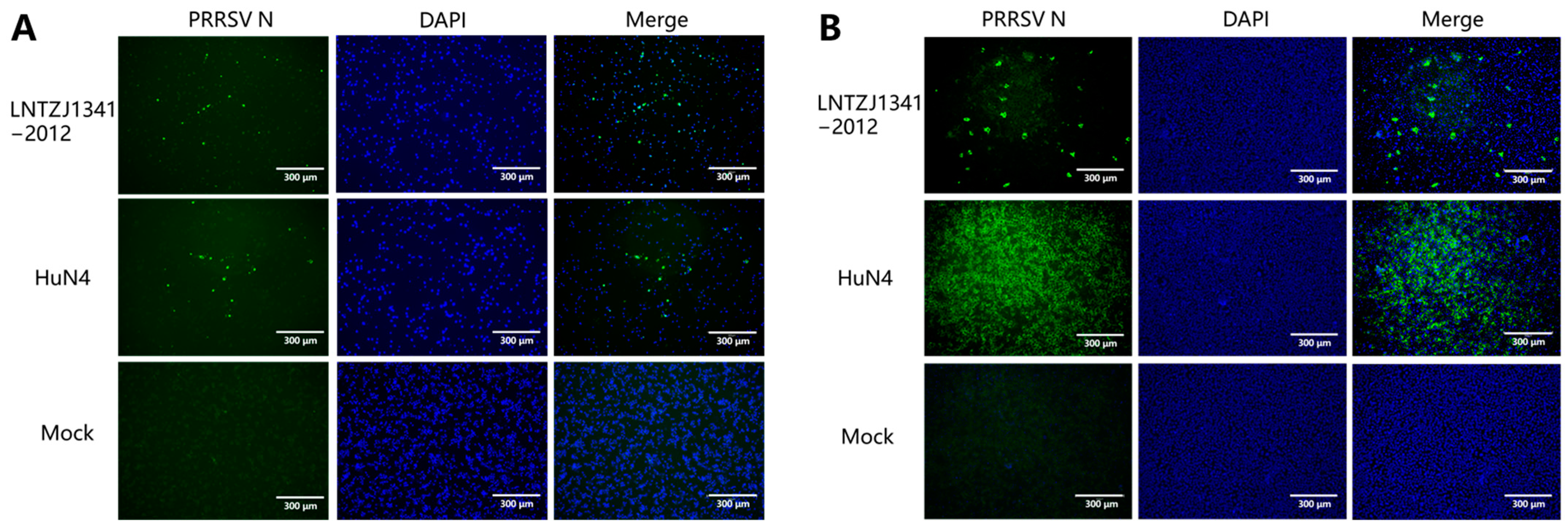
3.2. Virus Isolation and Identification
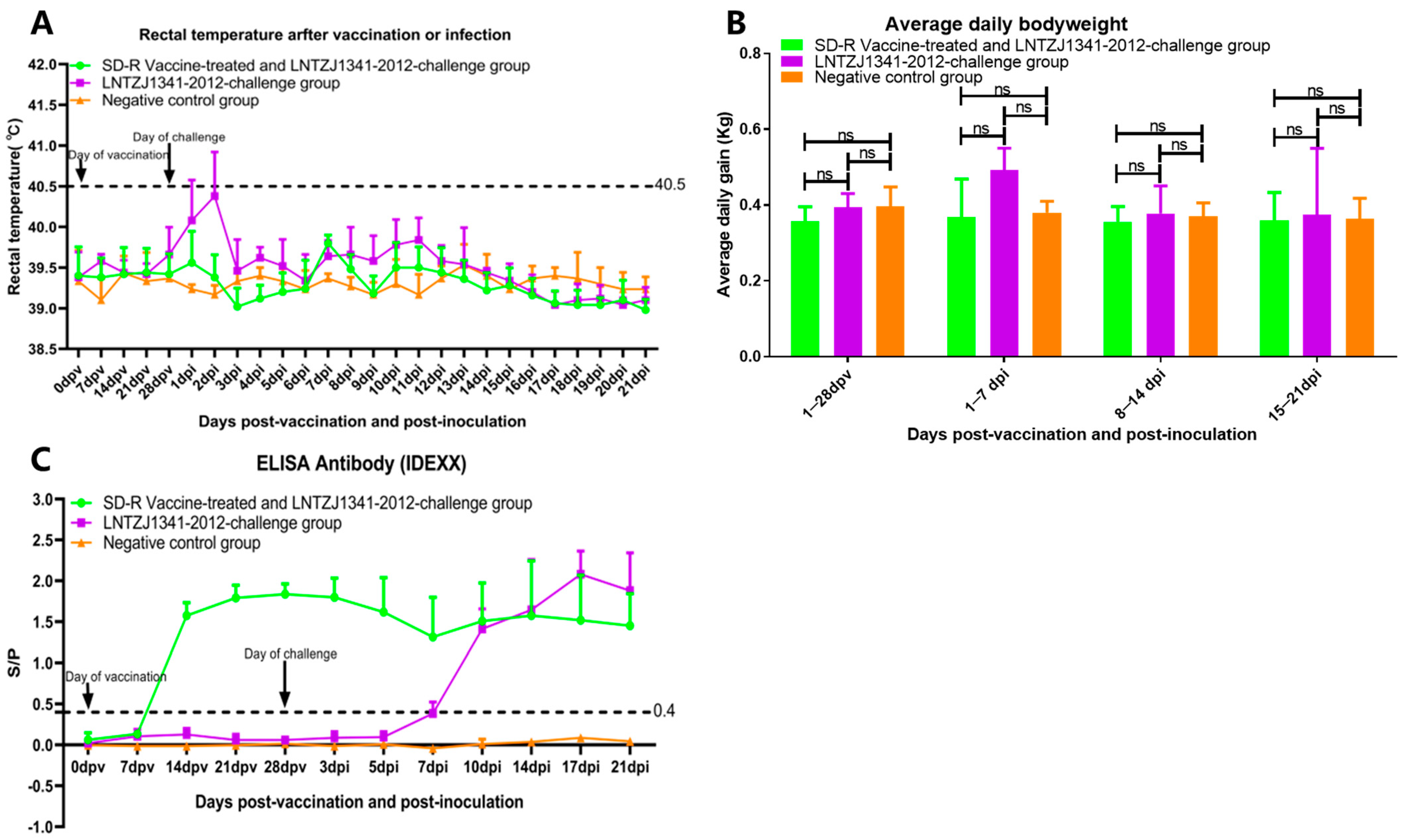
3.3. Clinical Reactions after Immunization and Challenge
3.4. Antibody Responses in Immunized or Challenged Piglets
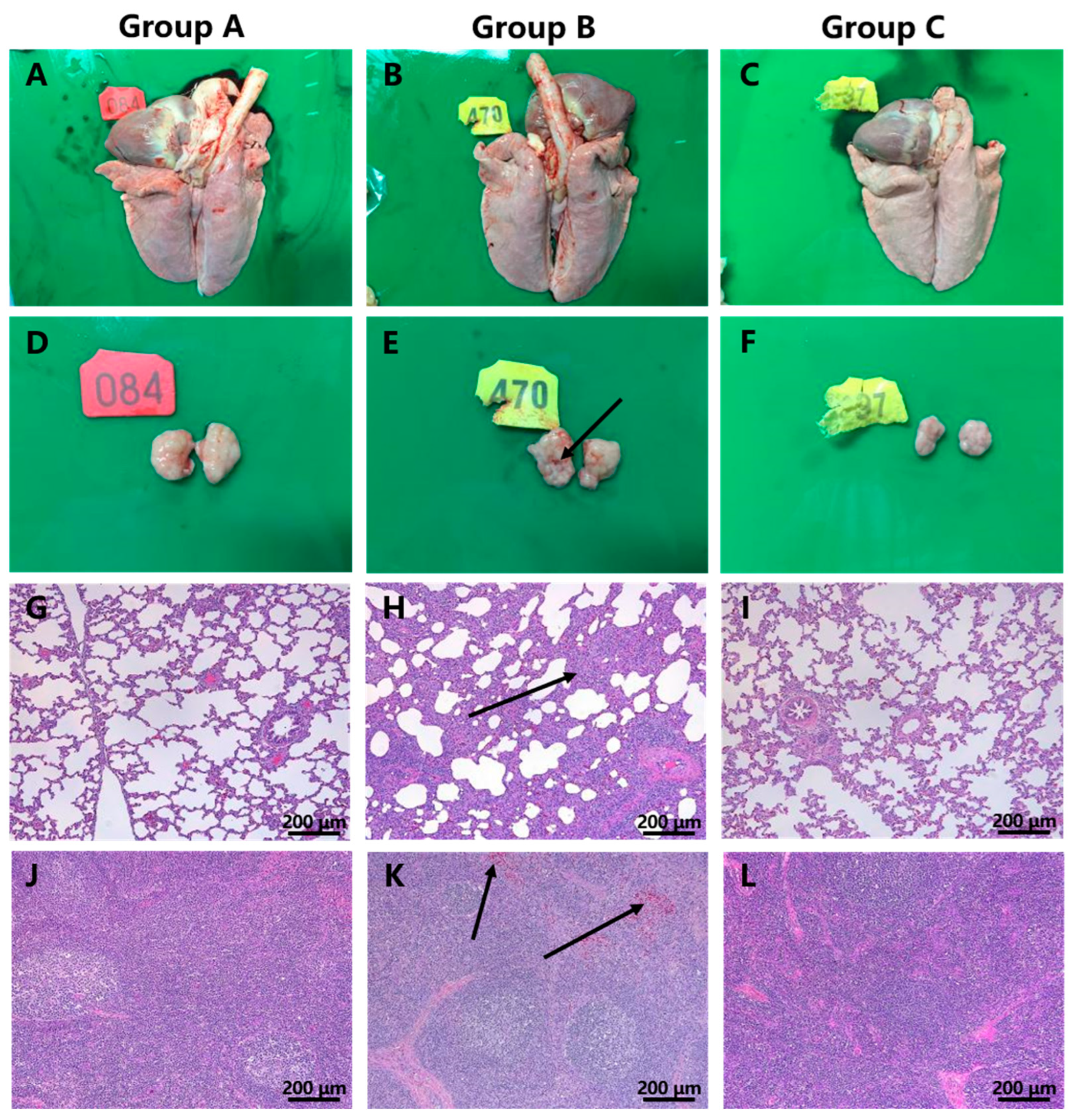
3.5. Macroscopic and Histopathological Lesions
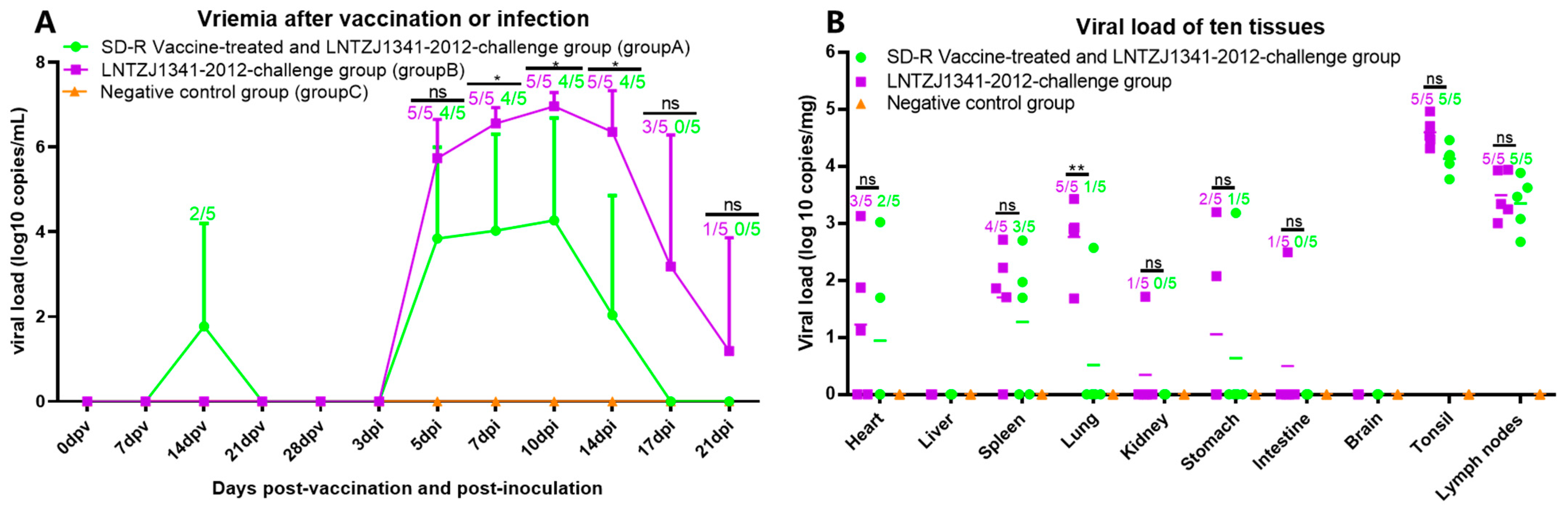
3.6. Viremia and Viral Tissue Distribution
3.7. Serum-Neutralizing Antibody Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Li, Z.; Li, H.; Yang, S.; Ren, F.; Bian, T.; Sun, L.; Zhou, B.; Zhou, L.; Qu, X. The economic impact of porcine reproductive and respiratory syndrome outbreak in four Chinese farms: Based on cost and revenue analysis. Front. Vet. Sci. 2022, 9, 1024720. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Snijder, E.J. The PRRSV replicase: Exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010, 154, 61–76. [Google Scholar] [CrossRef]
- Kappes, M.A.; Faaberg, K.S. PRRSV structure, replication and recombination: Origin of phenotype and genotype diversity. Virology 2015, 479–480, 475–486. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Stadejek, T.; Abrahante, J.E.; Lam, T.T.; Leung, F.C. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 18–30. [Google Scholar] [CrossRef]
- Stadejek, T.; Stankevicius, A.; Murtaugh, M.P.; Oleksiewicz, M.B. Molecular evolution of PRRSV in Europe: Current state of play. Vet. Microbiol. 2013, 165, 21–28. [Google Scholar] [CrossRef]
- Balka, G.; Podgorska, K.; Brar, M.S.; Balint, A.; Cadar, D.; Celer, V.; Denes, L.; Dirbakova, Z.; Jedryczko, A.; Marton, L.; et al. Genetic diversity of PRRSV 1 in Central Eastern Europe in 1994–2014: Origin and evolution of the virus in the region. Sci. Rep. 2018, 8, 7811. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lam, T.T.; Hon, C.C.; Hui, R.K.; Faaberg, K.S.; Wennblom, T.; Murtaugh, M.P.; Stadejek, T.; Leung, F.C. Molecular epidemiology of PRRSV: A phylogenetic perspective. Virus Res. 2010, 154, 7–17. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.; Li, J.; Wong, L.T.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef]
- Xu, H.; Xiang, L.; Tang, Y.D.; Li, C.; Zhao, J.; Gong, B.; Sun, Q.; Leng, C.; Peng, J.; Wang, Q.; et al. Genome-Wide Characterization of QYYZ-Like PRRSV During 2018–2021. Front. Vet. Sci. 2022, 9, 945381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, W.L.; Xiang, L.R.; Leng, C.L.; Tian, Z.J.; Tang, Y.D.; Cai, X.H. Emergence of novel porcine reproductive and respiratory syndrome viruses (ORF5 RFLP 1-7-4 viruses) in China. Vet. Microbiol. 2018, 222, 105–108. [Google Scholar] [CrossRef] [PubMed]
- van Geelen, A.G.M.; Anderson, T.K.; Lager, K.M.; Das, P.B.; Otis, N.J.; Montiel, N.A.; Miller, L.C.; Kulshreshtha, V.; Buckley, A.C.; Brockmeier, S.L.; et al. Porcine reproductive and respiratory disease virus: Evolution and recombination yields distinct ORF5 RFLP 1-7-4 viruses with individual pathogenicity. Virology 2018, 513, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Song, S.; Zhao, J.; Leng, C.; Fu, J.; Li, C.; Tang, Y.D.; Xiang, L.; Peng, J.; Wang, Q.; et al. A potential endemic strain in China: NADC34-like porcine reproductive and respiratory syndrome virus. Transbound. Emerg. Dis. 2020, 67, 1730–1738. [Google Scholar] [CrossRef]
- Liu, J.; Wei, C.; Lin, Z.; Xia, W.; Ma, Y.; Dai, A.; Yang, X. Full genome sequence analysis of a 1-7-4-like PRRSV strain in Fujian Province, China. PeerJ 2019, 7, e7859. [Google Scholar] [CrossRef]
- Bao, H.; Li, X. Emergence and spread of NADC34-like PRRSV in China. Transbound. Emerg. Dis. 2021, 68, 3005–3008. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Li, W.; Zhao, J.; Gong, B.; Sun, Q.; Tang, Y.D.; Xiang, L.; Leng, C.; Peng, J.; et al. characteristics of Chinese NADC34-like PRRSV during 2020–2021. Transbound. Emerg. Dis. 2022, 69, e3215–e3224. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Xia, Q.; Guan, Z.; Zhang, J.; Li, B.; Qiu, Y.; Liu, K.; Shao, D.; Ma, Z.; et al. Genetic characterization of porcine reproductive and respiratory syndrome virus from Eastern China during 2017–2022. Front. Microbiol. 2022, 13, 971817. [Google Scholar] [CrossRef]
- Li, P.; Shen, Y.; Wang, T.; Li, J.; Li, Y.; Zhao, Y.; Liu, S.; Li, B.; Liu, M.; Meng, F. Epidemiological survey of PRRS and genetic variation analysis of the ORF5 gene in Shandong Province, 2020–2021. Front. Vet. Sci. 2022, 9, 987667. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Wang, F.X.; Han, X.Y.; Guo, H.; Liu, C.Y.; Hou, L.N.; Wang, Y.X.; Zheng, H.; Wang, L.; Wen, Y.J. Recent advances in the study of NADC34-like porcine reproductive and respiratory syndrome virus in China. Front. Microbiol. 2022, 13, 950402. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.D.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Xu, Y.; Yang, Y.; Li, J.; Dai, A.; Huang, C.; Luo, M.; Wei, C. Molecular Characteristics and Pathogenicity of a Novel Recombinant Porcine Reproductive and Respiratory Syndrome Virus Strain from NADC30-, NADC34-, and JXA1-Like Strains That Emerged in China. Microbiol. Spectr. 2022, 10, e0266722. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Mo, Q.; Chen, G.; Lv, J.; Huang, J.; Pang, Y.; Wang, H.; Liu, W.; Huang, K.; et al. The Emergence and Pathogenesis of Recombinant Viruses Associated with NADC34-like Strains and the Predominant Circulating Strains of Porcine Reproductive and Respiratory Syndrome Virus in Southern China. Viruses 2022, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Liu, Y.; Yang, J.; Li, W.Z.; Yu, X.X.; Wang, S.Y.; Li, L.A.; Yu, H. Recombination between NADC34-like and QYYZ-like strain of porcine reproductive and respiratory syndrome virus with high pathogenicity for piglets in China. Transbound. Emerg. Dis. 2022, 69, e3202–e3207. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhu, Z.; Fan, J.; Liu, P.; Li, Y.; Li, Q.; Sun, Z.; Yu, X.; Lee, H.S.; Tian, K.; et al. High Pathogenicity of a Chinese NADC34-like PRRSV on Pigs. Microbiol. Spectr. 2022, 10, e0154122. [Google Scholar] [CrossRef]
- Xie, C.Z.; Ha, Z.; Zhang, H.; Zhang, Y.; Xie, Y.B.; Zhang, H.; Nan, F.L.; Wang, Z.; Zhang, P.; Xu, W.; et al. Pathogenicity of porcine reproductive and respiratory syndrome virus (ORF5 RFLP 1-7-4 viruses) in China. Transbound. Emerg. Dis. 2020, 67, 2065–2072. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, L.; Xu, H.; Li, C.; Tang, Y.D.; Gong, B.; Zhang, W.; Zhao, J.; Song, S.; Peng, J.; et al. Lineage 1 Porcine Reproductive and Respiratory Syndrome Virus Attenuated Live Vaccine Provides Broad Cross-Protection against Homologous and Heterologous NADC30-Like Virus Challenge in Piglets. Vaccines 2022, 10, 752. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Feng, W. Porcine Reproductive and Respiratory Syndrome Virus: Immune Escape and Application of Reverse Genetics in Attenuated Live Vaccine Development. Vaccines 2021, 9, 480. [Google Scholar] [CrossRef]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, B.; Xu, L.; Jin, H.; Ge, X.; Guo, X.; Han, J.; Yang, H. Efficacy evaluation of three modified-live virus vaccines against a strain of porcine reproductive and respiratory syndrome virus NADC30-like. Vet. Microbiol. 2017, 207, 108–116. [Google Scholar] [CrossRef]
- Wei, C.; Dai, A.; Fan, J.; Li, Y.; Chen, A.; Zhou, X.; Luo, M.; Yang, X.; Liu, J. Efficacy of Type 2 PRRSV vaccine against challenge with the Chinese lineage 1 (NADC30-like) PRRSVs in pigs. Sci. Rep. 2019, 9, 10781. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Chen, K.; Qian, J.; Hu, Y.; Fang, S.; Sun, Z.; Zhang, C.; Huang, L.; Zhang, J.; et al. Efficacy of the Synergy Between Live-Attenuated and Inactivated PRRSV Vaccines Against a NADC30-Like Strain of Porcine Reproductive and Respiratory Syndrome Virus in 4-Week Piglets. Front. Vet. Sci. 2022, 9, 812040. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, Y.; Xu, X.; Sun, Z.; Xiao, Y.; Ji, G.; Li, Y.; Tan, F.; Li, X.; Tian, K. Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine 2016, 34, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Zhou, L.; Bian, T.; Tian, X.X.; Ren, W.K.; Lu, C.; Zhang, L.; Li, X.L.; Cui, M.S.; Yang, H.C.; et al. Efficacy evaluation of two commercial modified-live virus vaccines against a novel recombinant type 2 porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2018, 216, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, P.; Song, Z.; Lv, L.; Li, L.; Bai, J. Pathogenicity and antigenicity of a novel NADC30-like strain of porcine reproductive and respiratory syndrome virus emerged in China. Vet. Microbiol. 2016, 197, 93–101. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, H.; Li, C.; Tang, Y.D.; An, T.Q.; Li, Z.; Liu, C.; Song, S.; Zhao, J.; Leng, C.; et al. Long-Term Genome Monitoring Retraces the Evolution of Novel Emerging Porcine Reproductive and Respiratory Syndrome Viruses. Front. Microbiol. 2022, 13, 885015. [Google Scholar] [CrossRef]
- Zhang, H.; Leng, C.; Ding, Y.; Zhai, H.; Li, Z.; Xiang, L.; Zhang, W.; Liu, C.; Li, M.; Chen, J.; et al. Characterization of newly emerged NADC30-like strains of porcine reproductive and respiratory syndrome virus in China. Arch. Virol. 2019, 164, 401–411. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, H.L.; Xu, H.; Tang, Y.D.; Leng, C.L.; Peng, J.M.; Wang, Q.; An, T.Q.; Cai, X.H.; Fan, J.H.; et al. Two novel recombinant porcine reproductive and respiratory syndrome viruses belong to sublineage 3.5 originating from sublineage 3.2. Transbound. Emerg. Dis. 2019, 66, 2592–2600. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Zhang, H.; Li, C.; Xu, H.; Gong, B.; Fu, J.; Guo, Z.; Peng, J.; Zhou, G.; et al. A Novel Immunochromatographic Strip Based on Latex Microspheres for the Rapid Detection of North American-Type Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2022, 13, 882112. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, M.A.; Perez, A.M.; Murtaugh, M.P.; Wang, X.; Morrison, R.B. Applications of Bayesian Phylodynamic Methods in a Recent U.S. Porcine Reproductive and Respiratory Syndrome Virus Outbreak. Front. Microbiol. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.B.; Graham, S.P.; Salguero, F.J.; Sanchez Cordon, P.J.; Mokhtar, H.; Rebel, J.M.; Weesendorp, E.; Bodman-Smith, K.B.; Steinbach, F.; Frossard, J.P. Increased pathogenicity of European porcine reproductive and respiratory syndrome virus is associated with enhanced adaptive responses and viral clearance. Vet. Microbiol. 2013, 163, 13–22. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, R.; Yu, J.; Xie, B.; Chen, C.; Li, X.; Xie, J.; Ye, Y.; Xiao, L.; Zhang, J.; et al. Genetic Characterization and Pathogenicity of a Novel Recombined Porcine Reproductive and Respiratory Syndrome Virus 2 among Nadc30-Like, Jxa1-Like, and Mlv-Like Strains. Viruses 2018, 10, 551. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. NADC30-like Strain of Porcine Reproductive and Respiratory Syndrome Virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Y.Y.; Pineyro, P.; Sanford, B.J.; Cossaboom, C.M.; Dryman, B.A.; Huang, Y.W.; Cao, D.J.; Meng, X.J. DNA shuffling of the GP3 genes of porcine reproductive and respiratory syndrome virus (PRRSV) produces a chimeric virus with an improved cross-neutralizing ability against a heterologous PRRSV strain. Virology 2012, 434, 96–109. [Google Scholar] [CrossRef]
- Vu, H.L.; Kwon, B.; Yoon, K.J.; Laegreid, W.W.; Pattnaik, A.K.; Osorio, F.A. Immune evasion of porcine reproductive and respiratory syndrome virus through glycan shielding involves both glycoprotein 5 as well as glycoprotein 3. J. Virol. 2011, 85, 5555–5564. [Google Scholar] [CrossRef]
| Groups | Number of Animals | Vaccination | Challenge |
|---|---|---|---|
| Group A: SD-R vaccinated and LNTZJ1341-2012 challenged | 5 (081; 082; 083; 084; 085) | 2 × 106.2 TCID50 per pig (SD-R) | 4 × 105.0 TCID50 per pig (LNTZJ1341-2012) |
| Group B: LNTZJ1341-2012 challenged | 5 (094; 458; 470; 481; 490) | DMEM | |
| Group C: Negative control group | 3 (097; 098; 101) | DMEM |
| Amino Acids/ Nucleotides | IA/2014/NADC34 (MF326985.1) | HLJDZD32-1901 (MN648449.1) | PRRSV-ZDXYL-China-2018-1 (MK453049.1) | JS2021NADC34 (MZ820388.1) | SD-R (ON25465031) |
|---|---|---|---|---|---|
| Whole genome | /96.6 | /95.4 | /95.1 | /94.1 | /84.4 |
| Nsp1α | 97.2/97.4 | 94.4/96.7 | 96.7/95.7 | 96.7/95.6 | 93.3/88.0 |
| Nsp1β | 93.6/95.5 | 91.6/93.9 | 89.1/92.4 | 90.1/91.7 | 75.7/80.2 |
| Nsp2 | 93.3/95.4 | 92.1/94.2 | 91.5/93.6 | 88.3/91.9 | 71.6/76.5 |
| Nsp3 | 97.4/96.7 | 96.5/95.5 | 97.0/95.5 | 94.8/93.9 | 89.6/84.2 |
| Nsp4 | 98.0/97.4 | 97.1/96.7 | 97.5/97.1 | 97.5/95.8 | 92.2/81.7 |
| Nsp5 | 97.6/96.5 | 96.5/95.3 | 94.7/94.1 | 93.5/91.6 | 89.4/84.3 |
| Nsp6 | 100/97.9 | 100/95.8 | 93.8/95.8 | 93.8/95.8 | 93.8/85.4 |
| Nsp7α | 98.7/96.6 | 98.0/93.7 | 97.3/95.3 | 96.6/92.6 | 93.3/81.4 |
| Nsp7β | 95.5/96.4 | 91.8/93.9 | 90.0/93.6 | 91.8/92.7 | 82.7/80.6 |
| Nsp8 | 97.8/95.6 | 95.6/94.8 | 93.3/93.3 | 97.8/93.3 | 93.3/90.4 |
| Nsp9 | 99.7/97.6 | 98.8/96.5 | 99.1/96.6 | 99.2/95.9 | 96.7/88.4 |
| Nsp10 | 99.5/97.1 | 99.5/96.4 | 99.1/95.8 | 99.3/95.8 | 98.0/88.9 |
| Nsp11 | 98.2/97.2 | 97.3/95.2 | 96.0/95.5 | 97.3/94.2 | 92.8/85.2 |
| Nsp12 | 97.4/97.0 | 97.4/96.1 | 96.8/96.1 | 96.8/95.5 | 89.0/84.4 |
| ORF2a | 97.3/97.4 | 94.6/96.0 | 94.9/95.5 | 93.8/94.8 | 81.7/84.8 |
| ORF2b | 97.3/97.7 | 98.6/97.3 | 94.6/95.9 | 93.2/95.9 | 90.5/86.9 |
| ORF3 | 94.9/96.9 | 94.1/96.2 | 93.7/95.8 | 92.5/94.6 | 83.9/86.4 |
| ORF4 | 98.3/96.8 | 96.6/96.3 | 98.3/96.6 | 97.8/95.2 | 93.9/92.6 |
| ORF5 | 95.0/95.9 | 94.0/94.7 | 92.0/94.2 | 92.0/92.7 | 87.4/86.9 |
| ORF5a | 98.1/96.8 | 98.1/96.2 | 94.2/94.9 | 94.2/94.9 | 94.1/82.8 |
| ORF6 | 97.1/97.5 | 97.1/96.6 | 97.7/96.6 | 97.1/96.6 | 93.1/92.1 |
| ORF7 | 98.4/97.6 | 96.8/96.5 | 96.0/96.8 | 94.4/95.2 | 94.4/92.7 |
| Group A | Serum Neutralization Titer | Group B | Serum Neutralization Titer | Group C | Serum Neutralization Titer | |||
|---|---|---|---|---|---|---|---|---|
| 3d | 7d | 3d | 7d | 3d | 7d | |||
| 081 28 dpv | 5.61 | 5 | 094 28 dpv | <1:4 | <1:4 | 097 28 dpv | <1:4 | <1:4 |
| 082 28 dpv | 8.03 | <1:4 | 458 28 dpv | <1:4 | <1:4 | 098 28 dpv | <1:4 | <1:4 |
| 083 28 dpv | 4 | <1:4 | 470 28 dpv | <1:4 | <1:4 | 101 28 dpv | <1:4 | <1:4 |
| 084 28 dpv | <1:4 | <1:4 | 481 28 dpv | <1:4 | <1:4 | 097 21 dpi | <1:4 | <1:4 |
| 085 28 dpv | 5.62 | <1:4 | 490 28 dpv | <1:4 | <1:4 | 098 21 dpi | <1:4 | <1:4 |
| 081 21 dpi | 10 | 10 | 094 21 dpi | <1:4 | <1:4 | 101 21 dpi | <1:4 | <1:4 |
| 082 21 dpi | 22.48 | 12.64 | 458 21 dpi | <1:4 | <1:4 | |||
| 083 21 dpi | 10 | 6.32 | 470 21 dpi | <1:4 | <1:4 | |||
| 084 21 dpi | 8 | 8 | 481 21 dpi | <1:4 | <1:4 | |||
| 085 21 dpi | 20 | 20 | 490 21 dpi | <1:4 | <1:4 | |||
| Group A | Serum Neutralization Titer | Group B | Serum Neutralization Titer | Group C | Serum Neutralization Titer | |||
|---|---|---|---|---|---|---|---|---|
| 3d | 7d | 3d | 7d | 3d | 7d | |||
| 081 28 dpv | <1:4 | <1:4 | 094 28 dpv | <1:4 | <1:4 | 097 28 dpv | <1:4 | <1:4 |
| 082 28 dpv | <1:4 | <1:4 | 458 28 dpv | <1:4 | <1:4 | 098 28 dpv | <1:4 | <1:4 |
| 083 28 dpv | <1:4 | <1:4 | 470 28 dpv | <1:4 | <1:4 | 101 28 dpv | <1:4 | <1:4 |
| 084 28 dpv | <1:4 | <1:4 | 481 28 dpv | <1:4 | <1:4 | 097 21 dpi | <1:4 | <1:4 |
| 085 28 dpv | <1:4 | <1:4 | 490 28 dpv | <1:4 | <1:4 | 098 21 dpi | <1:4 | <1:4 |
| 081 21 dpi | 8 | 5 | 094 21 dpi | 5.61 | <1:4 | 101 21 dpi | <1:4 | <1:4 |
| 082 21 dpi | 13.56 | 8 | 458 21 dpi | 5.61 | <1:4 | |||
| 083 21 dpi | 12.64 | 10 | 470 21 dpi | 8 | 5 | |||
| 084 21 dpi | 8 | 5.62 | 481 21 dpi | 6.32 | 5 | |||
| 085 21 dpi | 12.64 | 10 | 490 21 dpi | 5 | <1:4 | |||
| Infecting PRRSV Strain | Accession No. | Days Post-Inoculation (dpi) | Inoculated Dose | Parameters Evaluated | Challenge Group | Reference |
|---|---|---|---|---|---|---|
| LNTZJ1341-2012 | OL516360 | 21 | 4 × 105 TCID50 | Clinical symptoms | Mild clinical symptoms in | This study |
| Days of fever | 2/5 pigs fever for 2 days | |||||
| Pathological and histopathological lesions | Typical pathological changes in some piglets (2/5) | |||||
| Viremia | Peaked at 10 dpi, longer than 21 days | |||||
| IA/2014/NADC34 | MF326985.1 | 14 | 5 × 104 TCID50 | Clinical symptoms | Severe clinical symptoms, 2/14 pigs died by | [13] |
| Days of fever | 6 days | |||||
| Pathological and histopathological lesions | Severe pathological changes | |||||
| Viremia | Peaked at 4 dpi, longer than 14 days | |||||
| HLJDZD32-1901 | MN648449.1 | 14 | 4 × 104 TCID50 | Clinical symptoms | Mild clinical symptoms | [21] |
| Days of fever | No fever | |||||
| Pathological and histopathological lesions | Mild pathological changes | |||||
| Viremia | Peaked at 7 dpi, longer than 14 days | |||||
| PRRSV-ZDXYL-China-2018-1 | MK453049.1 | 28 | 4 × 103 TCID50 | Clinical symptoms | Mild clinical symptoms for | [26] |
| Days of fever | 2 days | |||||
| Pathological and histopathological lesions | Moderate pathological changes | |||||
| Viremia | Peaked at 11 dpi, longer than 28 days | |||||
| JS2021NADC34 | MZ820388.1 | 14 | 3 × 106 TCID50 | Clinical symptoms | Severe clinical symptoms, 3/4 pigs died by | [25] |
| Days of fever | 8 days | |||||
| Pathological and histopathological lesions | Severe pathological changes | |||||
| Viremia | Peaked at 7 dpi, viremia lasting for 14 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, C.; Gong, B.; Li, W.; Guo, Z.; Sun, Q.; Zhao, J.; Xiang, L.; Li, J.; Tang, Y.-D.; et al. Protective Efficacy of a Candidate Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus. Vaccines 2023, 11, 1349. https://doi.org/10.3390/vaccines11081349
Xu H, Li C, Gong B, Li W, Guo Z, Sun Q, Zhao J, Xiang L, Li J, Tang Y-D, et al. Protective Efficacy of a Candidate Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus. Vaccines. 2023; 11(8):1349. https://doi.org/10.3390/vaccines11081349
Chicago/Turabian StyleXu, Hu, Chao Li, Bangjun Gong, Wansheng Li, Zhenyang Guo, Qi Sun, Jing Zhao, Lirun Xiang, Jinhao Li, Yan-Dong Tang, and et al. 2023. "Protective Efficacy of a Candidate Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus" Vaccines 11, no. 8: 1349. https://doi.org/10.3390/vaccines11081349
APA StyleXu, H., Li, C., Gong, B., Li, W., Guo, Z., Sun, Q., Zhao, J., Xiang, L., Li, J., Tang, Y.-D., Leng, C., Wang, Q., Peng, J., Zhou, G., Liu, H., An, T., Cai, X., Tian, Z.-J., & Zhang, H. (2023). Protective Efficacy of a Candidate Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus. Vaccines, 11(8), 1349. https://doi.org/10.3390/vaccines11081349











