Mathematical Modeling Evaluates How Vaccinations Affected the Course of COVID-19 Disease Progression
Abstract
1. Introduction
2. Methods
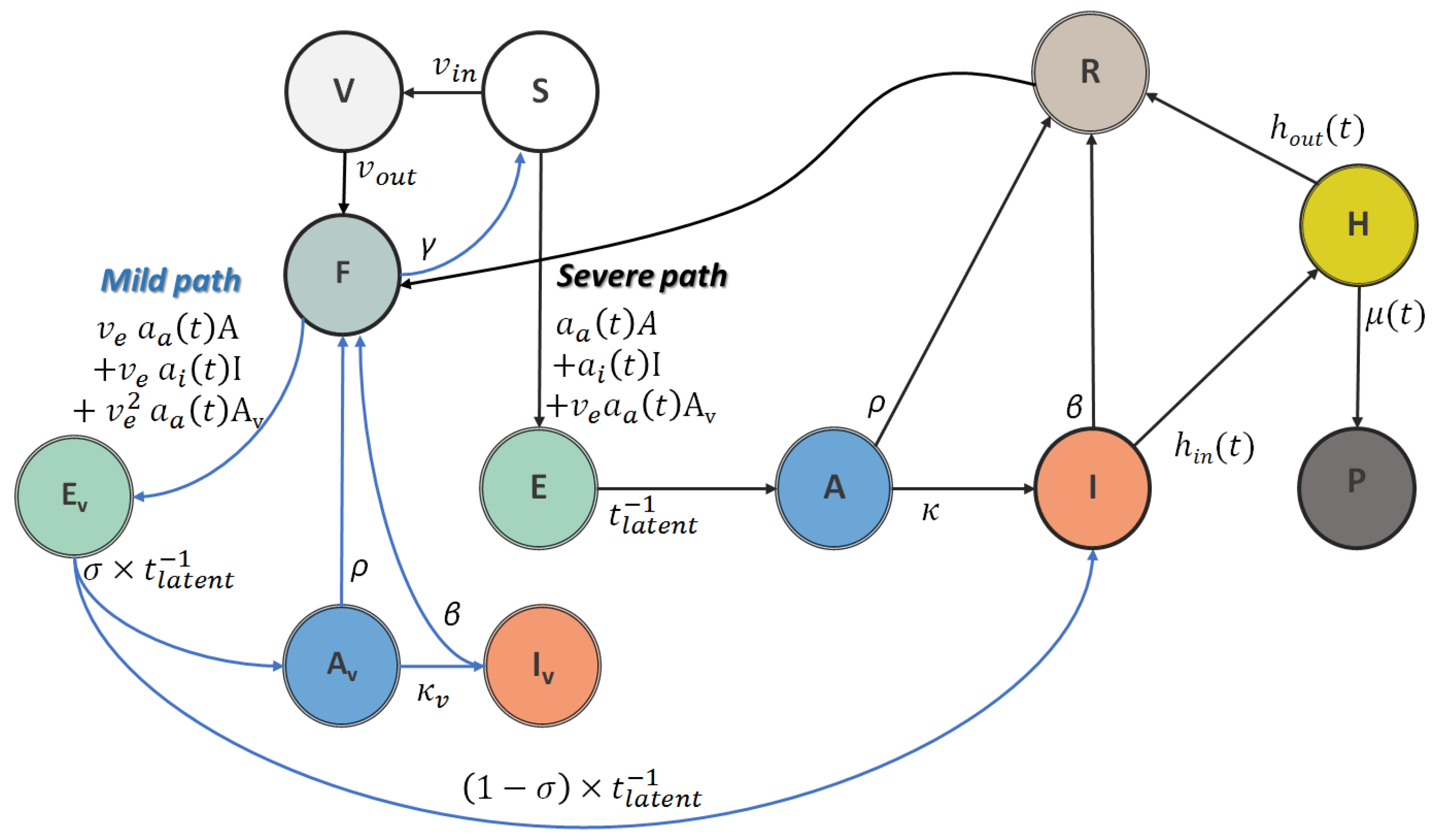
2.1. Adapted and Modified SEIR Model
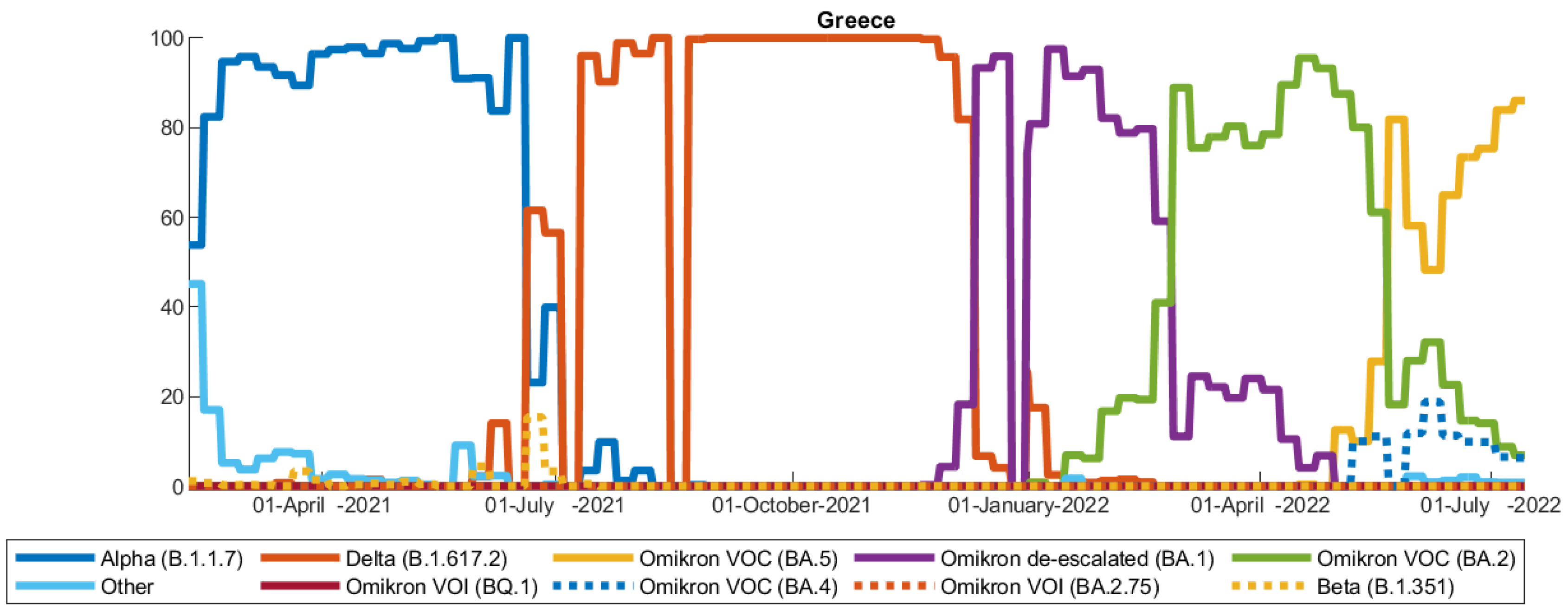
2.2. Data
2.3. Model Scenarios
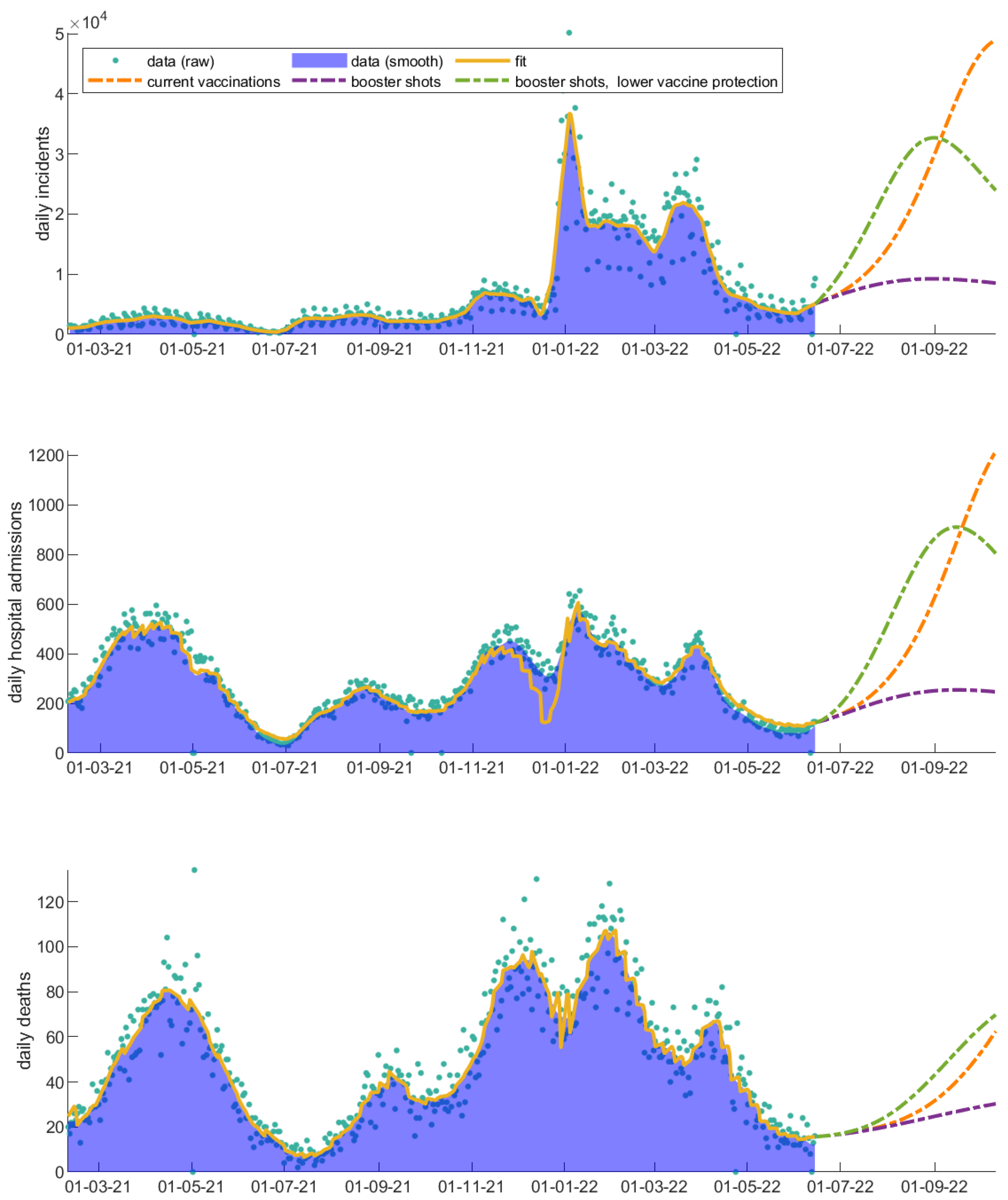
3. Results
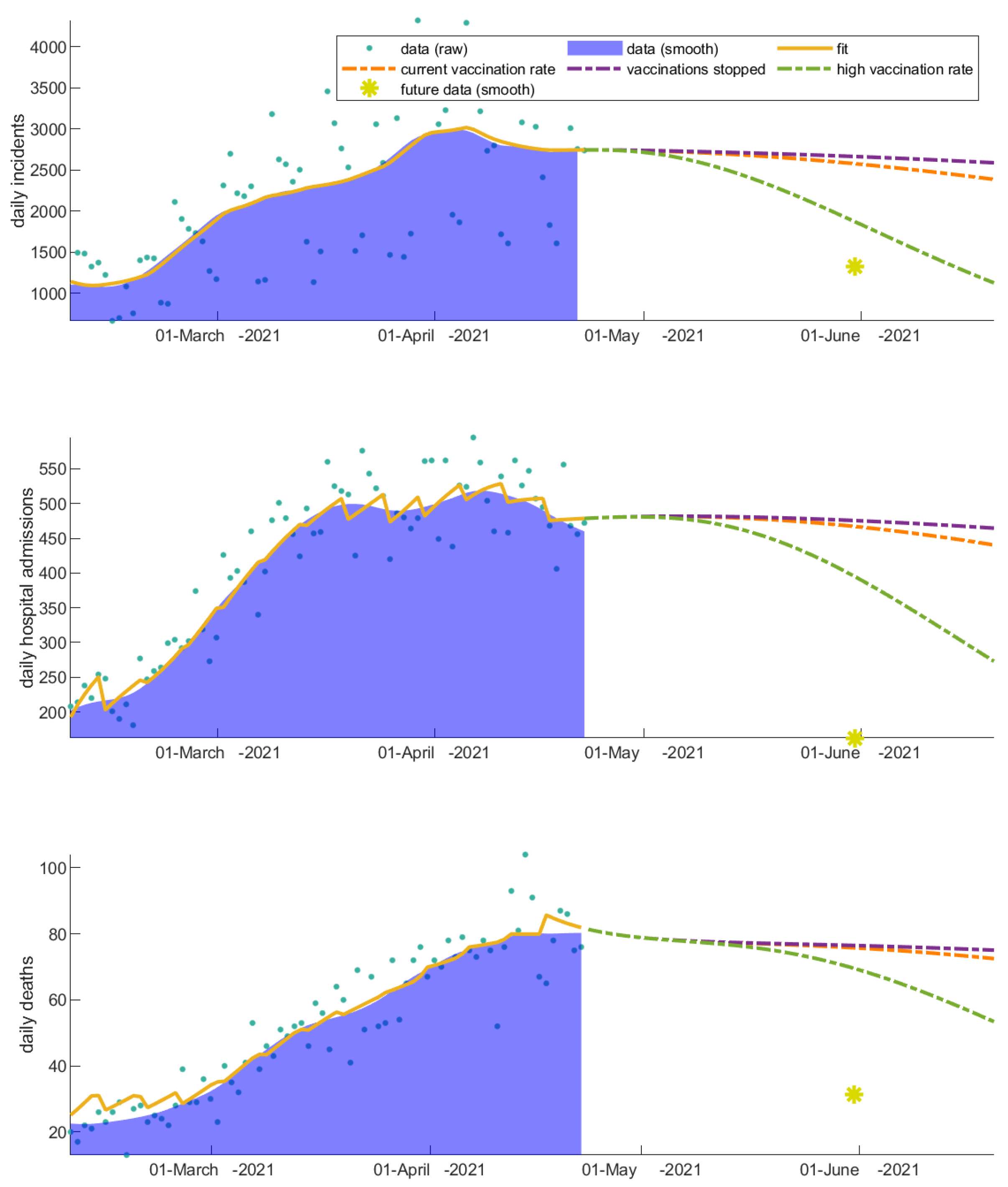
3.1. The Invaluable Win of Vaccinations
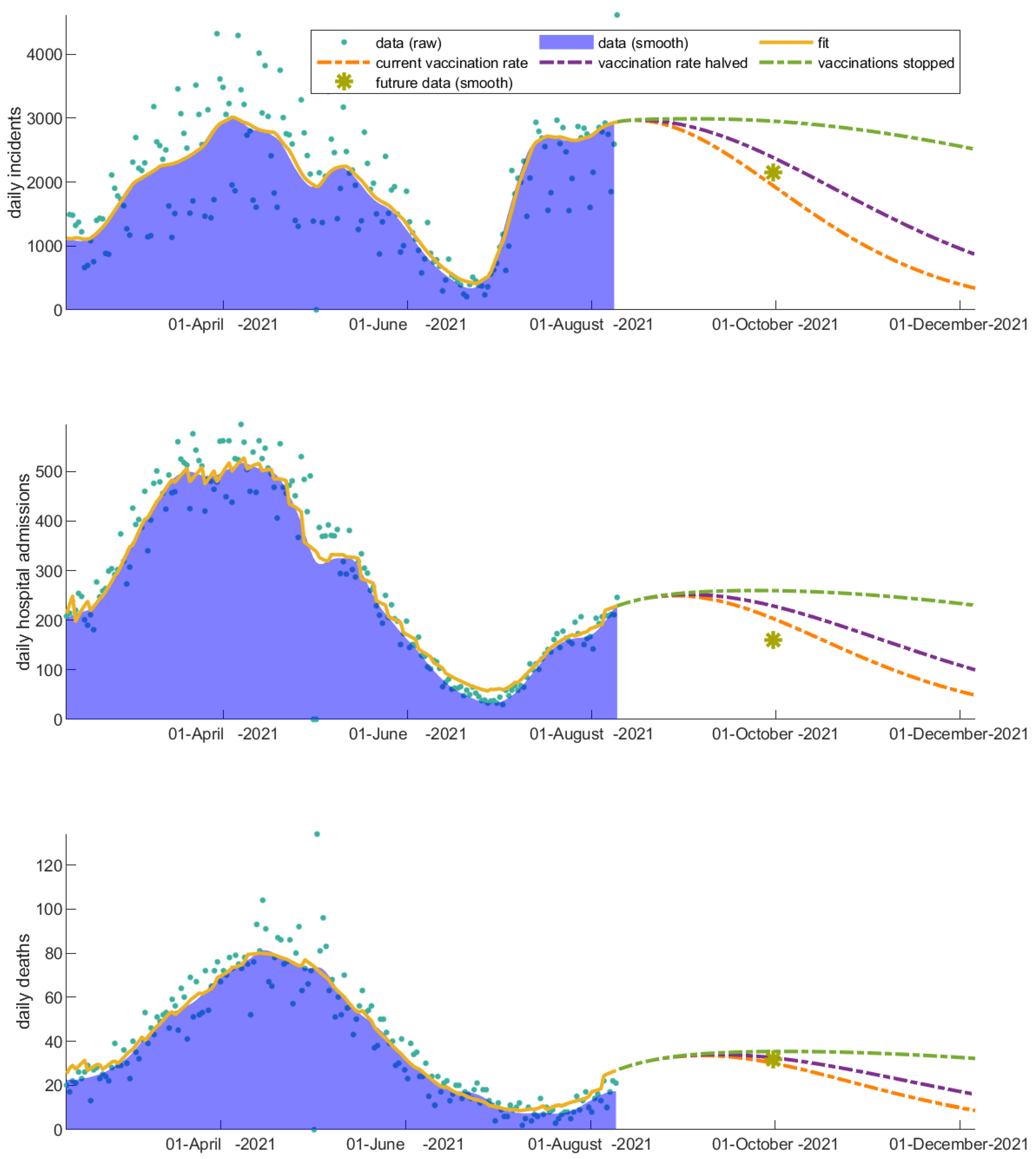
3.2. Vaccinated Individuals Play a Catalytic Role on COVID-19 Progression
3.3. Immunity Loss Feeds Back the Severity of the COVID-19 Waves
3.4. Evaluation of Vaccinations throughout COVID-19 Evolution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Azman, A.S.; Chen, X.; Zou, J.; Tian, Y.; Sun, R.; Xu, X.; Wu, Y.; Lu, W.; Ge, S.; et al. Global landscape of SARS-CoV-2 genomic surveillance and data sharing. Nat. Genet. 2022, 54, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Lopez-Cortes, A.; Gonzalez, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021, 892, 173751. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- De Gier, B.; Andeweg, S.; Backer, J.A.; Hahne, S.J.; van den Hof, S.; de Melker, H.E.; Knol, M.J. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Eur. Commun. Dis. Bull. 2021, 26, 2100977. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernan, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, P924–P944. [Google Scholar] [CrossRef]
- Mukaigawara, M.; Hassan, I.; Fernandes, G.; King, L.; Patel, J.; Sridhar, D. An equitable roadmap for ending the COVID-19 pandemic. Nat. Med. 2022, 28, 893–896. [Google Scholar] [CrossRef]
- Oraiopoulou, M.E.; Tzamali, E.; Tzedakis, G.; Liapis, E.; Zacharakis, G.; Vakis, A.; Papamatheakis, J.; Sakkalis, V. Integrating in vitro experiments with in silico approaches for Glioblastoma invasion: The role of cell-to-cell adhesion heterogeneity. Sci. Rep. 2018, 8, 16200. [Google Scholar] [CrossRef]
- Roniotis, A.; Marias, K.; Sakkalis, V.; Tsibidis, G.D.; Zervakis, M. A complete mathematical study of a 3D model of heterogeneous and anisotropic glioma evolution. Ann. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 2807–2810. [Google Scholar]
- Vytla, V.; Ramakuri, S.K.; Peddi, A.; Kalyan Srinivas, K.; Nithish Ragav, N. Mathematical Models for Predicting COVID-19 Pandemic: A Review. J. Phys. Conf. Ser. 2021, 1797, 012009. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- Davies, N.G.; Kucharski, A.J.; Eggo, R.M.; Gimma, A.; Edmunds, W.J. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: A modelling study. Lancet Public Health 2020, 5, e375–e385. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Colaneri, M.; Di Filippo, A.; Blanchini, F.; Bolzern, P.; De Nicolao, G.; Sacchi, P.; Colaneri, P.; Bruno, R. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat. Med. 2021, 27, 993–998. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Zou, Z.; Liu, Y.; Li, X.; Zhuang, G.; Shen, M.; Zhang, L. Evaluating the Impact of SARS-CoV-2 Variants on the COVID-19 Epidemic and Social Restoration in the United States: A Mathematical Modelling Study. Front. Public Health 2021, 9, 801763. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.M.; Dyson, L.; Tildesley, M.J.; Keeling, M.J. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. PLoS Comput. Biol. 2021, 17, e1008849. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and non-pharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Nixon, K.; Jindal, S.; Parker, F.; Reich, N.G.; Ghobadi, K.; Lee, E.C.; Truelove, S.; Gardner, L. An evaluation of prospective COVID-19 modelling studies in the USA: From data to science translation. Lancet Digit. Health 2022, 4, e738–e747. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Jorge, D.C.P.; Veiga, R.V.; Rodrigues, M.S.; Torquato, M.F.; da Silva, N.B.; Fiaccone, R.L.; Cardim, L.L.; Pereira, F.A.C.; de Castro, C.P.; et al. Mathematical modeling of COVID-19 in 14.8 million individuals in Bahia, Brazil. Nat. Commun. 2021, 12, 333. [Google Scholar] [CrossRef]
- Ojokoh, B.A.; Sarumi, O.A.; Salako, K.V.; Gabriel, A.J.; Taiwo, A.E.; Johnson, O.V.; Adegun, I.P.; Babalola, O.T. Modeling and predicting the spread of COVID-19: A continental analysis. In Data Science for COVID-19; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Tsay, C.; Lejarza, F.; Stadtherr, M.A.; Baldea, M. Modeling, state estimation, and optimal control for the US COVID-19 outbreak. Sci. Rep. 2020, 10, 10711. [Google Scholar] [CrossRef]
- Usherwood, T.; LaJoie, Z.; Srivastava, V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci. Rep. 2021, 11, 12051. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ghosh, S. A mathematical model for COVID-19 considering waning immunity, vaccination and control measures. Sci. Rep. 2023, 13, 3610. [Google Scholar] [CrossRef] [PubMed]
- Parolini, N.; Dede, L.; Ardenghi, G.; Quarteroni, A. Modelling the COVID-19 epidemic and the vaccination campaign in Italy by the SUIHTER model. Infect. Dis. Modell. 2022, 7, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Chowell, G. Fitting dynamic models to epidemic outbreaks with quantified uncertainty: A Primer for parameter uncertainty, identifiability, and forecasts. Infect. Dis. Modell. 2017, 2, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Rubner, Y.; Tomasi, C.; Guibas, L.J. The Earth Mover’s Distance as a Metric for Image Retrieval. Int. J. Comput. Vis. 2000, 40, 99–121. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Kulper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Eur. Commun. Dis. Bull. 2021, 26, 2100563. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Protection from the New Coronavirus—Everything You Need to Know. Available online: https://www.ynanp.gr/en/press-and-communications-office/protection-new-coronavirus-everything-you-need-know/ (accessed on 15 June 2022).
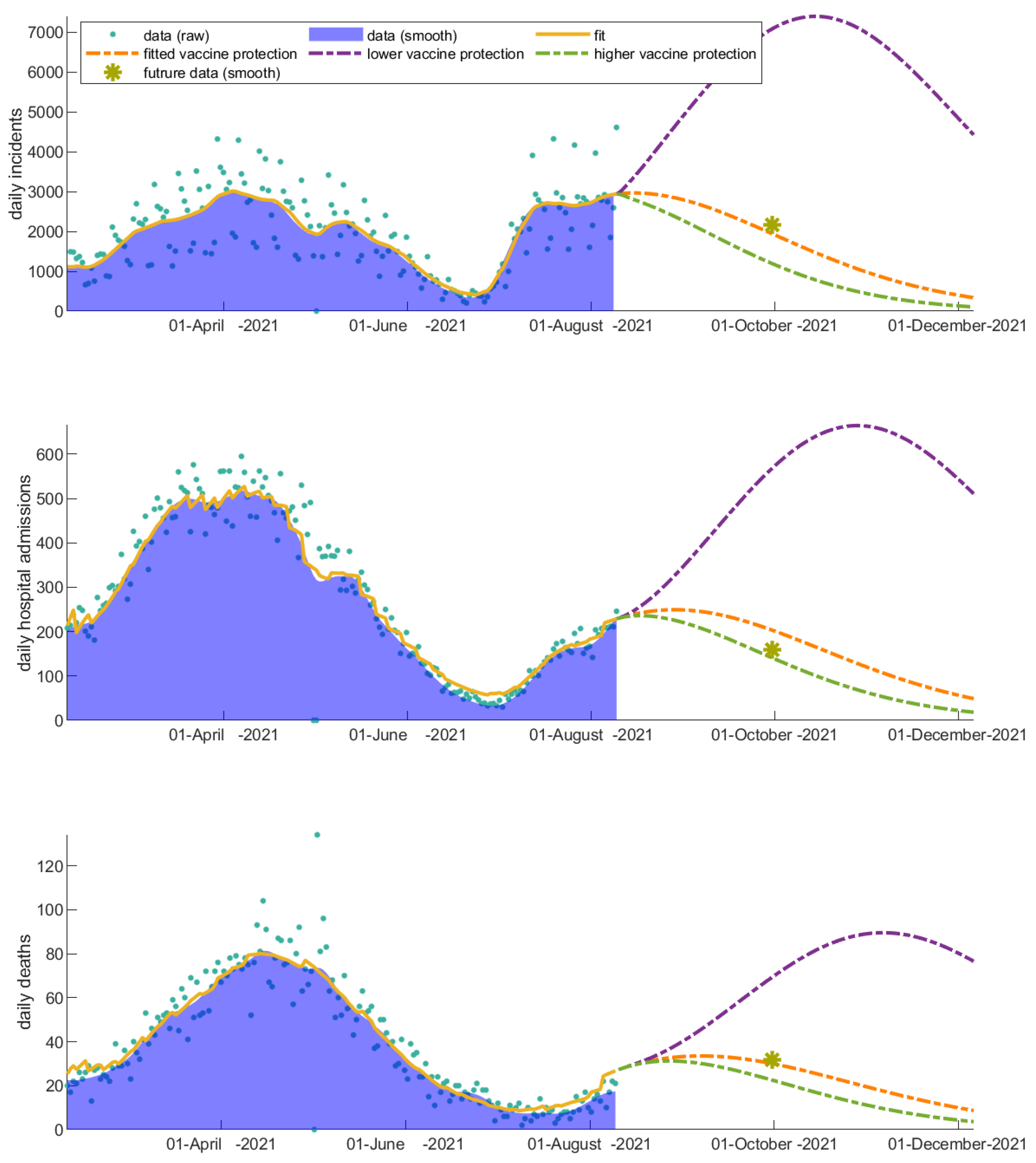
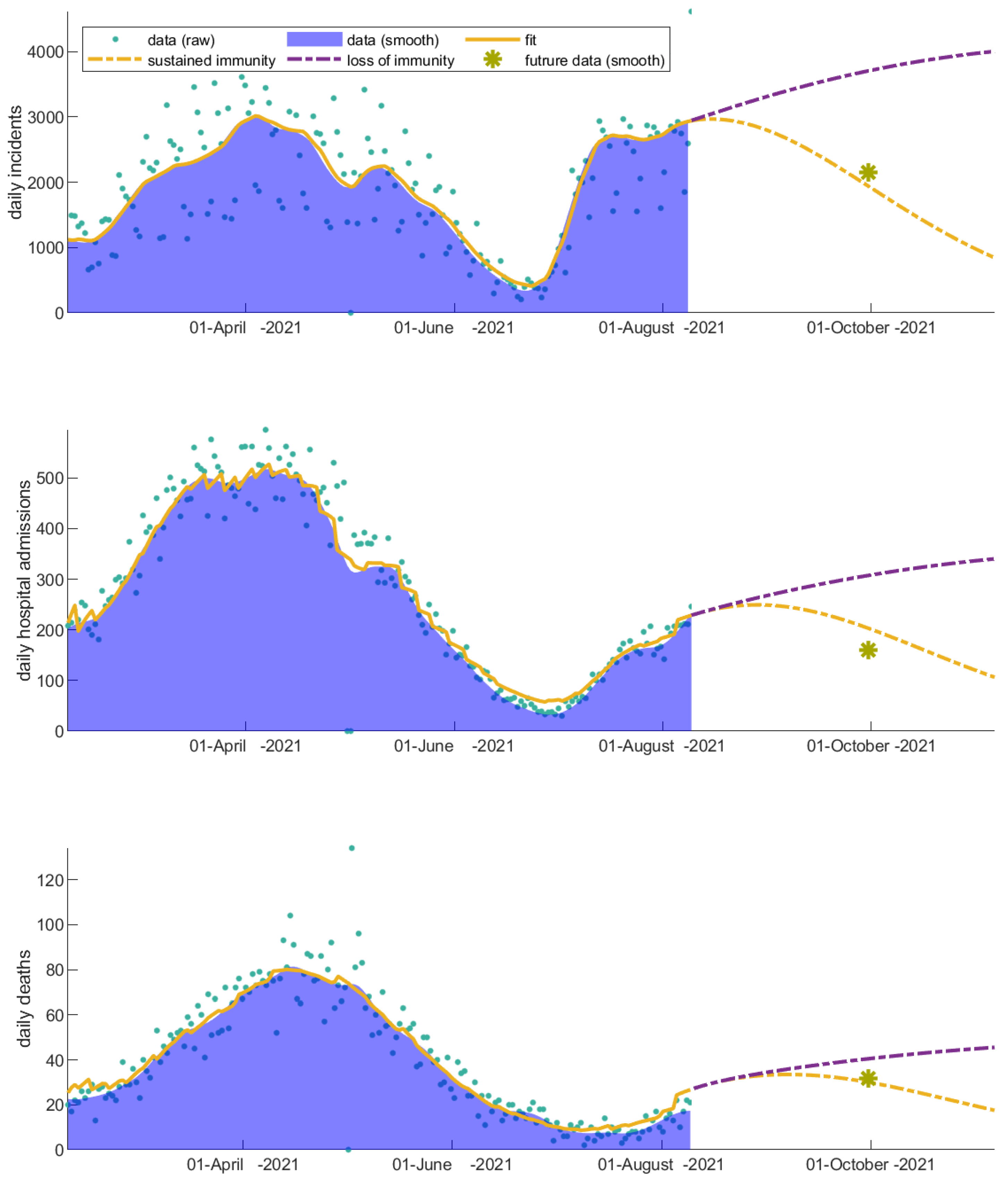
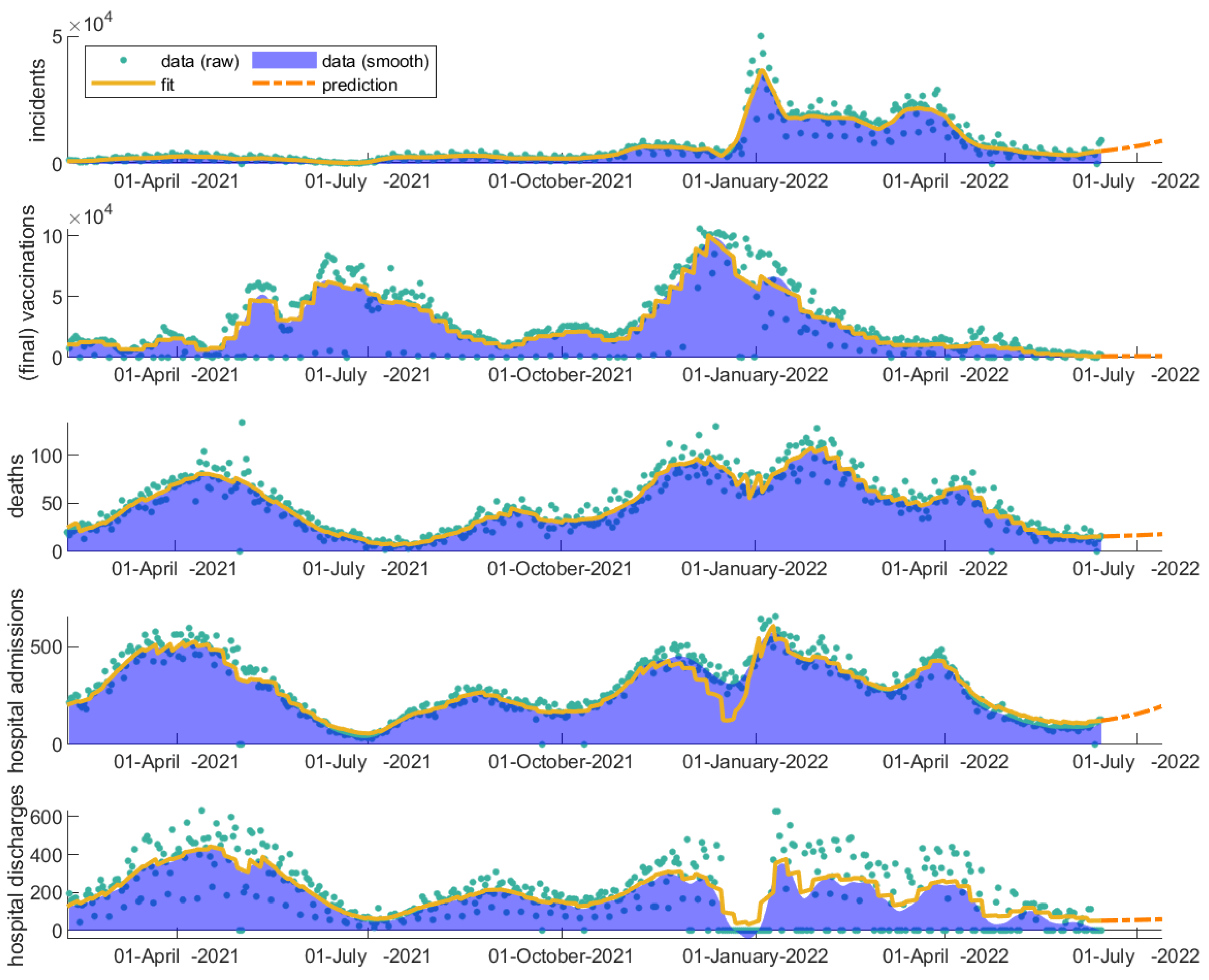

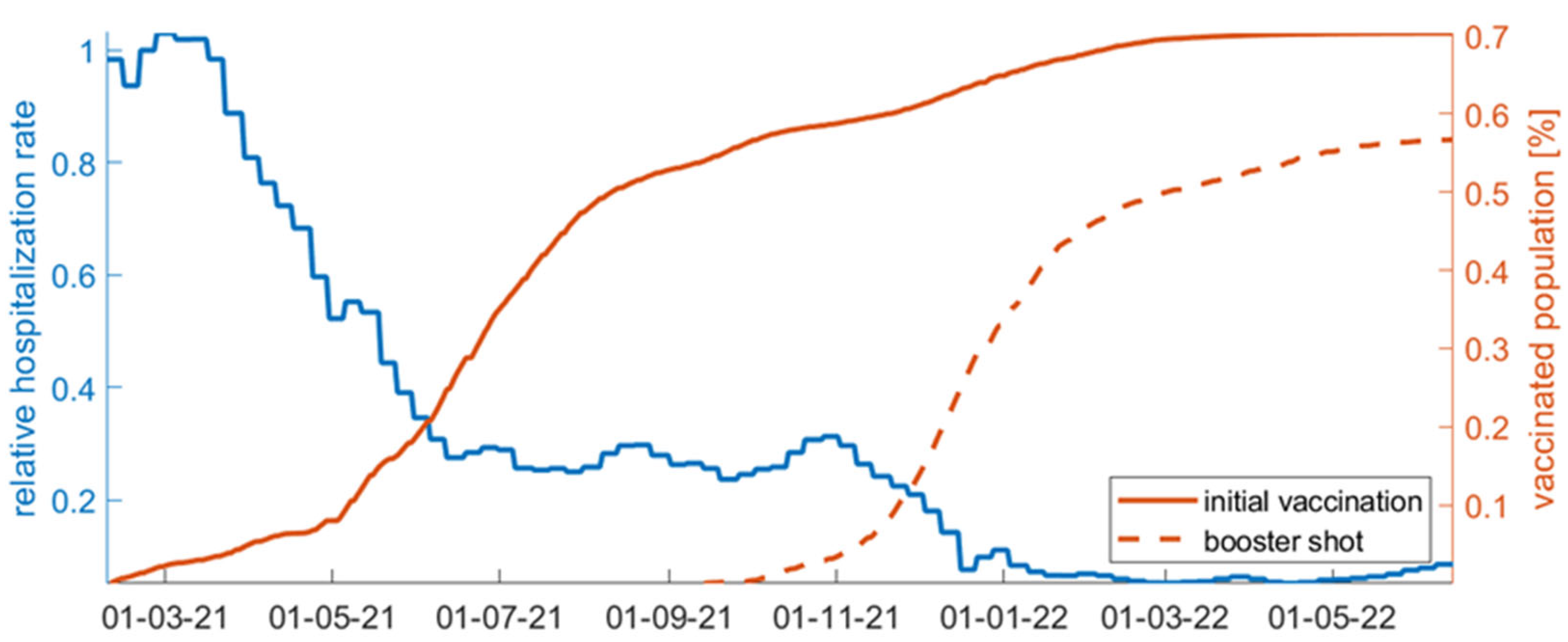
| Scenarios | Increase in Number of Incidents | Increase in Number of Hospitalizations | Increase in Number of Deaths |
|---|---|---|---|
| Vaccinations stop | 77,326 | 4792 | 501 |
| Infection after vaccination becomes twice as probable | 357,227 | 26,357 | 2960 |
| Vaccine protection to severe illness is compromised to 10% | 31,101 | 8966 | 1068 |
| Immunity is lost in 6 months | 135,823 | 8516 | 909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzamali, E.; Sakkalis, V.; Tzedakis, G.; Spanakis, E.G.; Tzanakis, N. Mathematical Modeling Evaluates How Vaccinations Affected the Course of COVID-19 Disease Progression. Vaccines 2023, 11, 722. https://doi.org/10.3390/vaccines11040722
Tzamali E, Sakkalis V, Tzedakis G, Spanakis EG, Tzanakis N. Mathematical Modeling Evaluates How Vaccinations Affected the Course of COVID-19 Disease Progression. Vaccines. 2023; 11(4):722. https://doi.org/10.3390/vaccines11040722
Chicago/Turabian StyleTzamali, Eleftheria, Vangelis Sakkalis, Georgios Tzedakis, Emmanouil G. Spanakis, and Nikos Tzanakis. 2023. "Mathematical Modeling Evaluates How Vaccinations Affected the Course of COVID-19 Disease Progression" Vaccines 11, no. 4: 722. https://doi.org/10.3390/vaccines11040722
APA StyleTzamali, E., Sakkalis, V., Tzedakis, G., Spanakis, E. G., & Tzanakis, N. (2023). Mathematical Modeling Evaluates How Vaccinations Affected the Course of COVID-19 Disease Progression. Vaccines, 11(4), 722. https://doi.org/10.3390/vaccines11040722








