Identification of a Potential Vaccine against Treponema pallidum Using Subtractive Proteomics and Reverse-Vaccinology Approaches
Abstract
1. Introduction:
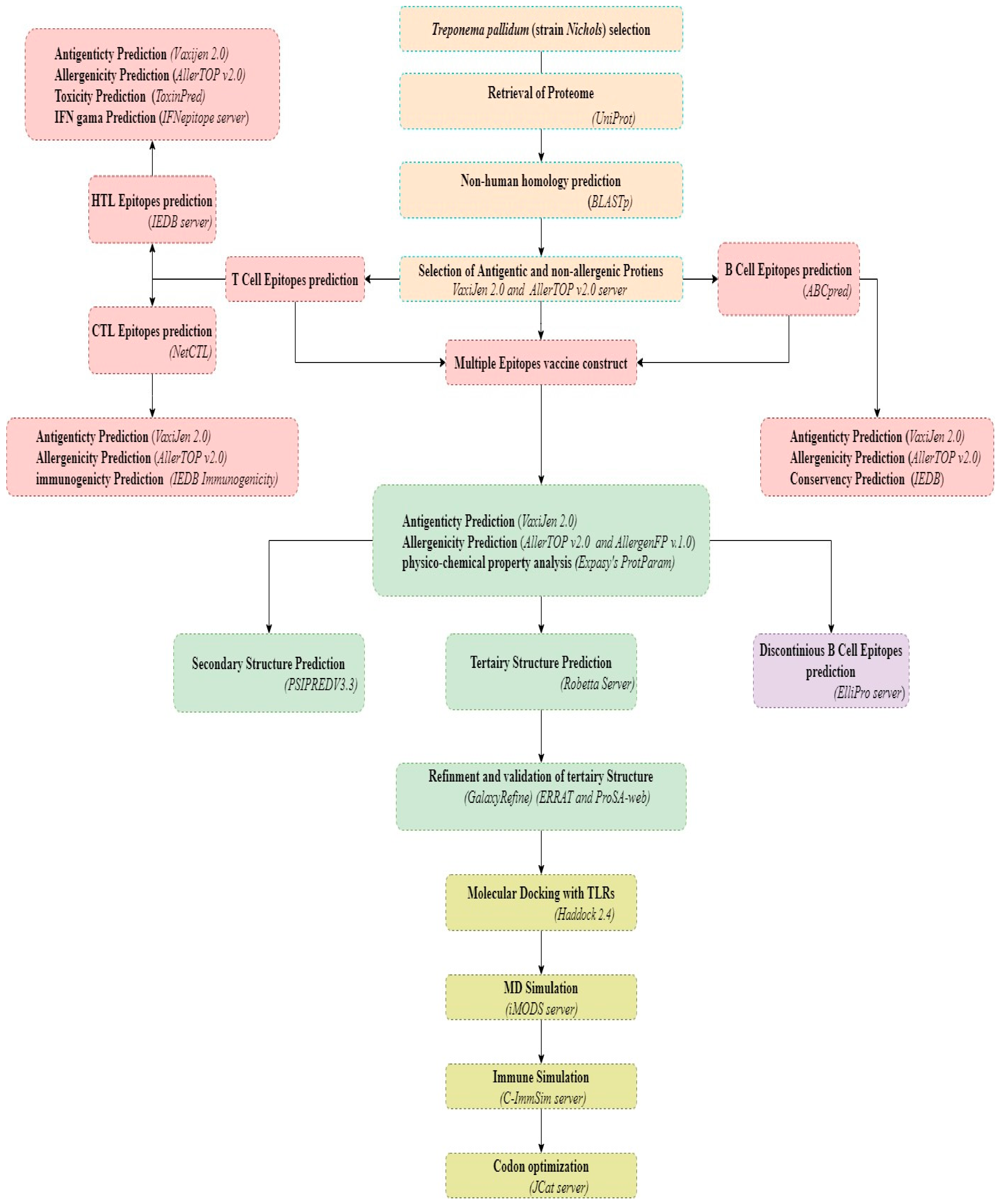
2. Materials and Methods
2.1. Protein Sequence Retrieval
2.2. Prioritization of Essential Genes
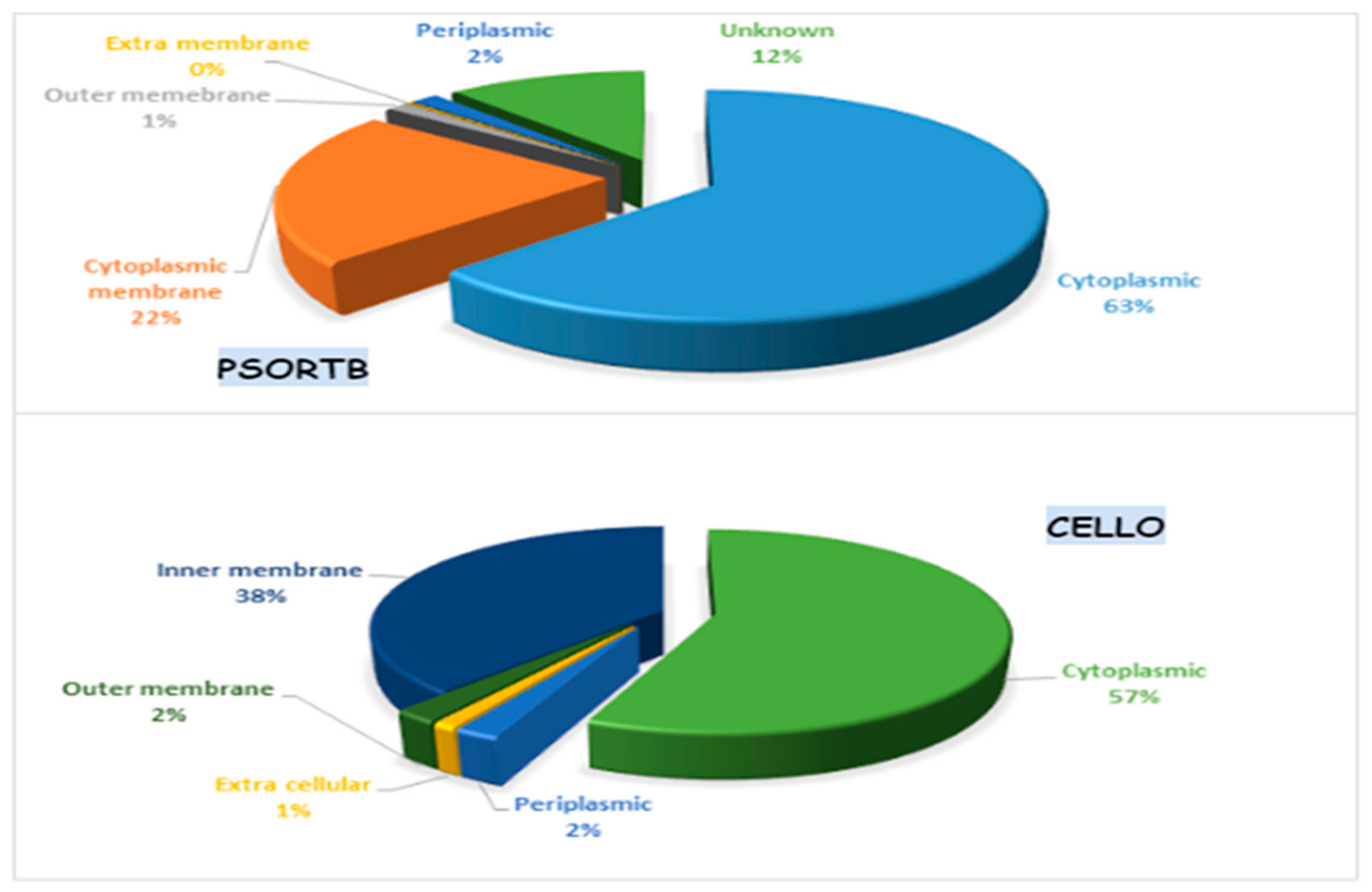
2.3. Subcellular Localization
2.4. Druggability of Cytoplasmic Membrane Proteins
2.5. Resistance Protein Analysis
2.6. Virulent Proteins Evaluation
2.7. Prediction of Antigenic Proteins
2.8. Protein–Protein Interaction Network Analysis
2.9. MHC-I Binding Epitopes (CTL) Prediction Epitopes
2.10. Evaluation of Predicted CTL Epitopes for Antigenicity, Allergenicity, and Immunogenicity
2.11. MHC-II Binding Epitopes (HTL) Prediction Epitopes
2.12. Evaluation of Predicted HTL Epitopes for Toxicity, Antigenicity, and Allergenicity
2.13. Identification of Cytokine-Inducing HTL Epitopes
2.14. Linear B Cell Epitope Prediction and Evaluation
2.15. Discontinues the Prediction of the B Cell Epitope
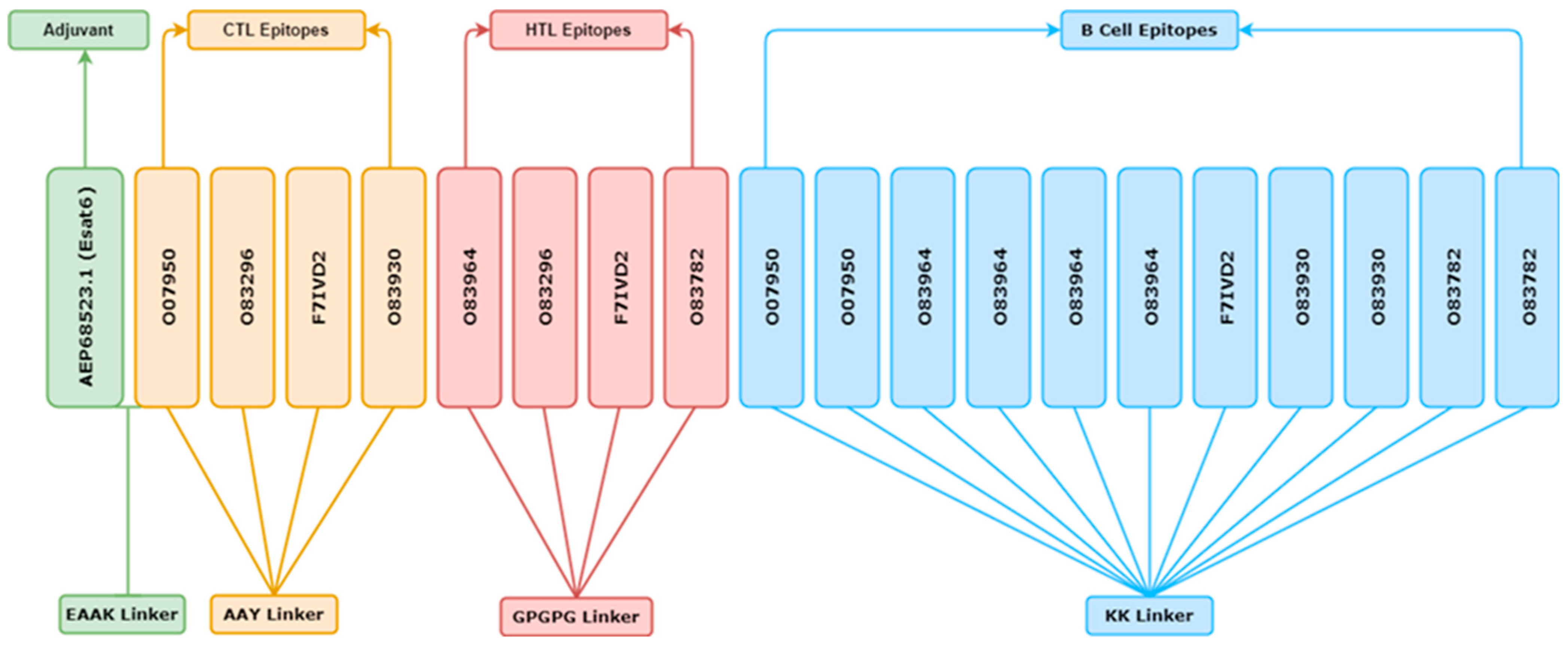
2.16. Assembling of Vaccine Construction Final Multi-Epitope
2.17. Evaluation of the Physicochemical Properties, Antigenicity, and Allergenicity of the Vaccine Construct
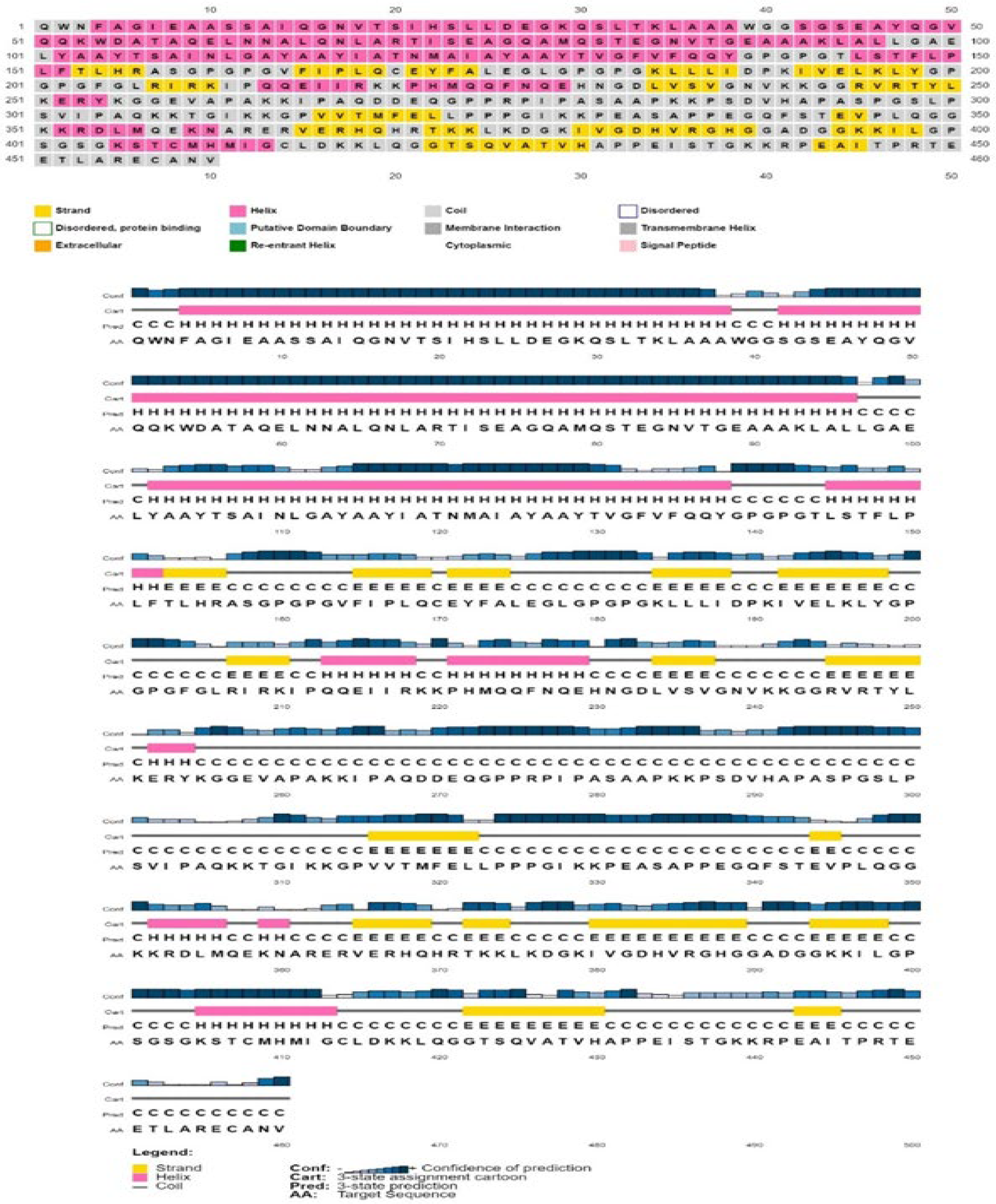
2.18. Prediction of the Secondary and Tertiary Structure of the Vaccine Design
2.19. Refinement and Validation of 3D Structure
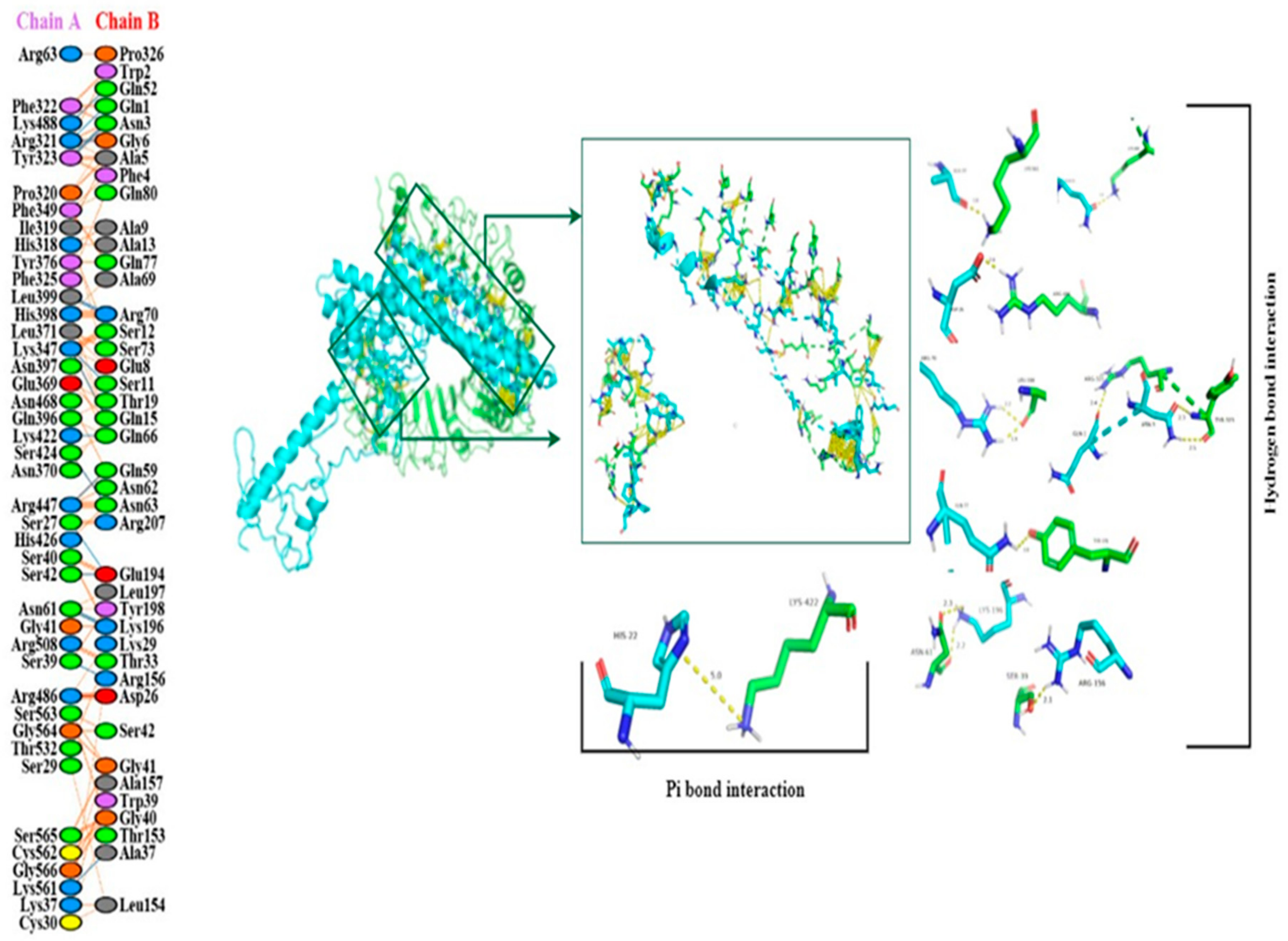
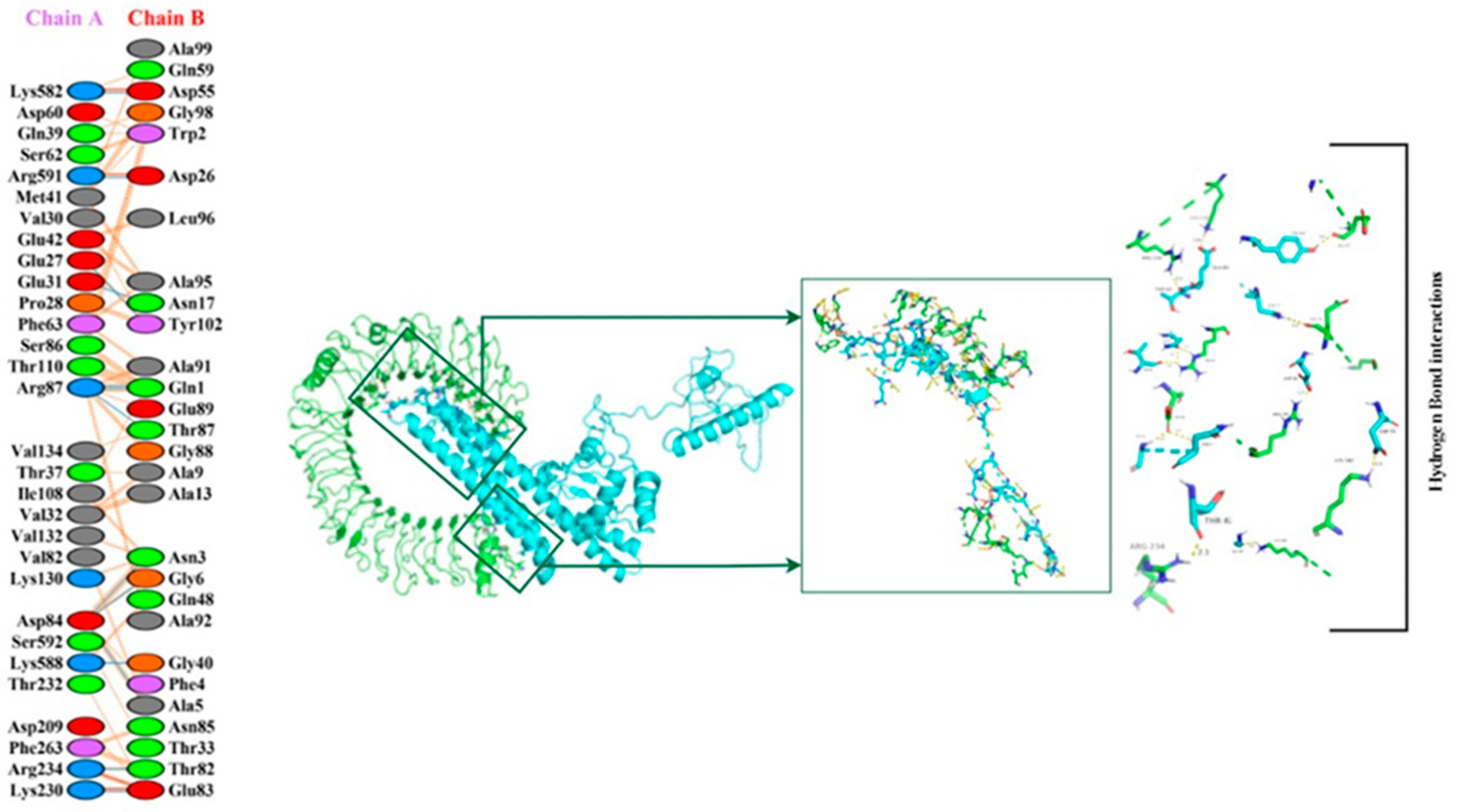
2.20. Molecular Docking of Constructed Vaccine with TLR2 and TLR-4
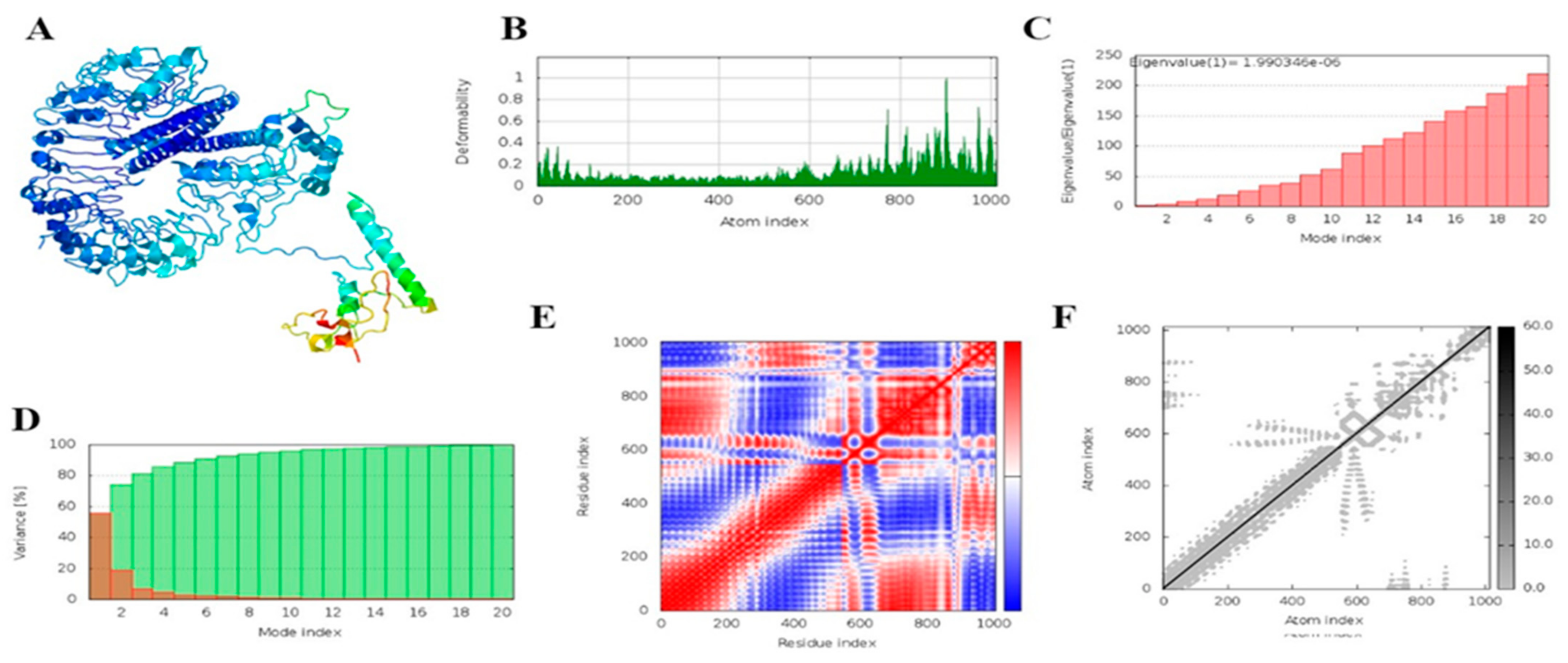
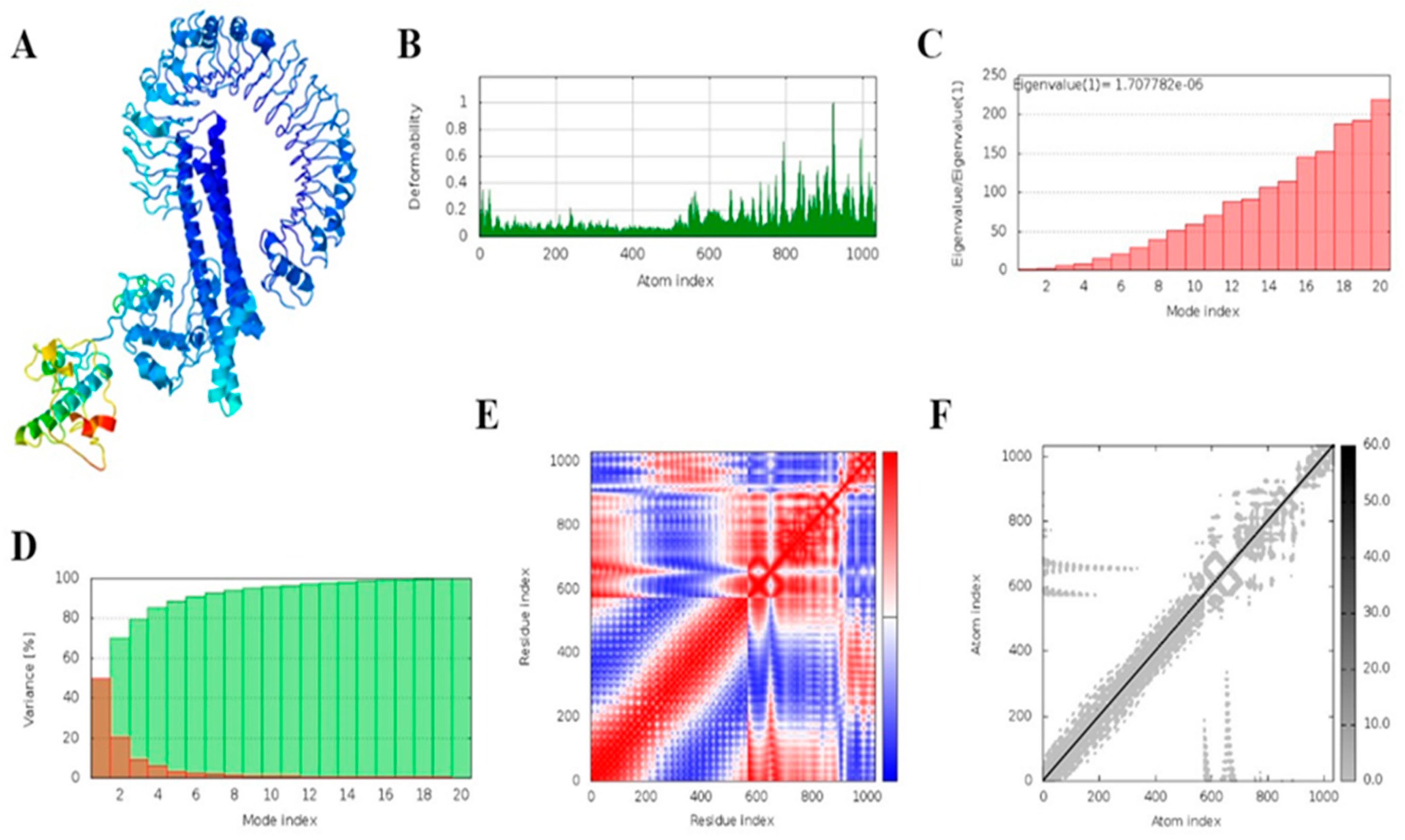
2.21. Molecular Dynamics Simulation
2.22. Immune Simulation
2.23. Codon Optimization of Vax Sequence and In Situ Cloning
3. Results
3.1. Proteome Collection
3.2. Removal of Homologous Proteins
3.3. Prediction of Paralogous Proteins
3.4. Essential Proteins Prediction
3.5. Subcellular Localization of the Essential Proteins
3.6. Druggability of Cytoplasmic Membrane Proteins
3.7. Resistance Protein Analysis
3.8. Virulent Protein Analysis
3.9. Vaccine Protein Prioritization
3.10. Protein–Protein Interaction Network Analysis
3.11. Selection and Evaluation of T-Cell Epitopes
3.12. Selection and Evaluation of B-Cell Epitopes
3.13. Epitope-Based Subunit Vaccine Construct
3.14. Antigenicity and Allergenicity Physicochemical Properties of the Vaccine Construct
3.15. Analysis of Secondary Structure
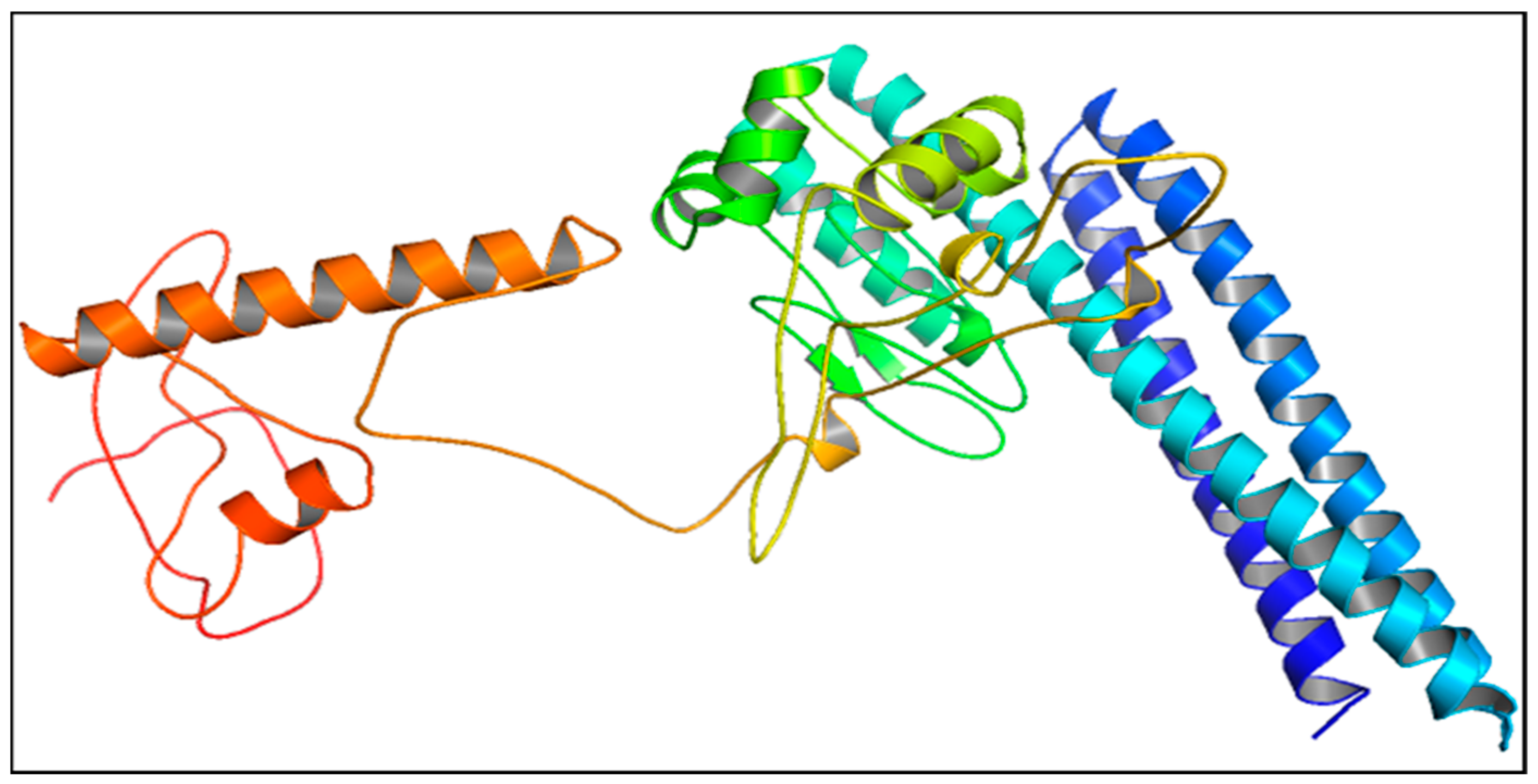
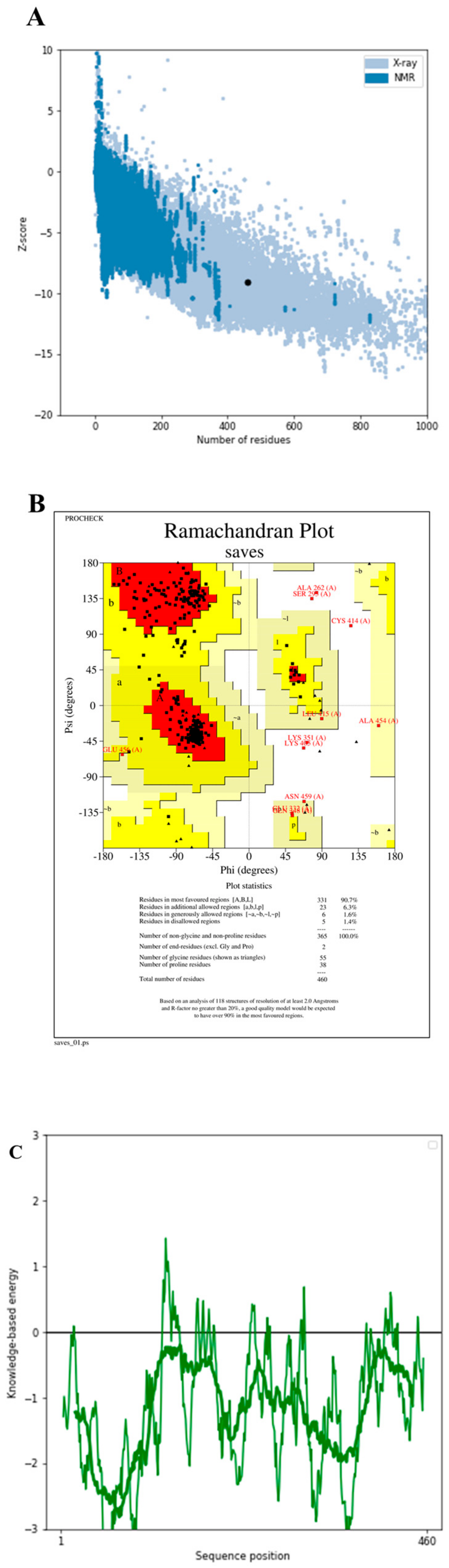
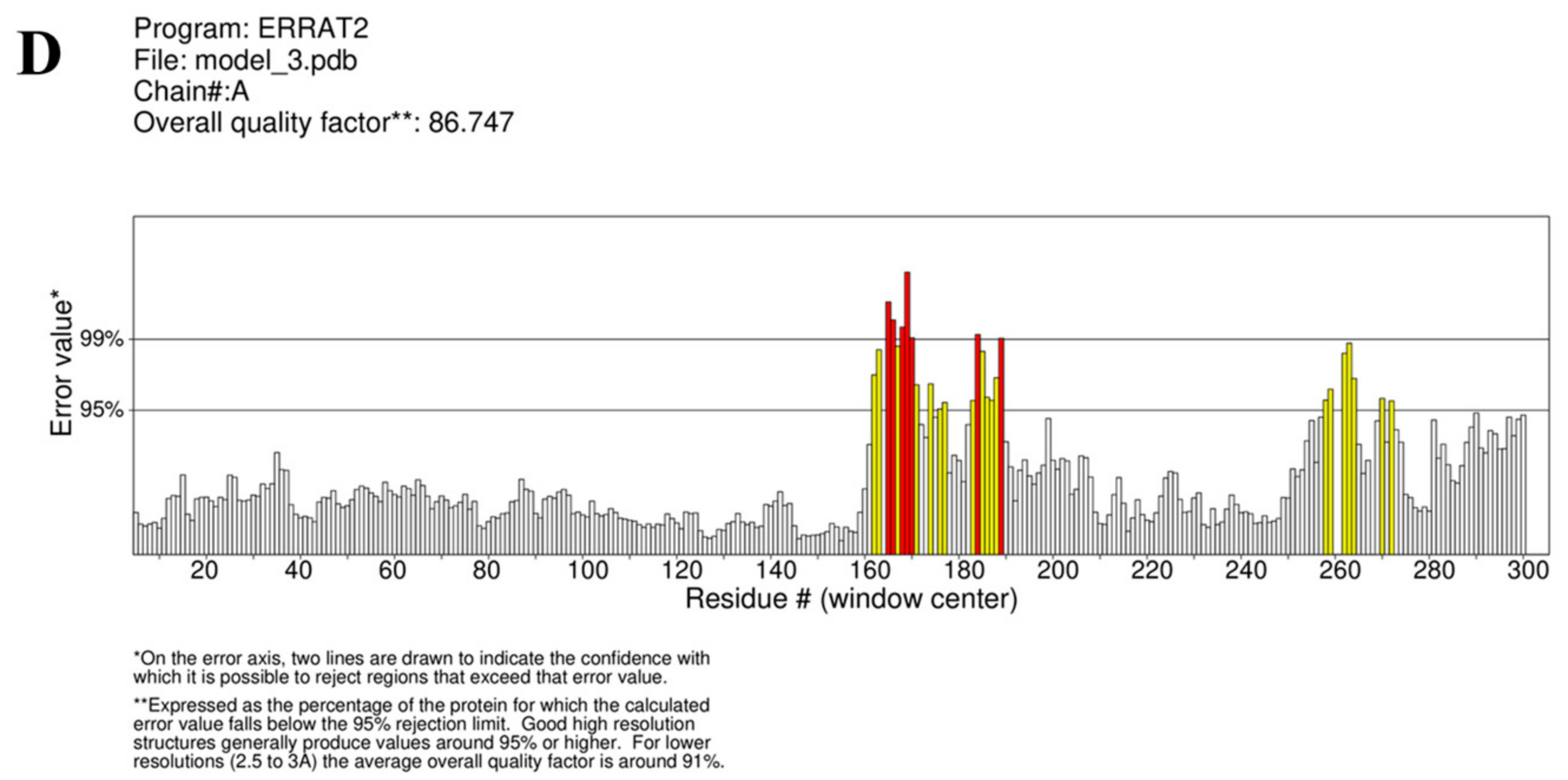
3.16. Tertiary Structure Prediction, Refinement, and Validation of Design Vaccine
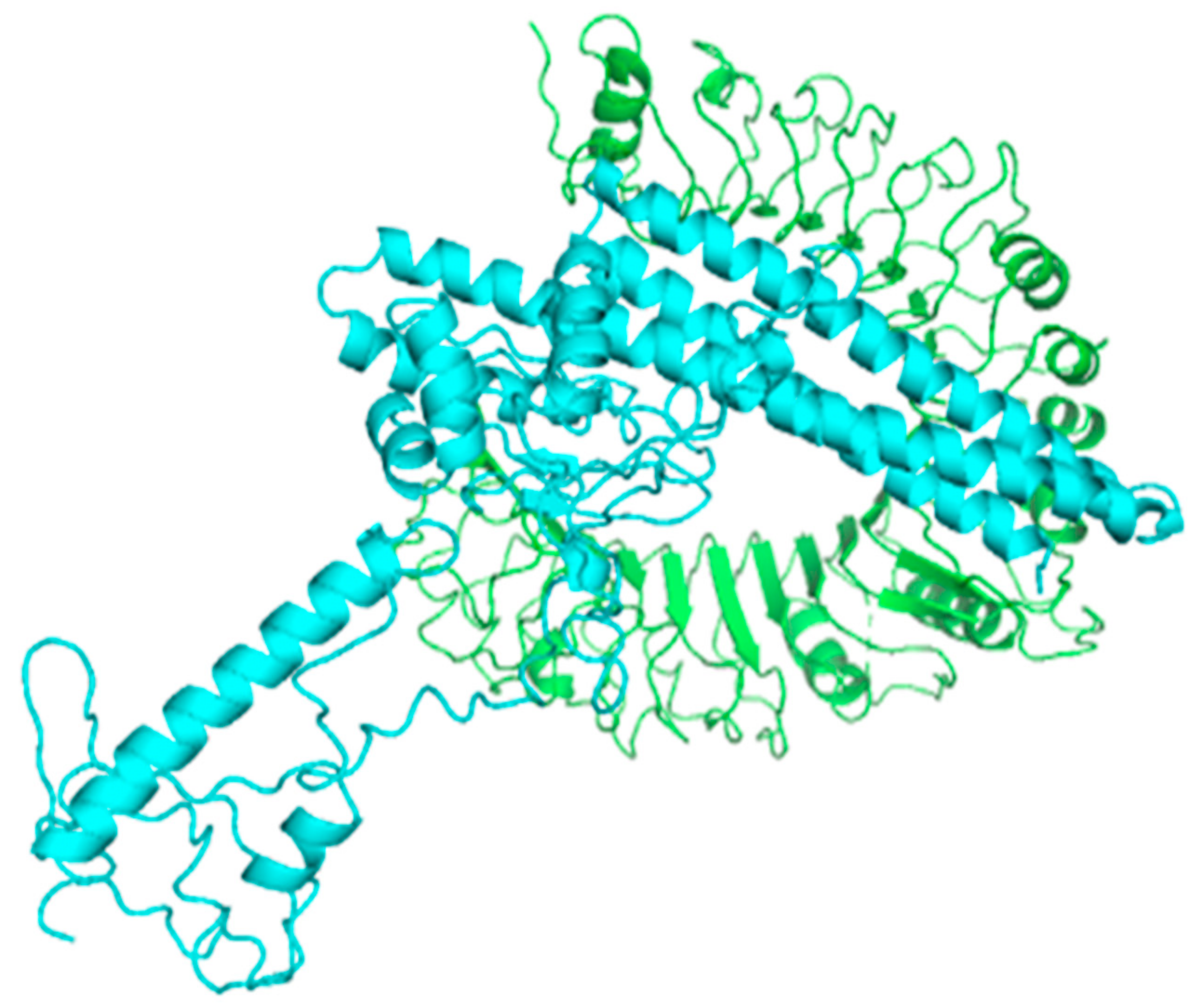
3.17. Molecular Docking of the Constructed Vaccine with Human TLR-2 and TLR-4
3.18. MD Simulation
3.19. Discontinuous B-Cell Epitope Prediction
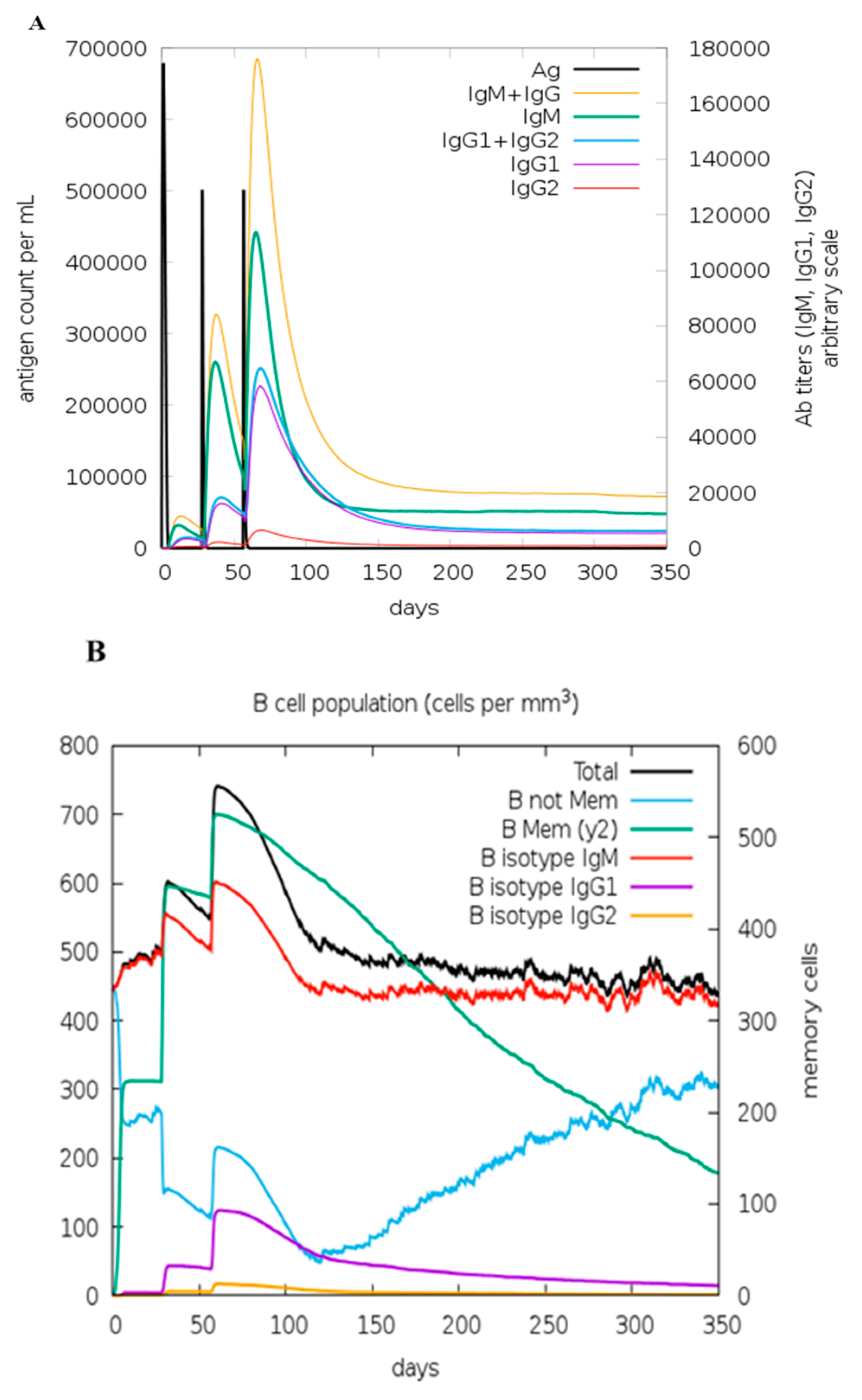
3.20. In Silico Immune Simulation
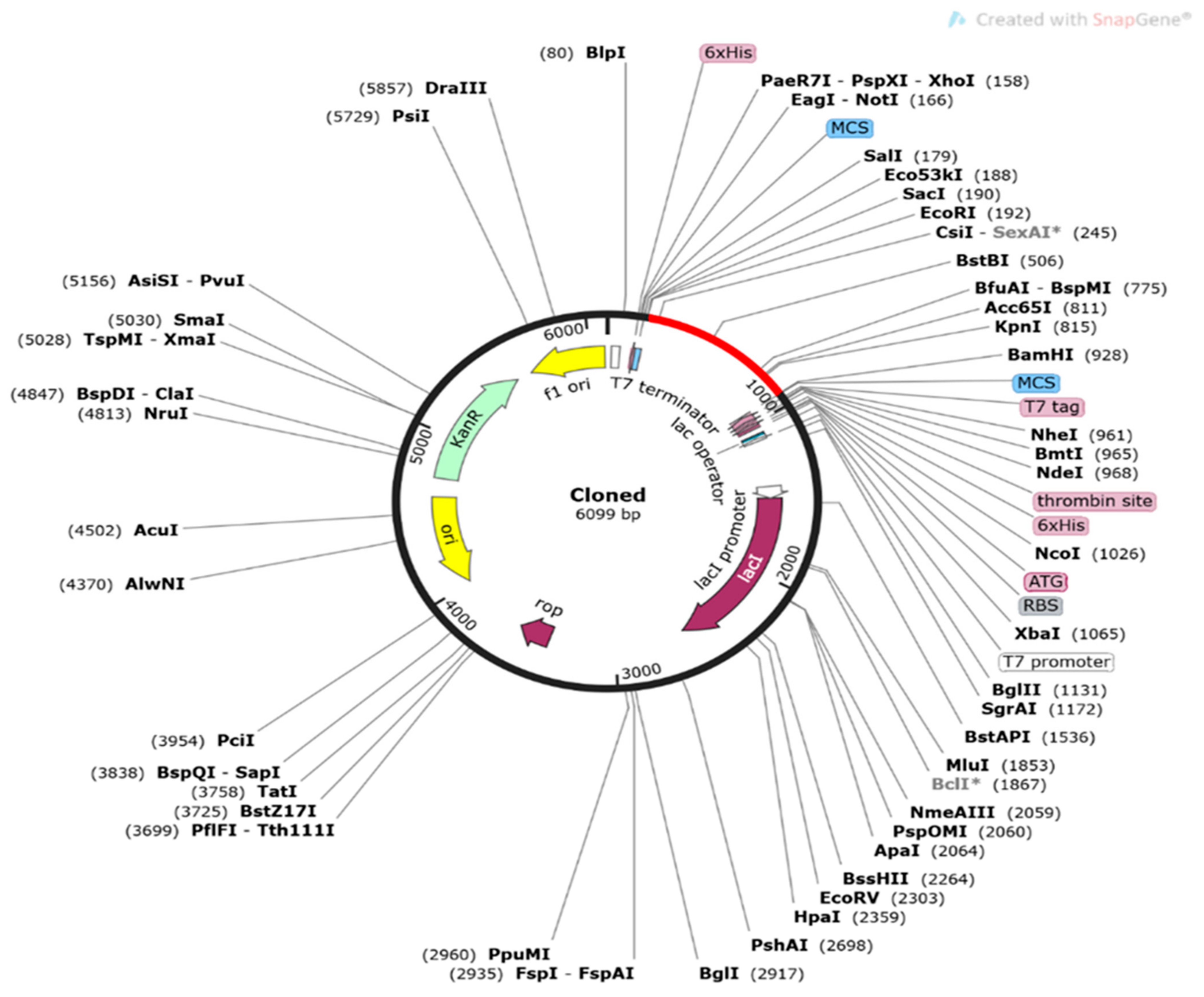
3.21. Codon Adaptation and In Silico Cloning of the Vaccine Construct
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stamm, L.V.; Bergen, H.L. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob. Agents Chemother. 2000, 44, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Norris, S.J.; Weinstock, G.M.; White, O.; Sutton, G.G.; Dodson, R.; Gwinn, M.; Hickey, E.K.; Clayton, R.; Ketchum, K.A.; et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 1998, 281, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Matějková, P.; Strouhal, M.; Šmajs, D.; Norris, S.J.; Palzkill, T.; Petrosino, J.F.; Sodergren, E.; Norton, J.E.; Singh, J.; Richmond, T.A.; et al. Complete genome sequence of Treponema pallidum ssp. Pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 2008, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Giacani, L.; Jeffrey, B.M.; Molini, B.J.; Le, H.T.; Lukehart, S.A.; Lara, A.C.; Rockey, D.D. Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J. Bacteriol. Res. 2010, 192, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. Available online: https://pubmed.ncbi.nlm.nih.gov/21413252/ (accessed on 2 September 2022).
- Ramos, A.N., Jr.; Matida, L.H.; Saraceni, V.; Veras, M.A.; Pontes, R.J. Control of mother-to-child transmission of infectious diseases in Brazil: Progress in HIV/AIDS and Failure in Congenital Syphilis. Cad Saude Publica 2007, 23, S370–S378. Available online: https://www.scielosp.org/article/ssm/content/raw/?resource_ssm_path=/media/assets/csp/v23s3/05.pdf (accessed on 2 September 2022). [CrossRef]
- Schmid, G.P.; Stoner, B.P.; Hawkes, S.; Broutet, N. The need and plan for global elimination of congenital syphilis. Sex. Transm. Dis. 2007, 34, S5–S10. Available online: https://www.jstor.org/stable/44969278 (accessed on 2 September 2022). [CrossRef]
- Zhao, F.; Wu, Y.; Zhang, X.; Yu, J.; Gu, W.; Liu, S.; Zeng, T.; Zhang, Y.; Wang, S. Enhanced immune response and protective efficacy of a Treponema pallidum Tp92 DNA vaccine vectored by chitosan nanoparticles and adjuvanted with IL-2. Hum. Vaccines 2011, 7, 1083–1089. [Google Scholar] [CrossRef][Green Version]
- Sellati, T.J.; Wilkinson, D.A.; Sheffield, J.S.; Koup, R.A.; Radolf, J.D.; Norgard, M.V. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J. Infect. Dis. 2000, 181, 283–293. [Google Scholar] [CrossRef]
- Heymans, R.; Helm, J.J.; Vries, H.J.; Fennema, H.S.; Coutinho, R.A.; Bruisten, S.M. Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J. Clin. Microbiol. 2010, 48, 497–502. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for the Treatment of Treponema pallidum (Syphilis). 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/249572/?sequence=1 (accessed on 3 September 2022).
- Chen, Z.-Q.; Zhang, G.-C.; Gong, X.-D.; Lin, C.; Gao, X.; Liang, G.-J.; Yue, X.-L.; Chen, X.-S.; Myron S Cohen, M.S. Syphilis in China: Results of a national surveillance programme. Lancet 2007, 369, 132–138. [Google Scholar] [CrossRef]
- Sharpe, T.T.; Harrison, K.M.; Dean, H.D. Summary of CDC consultation to address social determinants of health for prevention of disparities in HIV/AIDS, viral hepatitis, sexually transmitted diseases, and tuberculosis. Public Health Rep. 2010, 125 (Suppl. S4), 11–15. [Google Scholar] [CrossRef]
- Douglas, J.M. Penicillin treatment of syphilis. JAMA 2009, 301, 769–771. [Google Scholar] [CrossRef]
- South, M.A.; Short, D.H.; Knox, J.M. Failure of erythromycin estolate therapy in in utero syphilis. JAMA 1964, 190, 70–71. [Google Scholar] [CrossRef]
- Fenton, L.J.; Light, I.J. Congenital syphilis after maternal treatment with erythromycin. Obstet. Gynecol. 1976, 47, 492–494. [Google Scholar]
- Philipson, A.; Sabath, L.D.; Charles, D. Transplacental passage of erythromycin and clindamycin. N. Engl. J. Med. 1973, 288, 1219–1221. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Barrett, L.K.; Lukehart, S.A.; Schmidt, B.; Schriefer, M.; Cameron, C.E. Serodiagnosis of syphilis: Antibodies to recombinant Tp0453, Tp92, and Gpd proteins are sensitive and specific indicators of infection by Treponema pallidum. J. Clin. Microbiol. 2003, 41, 3668–3674. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, H.J.; Lee, M.G.; Lee, J.B. Virulent Treponema pallidum 47 kDa antigen regulates the expression of cell adhesion molecules and binding of T-lymphocytes to cultured human dermal microvascular endothelial cells. Yonsei Med. J. 2000, 41, 623–633. [Google Scholar] [CrossRef][Green Version]
- Cameron, C.E.; Castro, C.; Lukehart, S.A.; Voorhis, W.C. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect. Immun. 1998, 66, 5763–5770. [Google Scholar] [CrossRef]
- Cameron, C.E.; Castro, C.; Lukehart, S.A.; Voorhis, W.C. Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect. Immun. 1999, 67, 3168–3170. [Google Scholar] [CrossRef]
- Cameron, C.E.; Lukehart, S.A.; Castro, C.; Molini, B.; Godornes, C.; Voorhis, W.C. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 2000, 181, 1401–1413. [Google Scholar] [CrossRef]
- Hazlett, K.R.; Sellati, T.J.; Nguyen, T.T.; Cox, D.L.; Clawson, M.L.; Caimano, M.J.; Radolf, J.D. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 2001, 193, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A.; Lukehart, S.A.; Voorhis, W.C. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect. Immun. 2002, 70, 6811–6816. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.-K.; Kang, Y.-M.; Lee, H.-R.; Lee, S.-H.; Choi, B.-K. Highly conserved surface proteins of oral spirochetes as adhesins and potent inducers of proinflammatory and osteoclastogenic factors. Infect. Immun. 2008, 76, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Why certain vaccines have been delayed or not developed at all. Health Aff. 2005, 24, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Barh, D.; Tiwari, S.; Jain, N.; Ali, A.; Rodrigues Santos, A.; Narayan Misra, A.; Azevedo, V.; Kumar, A. In silico subtractive genomics for target identification in human bacterial pathogens. Drug Dev. Res. 2011, 72, 162–177. [Google Scholar] [CrossRef]
- Barh, D.; Gupta, K.; Jain, N.; Khatri, G.; León-Sicairos, N.; Canizalez-Roman, A.; Tiwari, S.; Verma, A.; Rahangdale, S.; Hassan, S.S.; et al. Conserved host–pathogen PPIs Globally conserved inter-species bacterial PPIs based conserved host-pathogen interactome derived novel target in C. pseudotuberculosis, C. diphtheriae, M. tuberculosis, C. ulcerans, Y. pestis, and E. coli targeted by Piper betel compounds. Integr. Biol. 2013, 5, 495–509. [Google Scholar] [CrossRef]
- Perumal, D.; Lim, C.S.; Sakharkar, K.R.; Sakharkar, M.K. Differential genome analyses of metabolic enzymes in Pseudomonas aeruginosa for drug target identification. Silico Biol. 2007, 7, 453–465. Available online: https://content.iospress.com/articles/in-silico-biology/isb00320 (accessed on 2 September 2022).
- Chander, S.; Pandey, R.K.; Penta, A.; Choudhary, B.S.; Sharma, M.; Malik, R.; Prajapati, V.K.; Murugesan, S. Molecular docking and molecular dynamics simulation based approach to explore the dual inhibitor against HIV-1 reverse transcriptase and Integrase. Comb. Chem. High Throughput Screen. 2017, 20, 734–746. [Google Scholar] [CrossRef]
- Baseer, S.; Ahmad, S.; Ranaghan, K.E.; Azam, S.S. Towards a peptide-based vaccine against Shigella sonnei: A subtractive reverse vaccinology based approach. Biologicals 2017, 50, 87–99. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Sanober, G.; Ahmad, S.; Azam, S.S. Identification of plausible drug targets by investigating the druggable genome of MDR Staphylococcus epidermidis. Gene Rep. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Zhang, R.; Ou, H.-Y.; Zhang, C.-T. DEG: A database of essential genes. Nucleic Acids Res. 2004, 32 (Suppl. S1), D271–D272. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J.J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2016, 4, 13. [Google Scholar] [CrossRef]
- Yu, N.; Wagner, J.R.; Laird, M.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of Protein Subcellular Localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Yu, C.-S.; Cheng, C.-W.; Su, W.-C.; Chang, S.-C.; Huang, S.-W.; Hwang, J.-K.; Lu, C.-H. CELLO2GO: A web server for protein subCELlular LOcalization prediction with functional gene ontology annotation. PLoS ONE 2014, 9, e99368. [Google Scholar] [CrossRef]
- Knox, C.; Law, V.; Jewison, T.; Liu, P.; Ly, S.; Frolkis, A.; Pon, A.; Banco, K.; Mak, C.; Neveu, V. DrugBank 3.0: A Comprehensive Resource for ‘Omics’ Research on Drugs. Nucleic Acids Res. 2010, 39, D1035–D1041. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.-M. ARG-ANNOT (Antibiotic Resistance Gene-ANNOTation), a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes 2. Antimicrob. Agents Chemother. 2013, 58, 212–220. [Google Scholar] [CrossRef]
- Solanki, V.; Tiwari, V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci. Rep. 2018, 8, 9044. [Google Scholar] [CrossRef]
- Zhou, C.; Smith, J.; Lam, M.; Zemla, A.; Dyer, M.; Slezak, T. MvirDB—A microbial database of protein toxins, virulence factors and antibiotic resistance genes for bio-defence applications. Nucleic Acids Res. 2007, 35 (Suppl. S1), D391–D394. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Ahmad, S.; Azam, S.S. Subtractive proteomics and immunoinformatics revealed novel B-cell derived T-cell epitopes against Yersinia enterocolitica: An etiological agent of Yersiniosis. Microb. Pathog. 2018, 125, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Mahmud, A.; Iqbal, A.; Hoque, S.F.; Hasan, M. Subtractive genomics approach towards the identification of novel therapeutic targets against human Bartonella bacilliformis. Inform. Med. Unlocked 2020, 20, 100385. [Google Scholar] [CrossRef]
- Magnan, C.N.; Zeller, M.; Kayala, M.A.; Vigil, A.; Randall, A.; Felgner, P.L.; Baldi, P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 2010, 26, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Yasri, S.; Wiwanitkit, V. Dose prediction of lopinavir/ritonavir based on mathematic modeling for 2019-novel coronavirus (2019-nCoV) infection. Asian Pac. J. Trop. Med. 2020, 13, 45–49. Available online: http://119.18.194.205:100/filesdown/xg/xgwk006.pdf (accessed on 5 July 2022). [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Larsen, M.V.; Lundegaard, C.; Lamberth, K.; Buus, S.; Lund, O.; Nielsen, M. Large-scale validation of methods for cytotoxic t-lymphocyte epitope prediction. BMC Bioinform. 2007, 8, 424. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Nielsen, M.; Lund, O. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinform. 2009, 10, 296. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Vir, P.; Raghava, G.P. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 8, 30. [Google Scholar] [CrossRef]
- Sanches, R.C.; Tiwari, S.; Ferreira, L.C.; Oliveira, F.M.; Lopes, M.D.; Passos, M.J.; Maia, E.H.; Taranto, A.G.; Kato, R.; Azevedo, V.A.; et al. Immunoinformatics design of multi-epitope peptide-based vaccine against Schistosoma mansoni using transmembrane proteins as a target. Front. Immunol. 2021, 12, 621706. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Bui, H.-H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A New Structure-Based Tool for the Prediction of Antibody Epitopes. BMC Bioinform. 2008, 9, 1–8. Available online: https://link.springer.com/article/10.1186/1471-2105-9-514 (accessed on 11 July 2022). [CrossRef]
- Khatoon, N.; Pandey, R.K.; Prajapati, V.K. Exploring Leishmania secretory proteins to design B and T cell multi-epitope subunit vaccine using immunoinformatics approach. Cytokine 2017, 7, 8285. [Google Scholar] [CrossRef]
- Jang, A.-R.; Shin, S.J.; Park, J.-H. Mycobacterium tuberculosis ESAT6 induces IFN-β gene expression in Macrophages via TLRs-mediated signaling. Cytokine 2018, 104, 104–109. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ved Prakash Dwivedi, V.P.; Yogesh Singh, Y.; Imran Siddiqui, I.; Sharma Kaer, L.V.; Hattopadhyay, D.; Das, G. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011, 7, e1002378. [Google Scholar] [CrossRef]
- Shams, N.; Gandabeh, Z.S.; Nazifi, N.; Forouharmehr, A.; Jaydari, A.; Rashidian, E. Computational design of different epitope-based vaccines against Salmonella typhi. Int. J. Pept. Res. Ther. 2020, 26, 1527–1539. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Patra, P.; Ghosh, P.; Sharma, G.; Patra, B.C.; Lee, S.-S.; Chakraborty, C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020, 92, 618–631. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, X.; Li, B.; Huang, J.; Zhan, B.; Zhu, X. Vaccination with a paramyosin-based multi-epitope vaccine elicits significant protective immunity against Trichinella spiralis infection in mice. Front. Microbiol. 2017, 8, 1475. [Google Scholar] [CrossRef]
- Arai, R.; Ueda, H.; Kitayama, A.; Kamiya, N.; Nagamune, T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001, 14, 529–532. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Dimitrov, I.; Naneva, L.; Doytchinova, I.; Bangov, I. AllergenFP: Allergenicity prediction by descriptor fingerprints. Bioinformatics 2014, 30, 846–851. [Google Scholar] [CrossRef] [PubMed]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Kim, D.E.; Chivian, D.; Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32 (Suppl. S2), W526–W531. [Google Scholar] [CrossRef] [PubMed]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35 (Suppl. S2), W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Colovos, C.; Yeates, T. ERRAT: An empirical atom-based method for validating protein structures. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Laskowski, R.A. PDBsum: Summaries and analyses of PDB structures. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef]
- Van Aalten, D.M.; De Groot, B.L.; Findlay, J.B.; Berendsen, H.J.; Amadei, A. A comparison of techniques for calculating protein essential dynamics. J. Comput. Chem. 1997, 18, 169–181. [Google Scholar] [CrossRef]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Orti, E.S.; Chacón, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef]
- Wüthrich, K.; Wagner, G.; Richarz, R.; Braun, W. Correlations between internal mobility and stability of globular proteins. Biophys. J. 1980, 32, 549–560. [Google Scholar] [CrossRef]
- Rapin, N.; Lund, O.; Bernaschi, M.; Castiglione, F. Computational immunology meets bioinformatics: The use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 2010, 5, e9862. [Google Scholar] [CrossRef]
- Castiglione, F.; Mantile, F.; Berardinis, P.D.; Prisco, A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput. Math. Methods Med. 2012, 2012, 842329. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.-H. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Nagpal, G.; Usmani, S.S.; Raghava, G.P. A web resource for designing subunit vaccine against major pathogenic species of bacteria. Front. Immunol. 2018, 9, 2280. [Google Scholar] [CrossRef]
- Arumugam, S.; Varamballi, P. In-silico design of envelope based multi-epitope vaccine candidate against Kyasanur forest disease virus. Sci. Rep. 2021, 11, 17118. [Google Scholar] [CrossRef]
- Dar, H.A.; Zaheer, T.; Shehroz, M.; Ullah, N.; Naz, K.; Muhammad, S.A.; Zhang, T.; Ali, A. Immunoinformatics-aided design and evaluation of a potential multi-epitope vaccine against Klebsiella pneumoniae. Vaccines 2019, 7, 88. [Google Scholar] [CrossRef]
- Soltan, M.A.; Magdy, D.; Solyman, S.M.; Hanora, A. Design of Staphylococcus aureus New Vaccine Candidates with B and T Cell Epitope Mapping, Reverse Vaccinology, and Immunoinformatics. Omi. A J. Integr. Biol. 2020, 24, 195–204. [Google Scholar] [CrossRef]
- Khan, M.K.; Zaman, S.; Chakraborty, S.; Chakravorty, R.; Alam, M.M.; Bhuiyan, T.R.; Rahman, M.J.; Fernández, C.; Qadri, F.; Seraj, Z.I. In silico predicted mycobacterial epitope elicits in vitro T-cell responses. Mol. Immunol. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Leow, C.Y.; Kazi, A.; Ismail, C.M.K.H.; Chuah, C.; Lim, B.H.; Leow, C.H.; Singh, K.K.B. Reverse vaccinology approach for the identification and characterization of outer membrane proteins of shigella flexneri as potential cellular-and antibody-dependent vaccine candidates. Clin. Exp. Vaccine Res. 2000, 22, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Solanki, V.; Tiwari, M.; Tiwari, V. Prioritization of potential vaccine targets using comparative proteomics and designing of the chimeric multi-epitope vaccine against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 5240. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.A.; Elbassiouny, N.; Gamal, H.; Elkaeed, E.B.; Eid, R.A.; Eldeen, M.A.; Al-Karmalawy, A.A. In Silico Prediction of a Multitope Vaccine against Moraxella catarrhalis: Reverse Vaccinology and Immunoinformatics. Vaccines 2021, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhu, Y.; Li, Y.; Chen, Z.; Sha, T.; Li, Z.; Zhang, F.; Ding, J. Design of a Novel Multi-Epitope Vaccine Against Echinococcus granulosus in Immunoinformatics. Front. Immunol. 2021, 12, 668492. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Habibi, M.; Shokrgozar, M.A.; Ahangari Cohan, R.; Ahmadi, K.; Asadi Karam, M.R.; Bouzari, S. In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli. Sci. Rep. 2020, 10, 16258. [Google Scholar] [CrossRef]


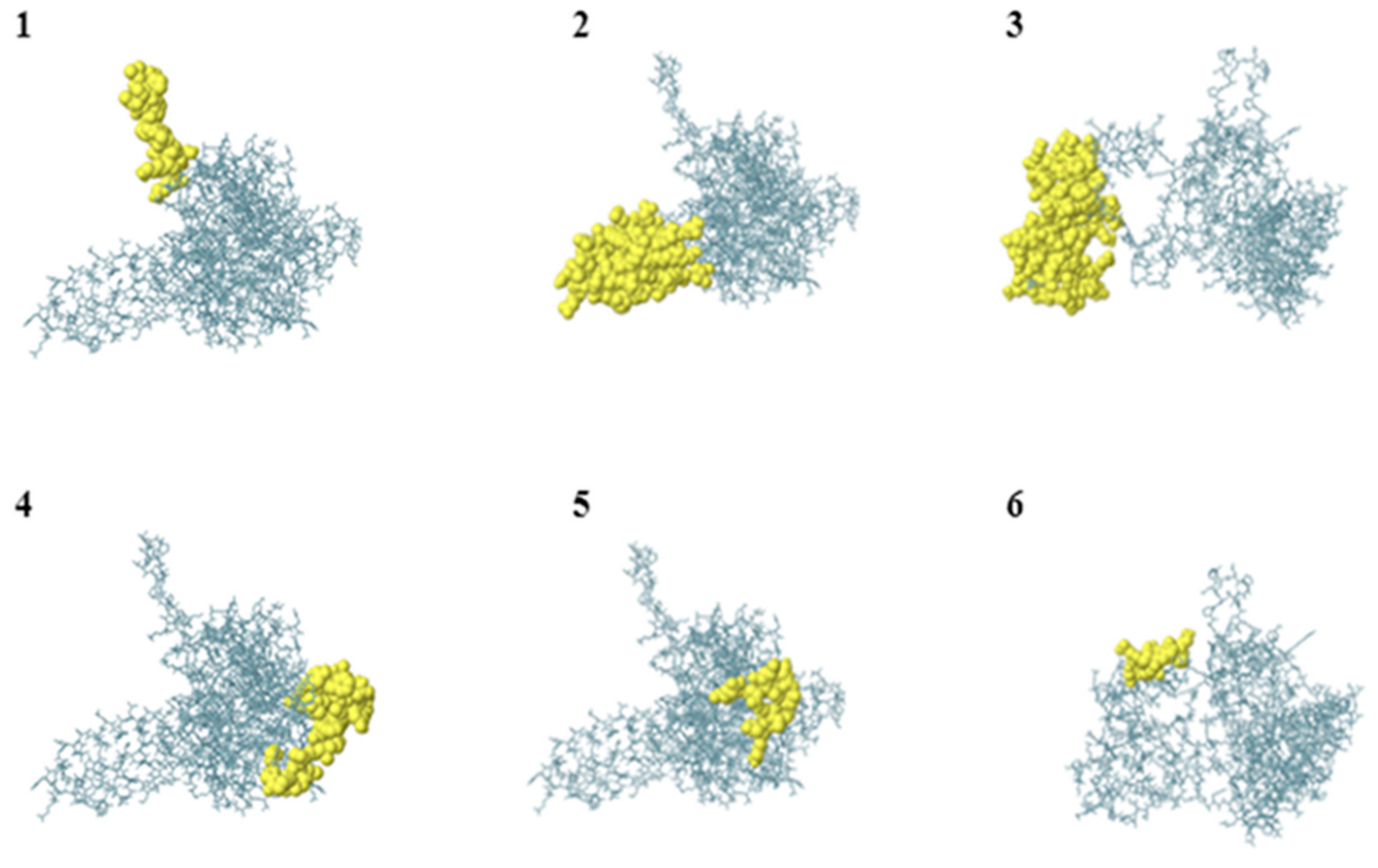

| Cluster | Size | Protein ID | % Similarity |
|---|---|---|---|
| >Cluster 0 0 1 | |||
| 215aa 48aa | P56822 O83336 | 98.14% 93.75% | |
| >Cluster 1 0 >Cluster 2 0 | |||
| 756aa 598aa | O83337 O88138 | 85.45% 81.10% | |
| Protein Name | Protein ID | Peptide Sequence | MHC Binding Affinity | Rescale Binding Affinity | C-Terminal Cleavage Affinity | Transport Affinity | Prediction Score | MHC-I Binding | VaxiJen Score | AllerTOP v.2.0 | Immunogenicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FTSK_TREPA DNA translocase FtsK | O83964 | LALLGAELY | 0.178 | 0.7558 | 0.7357 | 3.047 | 1.0185 | Yes | 0.5305 | Non-allergen | 0.13309 |
| SOJ_TREPA Protein | O83296 | TSAINLGAY | 0.6054 | 2.5705 | 0.4577 | 2.971 | 2.7877 | Yes | 0.4485 | Non-allergen | 0.18134 |
| TREPA Site-determining protein | F7IVD2 | IATNMAIAY | 0.2248 | 0.9546 | 0.539 | 3.105 | 1.1907 | Yes | 0.6396 | Non-allergen | 0.0071 |
| TREPA ABC transporter, ATP-binding protein | O83930 | TVGFVFQQY | 0.1452 | 0.6164 | 0.9747 | 3.011 | 0.9131 | Yes | 0.4966 | Non-allergen | 0.11376 |
| Name | Uniport ID | Start | End | Alleles | Peptide Sequence | Method | Toxicity | Antigenicity | Allergenicity | IFN-γ |
|---|---|---|---|---|---|---|---|---|---|---|
| FTSK_TREPA DNA translocase FtsK | O83964 | 26 | 40 | HLA-DRB5*01:01 | TLSTFLPLFTLHRAS | Consensus (smm/nn/sturniolo) | Non-toxic | 0.589 | Non-allergenic | Positive |
| SOJ_TREPA Protein | O83296 | 141 | 155 | HLA-DRB4*01:01 | VFIPLQCEYFALEGL | Consensus (comb.lib./smm/nn) | Non-toxic | 0.7306 | Non-allergenic | Positive |
| TREPA Site-determining protein | F7IVD2 | 34 | 48 | HLA-DRB1*03:01 | KLLLIDPKIVELKLY | Consensus (smm/nn/sturniolo) | Non-toxic | 1.3598 | Non-allergenic | Positive |
| TREPA Sugar ABC superfamily ATP-binding cassette transporter | O83782 | 39 | 53 | HLA-DRB4*01:01 | FGLRIRKIPQQEIIR | Consensus (comb.lib./smm/nn) | Non-toxic | 0.6532 | Non-allergenic | Positive |
| Peptide | Protein | Score | Antigenicity | Conservancy % |
|---|---|---|---|---|
| PHMQQFNQEHNGDLVSVGNV | TPN32_TREPA membrane lipoprotein TpN32 | 0.983 | 0.408 | 100.00% |
| GGRVRTYLKERYKGGEVAPA | TPN32_TREPA Membrane lipoprotein TpN32 | 0.901 | 0.7478 | 100.00% |
| IPAQDDEQGPPRPIPASAAP | FTSK_TREPA DNA translocase FtsK | 1 | 0.6798 | 100.00% |
| PSDVHAPASPGSLPSVIPAQ | FTSK_TREPA DNA translocase FtsK | 0.998 | 0.4694 | 100.00% |
| TGIKKGPVVTMFELLPPPGI | FTSK_TREPA DNA translocase FtsK | 0.996 | 0.7765 | 100.00% |
| PEASAPPEGQFSTEVPLQGG | FTSK_TREPA DNA translocase FtsK | 0.99 | 0.6035 | 100.00% |
| RDLMQEKNARERVERHQHRT | TREPA site-determining protein | 0.967 | 0.8618 | 100.00% |
| LKDGKIVGDHVRGHGGADGG | TREPA ABC transporter, ATP-binding protein | 0.981 | 1.5311 | 100.00% |
| ILGPSGSGKSTCMHMIGCLD | TREPA ABC transporter, ATP-binding protein | 0.948 | 0.9457 | 100.00% |
| LQGGTSQVATVHAPPEISTG | TREPA Sugar ABC superfamily ATP-binding cassette transporter | 0.966 | 0.9404 | 100.00% |
| RPEAITPRTEETLARECANV | TREPA Sugar ABC superfamily ATP-binding cassette transporter | 0.946 | 0.7421 | 100.00% |
| Cluster 1 | |
|---|---|
| HADDOCK score Cluster size RMSD from the overall lowest-energy structure Van der Waals energy Electrostatic energy Desolvation energy Restraint’s violation of energy Buried Surface Area Z-Score | −52.2 +/− 6.4 18 2.5 +/− 1.4 −118.9 +/− 12.7 −428.7 +/− 52.0 −4.9 +/− 4.2 1573.1 +/− 143.0 3926.3 +/− 189.9 −2.1 |
| Cluster 10 | |
|---|---|
| HADDOCK score Cluster size RMSD from the overall lowest-energy structure Van der Waals energy Electrostatic energy Desolvation energy Restraint’s violation of energy Buried Surface Area Z-Score | 17.7 +/− 17.5 5 1.7 +/− 1.6 −40.1 +/− 5.1 −326.1 +/− 93.0 −5.5 +/− 1.8 1286.0 +/− 213.8 2678.3 +/− 408.6 −1.0 |
| No. Residues | Number of Residues Score | Score |
|---|---|---|
| 1 | A:K286, A:S288, A:D289, A:V290, A:H291, A:A292, A:P293, A:A294, A:S295, A:P296, A:G297, A:S298, A:L299, A:P300, A:S301, A:V302, A:I303, A:P304, A:A305, A:Q306, A:K307 | 0.801 |
| 2 | A:Q1, A:W2, A:N3, A:F4, A:A5, A:G6, A:I7, A:E8, A:A9, A:A10, A:S11, A:S12, A:A13, A:I14, A:Q15, A:G16, A:T19, A:N63, A:Q66, A:N67, A:L68, A:A69, A:R70, A:T71, A:I72, A:S73, A:E74, A:A75, A:G76, A:Q77, A:A78, A:M79, A:Q80, A:S81, A:T82, A:E83, A:G84, A:N85, A:V86, A:T87, A:G88, A:E89, A:A90, A:A91, A:A92, A:K93, A:L94, A:A95, A:L96, A:L97, A:G98, A:A99, A:E100, A:L101 | 0.798 |
| 3 | A:P337, A:E338, A:G339, A:Q340, A:F341, A:V365, A:E366, A:H368, A:Q369, A:H370, A:R371, A:T372, A:K373, A:K374, A:L375, A:K376, A:D377, A:G378, A:K379, A:I380, A:V381, A:G382, A:D383, A:H384, A:V385, A:R386, A:H388, A:G390, A:A391, A:D392, A:G393, A:G394, A:K395, A:K396, A:I397, A:L398, A:G399, A:P400, A:S401, A:G402, A:S403, A:G404, A:K405, A:S406, A:T407, A:C408, A:M409, A:H410, A:M411, A:I412, A:G413, A:C414, A:L415, A:D416, A:K417, A:K418, A:L419, A:Q420, A:G421, A:G422, A:T423, A:S424, A:Q425, A:V426, A:A427, A:T428, A:V429, A:H430, A:A431, A:P432, A:P433, A:E434, A:I435, A:S436, A:T437, A:G438, A:K439, A:R441, A:P442, A:E443, A:A444, A:I445, A:T446, A:P447, A:R448, A:T449, A:E450, A:E451, A:T452, A:L453, A:A454, A:R455, A:E456, A:C457, A:A458, A:N459, A:V460 | 0.754 |
| 4 | A:Y129, A:T130, A:V131, A:G132, A:F133, A:V134, A:F135, A:Q136, A:Q137, A:Y138, A:G139, A:P140, A:G141, A:P142, A:G143, A:T144, A:L145, A:S146, A:T147, A:F148, A:L151, A:L154, A:H155, A:A157, A:S158, A:G159, A:P160, A:G161, A:G163, A:Q169 | 0.64 |
| 5 | A:T33, A:K34, A:A36, A:A37, A:A38, A:W39, A:G40, A:G41, A:S42, A:G43, A:S44, A:E45, A:Q48, A:Q52 | 0.612 |
| 6 | A:S342, A:T343, A:E344, A:V345, A:P346, A:L347, A:Q348, A:K351, A:E358, A:R362 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Rizwan, M.; Zeb, A.; Eldeen, M.A.; Hassan, S.; Ur Rehman, A.; A. Eid, R.; Samir A. Zaki, M.; M. Albadrani, G.; E. Altyar, A.; et al. Identification of a Potential Vaccine against Treponema pallidum Using Subtractive Proteomics and Reverse-Vaccinology Approaches. Vaccines 2023, 11, 72. https://doi.org/10.3390/vaccines11010072
Khan S, Rizwan M, Zeb A, Eldeen MA, Hassan S, Ur Rehman A, A. Eid R, Samir A. Zaki M, M. Albadrani G, E. Altyar A, et al. Identification of a Potential Vaccine against Treponema pallidum Using Subtractive Proteomics and Reverse-Vaccinology Approaches. Vaccines. 2023; 11(1):72. https://doi.org/10.3390/vaccines11010072
Chicago/Turabian StyleKhan, Siyab, Muhammad Rizwan, Adnan Zeb, Muhammad Alaa Eldeen, Said Hassan, Ashfaq Ur Rehman, Refaat A. Eid, Mohamed Samir A. Zaki, Ghadeer M. Albadrani, Ahmed E. Altyar, and et al. 2023. "Identification of a Potential Vaccine against Treponema pallidum Using Subtractive Proteomics and Reverse-Vaccinology Approaches" Vaccines 11, no. 1: 72. https://doi.org/10.3390/vaccines11010072
APA StyleKhan, S., Rizwan, M., Zeb, A., Eldeen, M. A., Hassan, S., Ur Rehman, A., A. Eid, R., Samir A. Zaki, M., M. Albadrani, G., E. Altyar, A., Nouh, N. A. T., Abdel-Daim, M. M., & Ullah, A. (2023). Identification of a Potential Vaccine against Treponema pallidum Using Subtractive Proteomics and Reverse-Vaccinology Approaches. Vaccines, 11(1), 72. https://doi.org/10.3390/vaccines11010072








