Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- vaccinated individuals undergoing COVID-19 infection (“COVID-19 Vaccinated”);
- unvaccinated individuals undergoing COVID-19 infection (“COVID-19 Unvaccinated”);
- vaccinated individuals who did not have COVID-19 infection (“Non-COVID-19 Vaccinated”);
- unvaccinated individuals who did not have COVID-19 infection (“Non-COVID-19 Unvaccinated”).
2.2. Sample Collection
2.3. Detection of SARS-CoV-2 Antibody
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Characteristics of the Studied Population
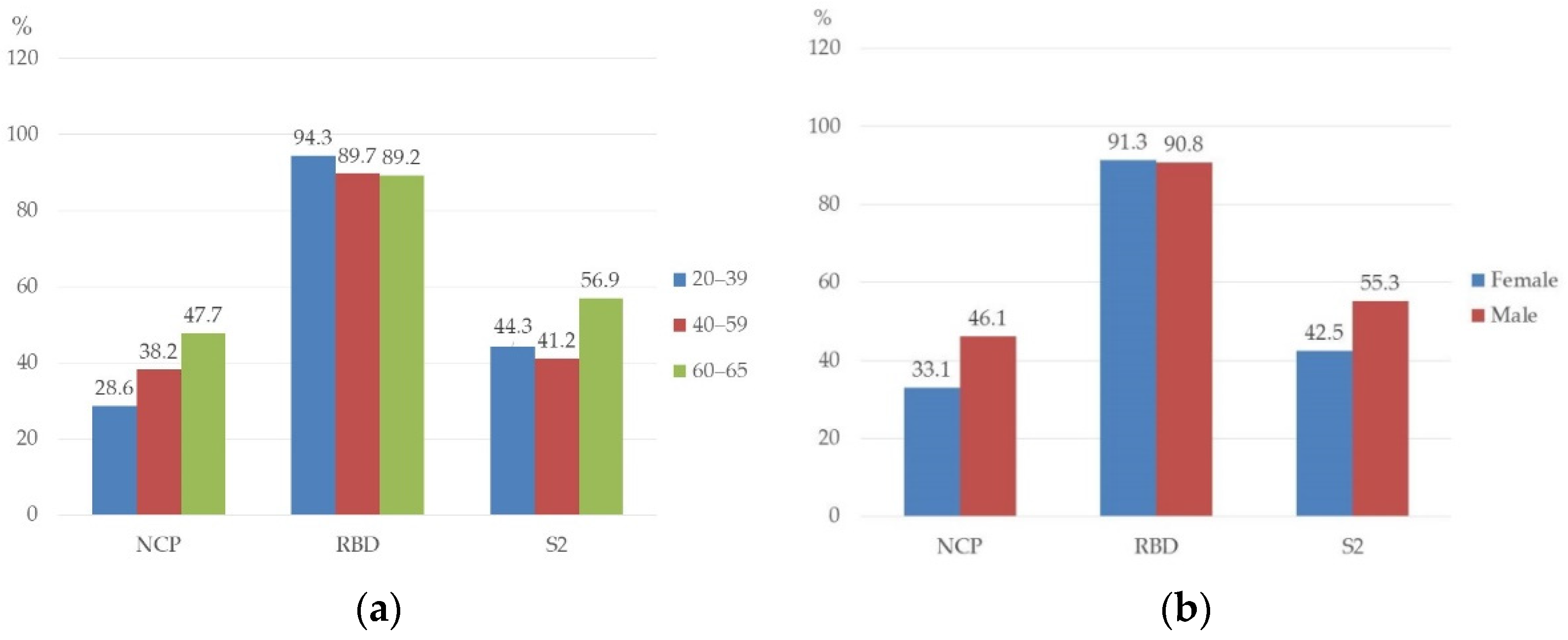
3.2. Prevalence of NCP, RBD, and S2 Antibodies in Studied Persons
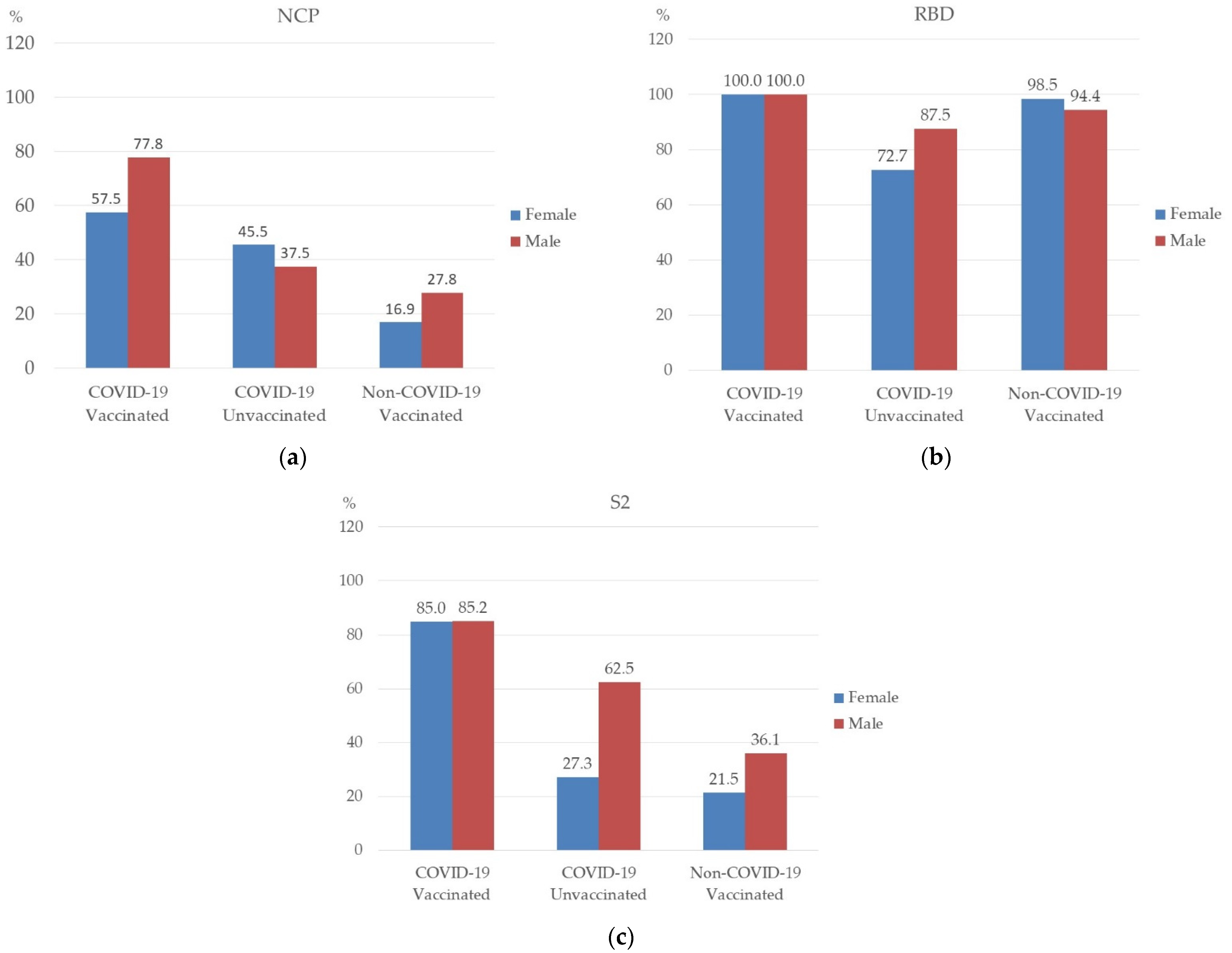
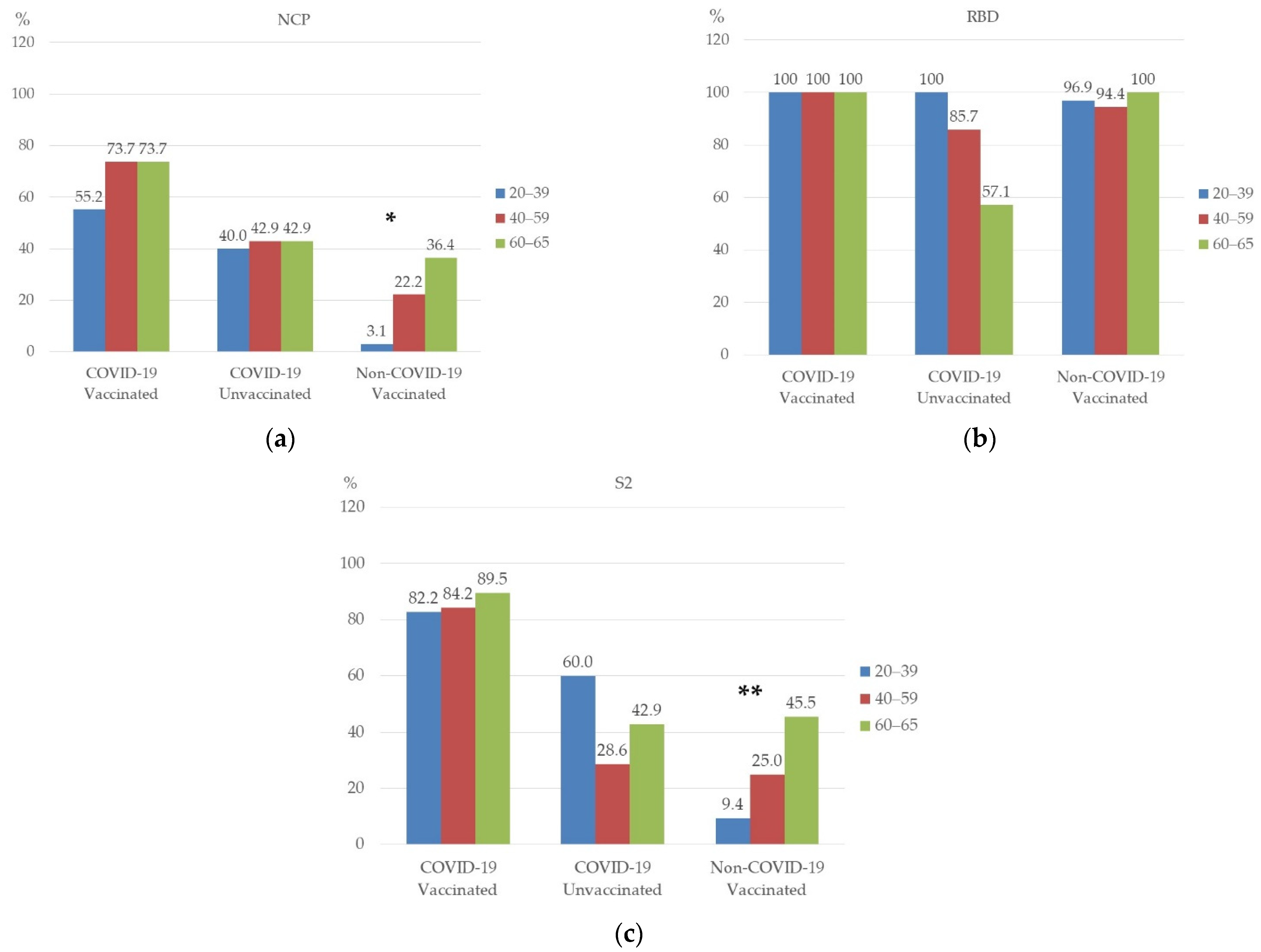
3.3. Prevalence of NCP, RBD, and S2 Antibodies in Studied Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19—18 May 2022. Edition 92. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---18-may-2022 (accessed on 20 May 2022).
- European Medicines Agency. COVID-19 mRNA Vaccine (Comirnaty): EU Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 20 May 2022).
- Sasso, B.L.; Giglio, R.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.; Ciaccio, A.; Agnello, L.; Ciaccio, M. Evaluation of anti-SARS-CoV-2 S-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Jacofsky, D.; Jacofsky, E.M.; Jacofsky, M. Understanding antibody testing for COVID-19. J. Arthroplast. 2020, 35, S74–S81. [Google Scholar] [CrossRef]
- Sauer, K.; Harris, T. An effective COVID-19 vaccine needs to engage T cells. Front. Immunol. 2020, 11, 581807. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. Domains and functions of spike protein in SARS-CoV-2 in the context of vaccine design. Viruses 2021, 13, 109. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Angulo, F.J.; Finelli, L.; Swerdlow, D.L. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw. Open 2021, 4, e2033706. [Google Scholar] [CrossRef]
- Ong, D.S.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, J.-H.; Hsueh, P.-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Slev, P.; Wheeler, S.; Couturier, M.R.; Wong, S.J.; Kadkhoda, K. The role of antibody testing for SARS-CoV-2: Is there one? J. Clin. Microbiol. 2020, 58, e00797-20. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef] [PubMed]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Nicol, T.; Lefeuvre, C.; Serri, O.; Pivert, A.; Joubaud, F.; Dubée, V.; Kouatchet, A.; Ducancelle, A.; Lunel-Fabiani, F.; Le Guillou-Guillemette, H. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 2020, 129, 104511. [Google Scholar] [CrossRef]
- Montesinos, I.; Dahma, H.; Wolff, F.; Dauby, N.; Dalunoy, S.; Wuyts, M.; Detemmerman, C.; Duterme, C.; Vandenberg, O.; Martin, C.; et al. Neutralizing antibody responses following natural SARS-CoV-2 infection: Dynamics and correlation with commercial serologic tests. J. Clin. Virol. 2021, 144, 104988. [Google Scholar] [CrossRef]
- Wolff, F.; Dahma, H.; Duterme, C.; Van den Wijngaert, S.; Vandenberg, O.; Cotton, F.; Montesinos, I. Monitoring antibody response following SARS-CoV-2 infection: Diagnostic efficiency of 4 automated immunoassays. Diagn. Microbiol. Infect. Dis. 2020, 98, 115140. [Google Scholar] [CrossRef]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: A systematic review and meta-analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef]
- Sahu, A.K.; Amrithanand, V.; Mathew, R.; Aggarwal, P.; Nayer, J.; Bhoi, S. COVID-19 in health care workers—A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 1727–1731. [Google Scholar] [CrossRef]
- Lorent, D.; Nowak, R.; Roxo, C.; Lenartowicz, E.; Makarewicz, A.; Zaremba, B.; Nowak, S.; Kuszel, Ł.; Stefaniak, J.; Kierzek, R.; et al. Prevalence of anti-SARS-CoV-2 antibodies in Poznań, Poland, after the first wave of the COVID-19 pandemic. Vaccines 2021, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, J.; Vollmer, T.; Fischer, B.; Becher, H.; Becker, A.-K.; Honarpisheh, H.; Guraya, S.Y.; Strate, T.; Knabbe, C. SARS-CoV-2-antibody response in health care workers after vaccination or natural infection in a longitudinal observational study. Vaccine 2021, 40, 206–212. [Google Scholar] [CrossRef]
- Sonmezer, M.C.; Erul, E.; Sahin, T.K.; Al, I.R.; Cosgun, Y.; Korukluoglu, G.; Zengin, H.; Dizman, G.T.; Inkaya, A.C.; Unal, S. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers before the era of vaccination at a tertiary care hospital in Turkey. Vaccines 2022, 10, 258. [Google Scholar] [CrossRef]
- Houlihan, C.F.; Vora, N.; Byrne, T.; Lewer, D.; Kelly, G.; Heaney, J.; Gandhi, S.; Spyer, M.J.; Beale, R.; Cherepanov, P. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 2020, 396, e6–e7. [Google Scholar] [CrossRef]
- Shields, A.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Aldera, E.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Bosworth, A.; Dunbar, L.A.; et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: A cross-ectional study. Thorax 2020, 75, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Kwiecińska-Piróg, J.; Przekwas, J.; Kraszewska, Z.; Sękowska, A.; Brodzka, S.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Zacharski, M.; Mańkowska-Cyl, A.; et al. The differences in the level of anti-SARS-CoV-2 antibodies after mRNA vaccine between convalescent and non-previously infected people disappear after the second dose—Study in healthcare workers group in Poland. Vaccines 2021, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, E.; Imaizumi, H.; Oshida, S.; Igarashi, K.; Yoshida, M.; Yanase, N. Survey of spike-specific immunoglobulin G antibodies at approximately 3 months and 9 months after vaccination against coronavirus disease 2019 (severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2]) in health care workers. Sangyo Eiseigaku Zasshi 2022. Epub ahead of printing (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Bleier, B.S.; Ramanathan, M., Jr.; Lane, A.P. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 2021, 164, 305–307. [Google Scholar] [CrossRef]
- Noda, K.; Matsuda, K.; Yagishita, S.; Maeda, K.; Akiyama, Y.; Terada-Hirashima, Y.; Matsushita, H.; Iwata, S.; Yamashita, K.; Atarashi, Y.; et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci. Rep. 2021, 11, 5198. [Google Scholar] [CrossRef]
- The U.S. Food and Drug Administration; 2021 Safety Communications. Antibody Testing Is Not Currently Recommended to Assess Immunity after COVID-19 Vaccination: FDA Safety Communication. 2021. Available online: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-COVID-19-vaccination-fda-safety (accessed on 20 May 2022).
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef]
- Kumar, N.; Bhartiya, S.; Desai, S.; Mutha, A.; Beldar, A.; Singh, T. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in Mumbai, India. Asia Pac. J. Public Health 2021, 33, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Balfe, P.; Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Warren, F.; Crook, D.W.; Jeffery, K.; Matthews, P.C.; Klerman, E.B.; et al. Time of day of vaccination affects SARS-CoV-2 antibody responses in an observational study of health care workers. J. Biol. Rhythm. 2022, 37, 124–129. [Google Scholar] [CrossRef] [PubMed]
| N | % | ||
|---|---|---|---|
| Sex | Female | 127 | 62.6 |
| Male | 76 | 37.4 | |
| Age | 20–39 | 70 | 34.5 |
| 40–59 | 68 | 33.5 | |
| 60–65 | 65 | 32.0 | |
| COVID-19 | Yes | 86 | 42.4 |
| No | 117 | 57.6 | |
| Vaccinated | Yes | 168 | 82.8 |
| No | 35 | 17.2 | |
| Physicians | 108 | 53.2 | |
| Nurses | 95 | 46.8 | |
| Participants’ Group | NCP | RBD | S2 | Total Participants N = 203 | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| COVID-19 Vaccinated | 44 | 65.7 | 67 | 100.0 | 57 | 85.1 | 67 | 33.0 |
| COVID-19 Unvaccinated | 8 | 42.1 | 15 | 78.9 | 8 | 42.1 | 19 | 9.4 |
| Non-COVID-19 Vaccinated | 21 | 20.8 | 98 | 97.0 | 27 | 26.7 | 101 | 49.7 |
| Non-COVID-19 Unvaccinated | 4 | 25.0 | 4 | 25.0 | 4 | 25.0 | 16 | 7.9 |
| Participants’ Group | NCP | RBD | S2 |
|---|---|---|---|
| COVID-19 Vaccinated | 849.2 (399.8–989.2) | 965.3 (224.8–976.1) | 771.6 (478.3–833.0) |
| COVID-19 Unvaccinated | 559.1 (196.3–969.1) | 696.9 (210.0–999.9) | 476.1 (187.3–986.1) |
| Non-COVID-19 Vaccinated | 558.7 (196.3–969.1) | 888.7 (190.2–1000.5) | 472.5 (189.0–1000.0) |
| Participants’ Group | NCP | RBD | S2 | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| COVID-19 Vaccinated | 769.9 | 928.5 | 852.8 | 965.3 | 774.9 | 768.2 |
| (399.8–989.2) | (928.5–928.5) | (224.8–976.1) | (965.3–965.3) | (478.3–833.0) | (768.2–768.2) | |
| COVID-19 Unvaccinated | 457.1 | 623.5 | 666.8 | 767.5 | 474.6 | 506.7 |
| (196.3–880.2) | (265.9–969.1) | (227.1–995.6) | (210.0–999.9) | (254.6–797.5) | (187.3–986.1) | |
| Non-COVID-19 Vaccinated | 439.8 | 454.8 | 871.6 | 920.5 | 503.9 | 464.2 |
| (210.0–835.1) | (202.2–928.5) | (190.2–1000.5) | (210.0–998.9) | (189.0–1000) | (199.5–995.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczuk, A.; Michalski, A.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers. Vaccines 2022, 10, 1169. https://doi.org/10.3390/vaccines10081169
Błaszczuk A, Michalski A, Malm M, Drop B, Polz-Dacewicz M. Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers. Vaccines. 2022; 10(8):1169. https://doi.org/10.3390/vaccines10081169
Chicago/Turabian StyleBłaszczuk, Agata, Aleksander Michalski, Maria Malm, Bartłomiej Drop, and Małgorzata Polz-Dacewicz. 2022. "Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers" Vaccines 10, no. 8: 1169. https://doi.org/10.3390/vaccines10081169
APA StyleBłaszczuk, A., Michalski, A., Malm, M., Drop, B., & Polz-Dacewicz, M. (2022). Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers. Vaccines, 10(8), 1169. https://doi.org/10.3390/vaccines10081169







