A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers
Abstract
:1. Introduction
2. Materials and Methods
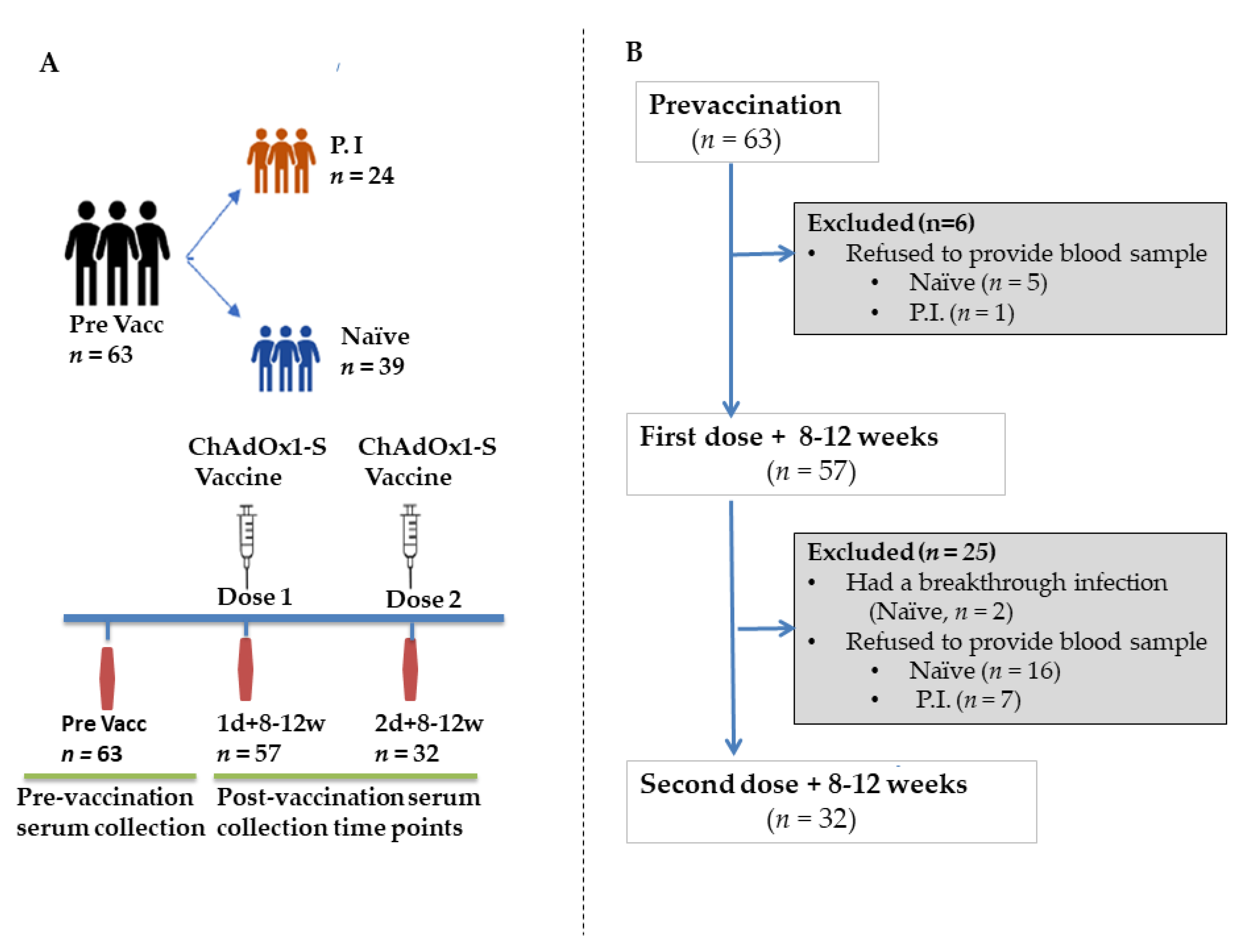
2.1. Study Design and Participants
2.2. ELISA Methods
2.3. Statistical Analysis
3. Results
3.1. Post-Vaccination Seropositivity
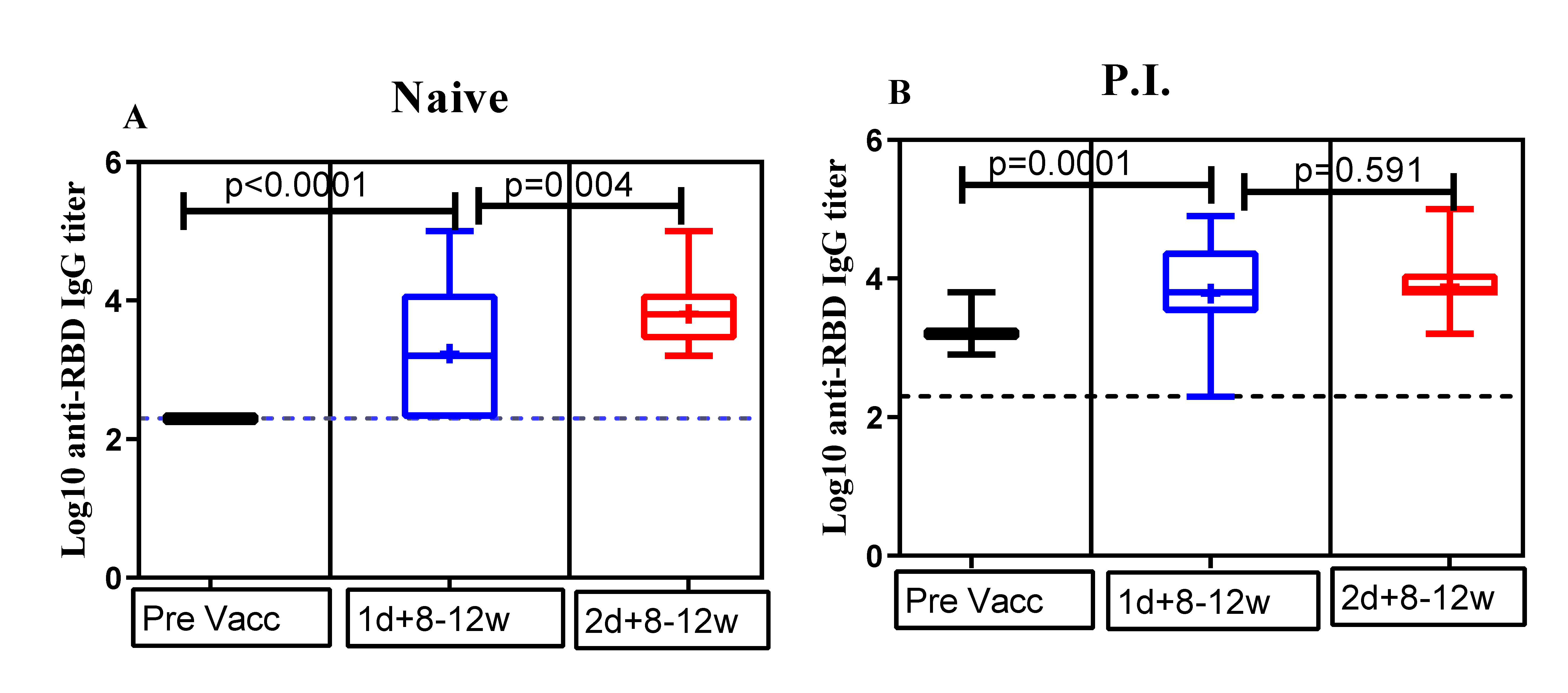
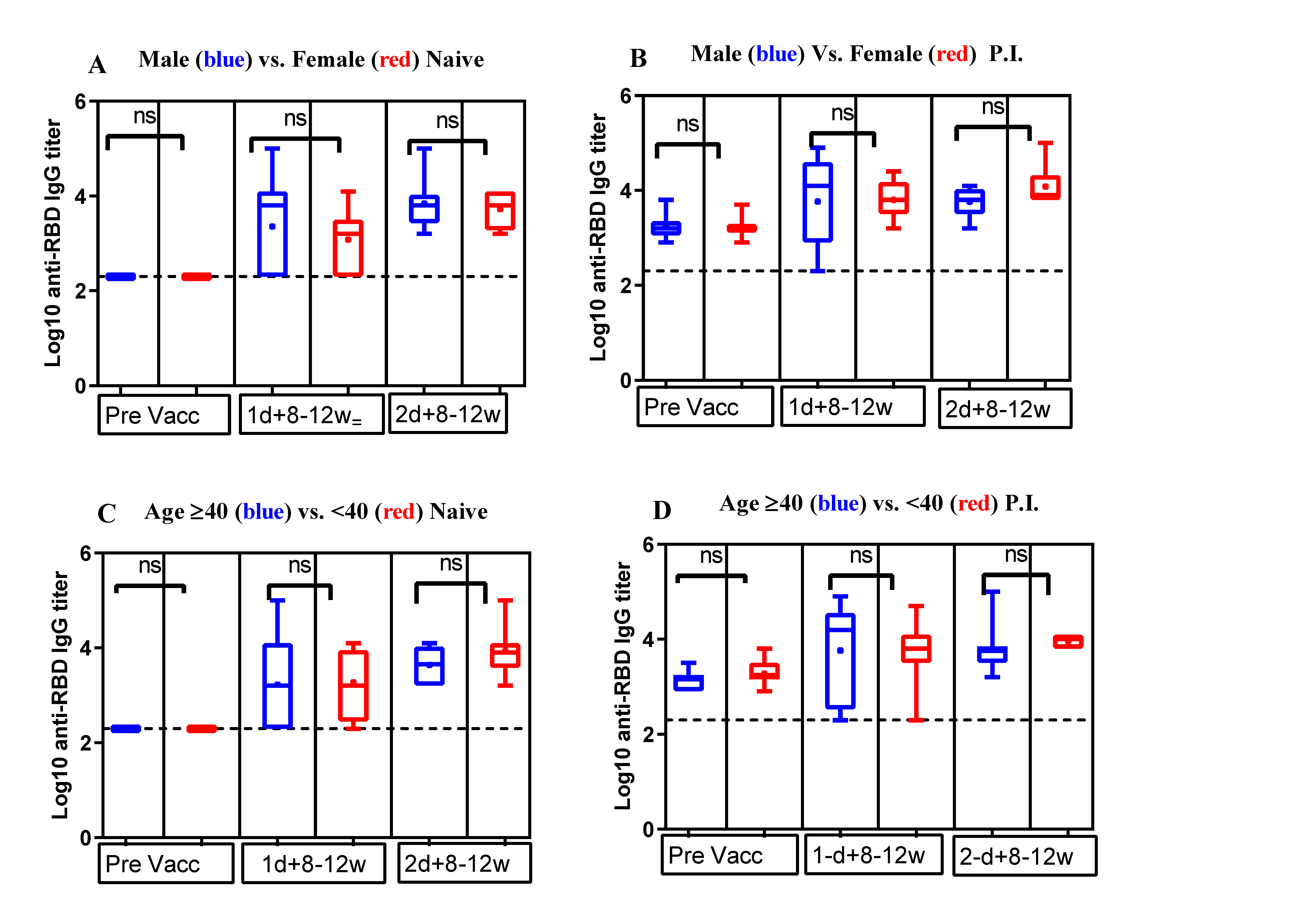
3.2. Comparison of Anti-RBD IgG Antibody Titers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, T.; Beardsley, J.; Marais, B.J.; Nguyen, T.A.; Fox, G.J. The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines. Vaccines 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in HIV Infection: A Single-Arm Substudy of a Phase 2/3 Clinical Trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, NEJMoa2035389. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. Lancet Infect Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 MRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously Infected and SARS-CoV-2-Naive UK Health-Care Workers: A Multicentre Prospective Cohort Study. Lancet Microbe 2021, 3, e21–e31. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A Systematic Review of Antibody Mediated Immunity to Coronaviruses: Kinetics, Correlates of Protection, and Association with Severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef]
- Rajesh, T. Gandhi, M.D. Toward Defining an Immune Correlate of Protection Against SARS-CoV-2. NEJM J. Watch. 2021, 2021. [Google Scholar] [CrossRef]
- Sui, Y.; Bekele, Y.; Berzofsky, J.A. Potential SARS-CoV-2 Immune Correlates of Protection in Infection and Vaccine Immunization. Pathogens 2021, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Levi, R.; Azzolini, E.; Pozzi, C.; Ubaldi, L.; Lagioia, M.; Mantovani, A.; Rescigno, M. One Dose of SARS-CoV-2 Vaccine Exponentially Increases Antibodies in Individuals Who Have Recovered from Symptomatic COVID-19. J. Clin. Investig. 2021, 1131, e149154. [Google Scholar] [CrossRef] [PubMed]
- Carbonare, L.D.; Valenti, M.T.; Bisoffi, Z.; Piubelli, C.; Pizzato, M.; Accordini, S.; Mariotto, S.; Ferrari, S.; Minoia, A.; Bertacco, J.; et al. Antibody Response to BTN162b2 MRNA Vaccination in Naïve versus SARS-CoV-2 Infected Subjects with and without Waning Immunity. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Shenoy, P.; Ahmed, S.; Paul, A.; Cherian, S.; Umesh, R.; Shenoy, V.; Vijayan, A.; Babu, S.; Nivin, S.; Thambi, A. Hybrid Immunity versus Vaccine-Induced Immunity against SARS-CoV-2 in Patients with Autoimmune Rheumatic Diseases. Lancet Rheumatol. 2021, 4, e80–e82. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mendonça, L.; Allen, E.R.; Howe, A.; Lee, M.; Allen, J.D.; Chawla, H.; Pulido, D.; Donnellan, F.; Davies, H.; et al. Native-like SARS-CoV-2 Spike Glycoprotein Expressed by ChAdOx1 NCoV-19/AZD1222 Vaccine. ACS Cent. Sci. 2021, 9, 594–602. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA Vaccine Protection against SARS-CoV-2 in Rhesus Macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of Serum and Saliva Antibody Responses to SARS-CoV-2 Spike Antigens in COVID-19 Patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef]
- Gelanew, T.; Seyoum, B.; Mulu, A.; Mihret, A.; Abebe, M.; Wassie, L.; Gelaw, B.; Sorsa, A.; Merid, Y.; Muchie, Y.; et al. High Seroprevalence of Anti-SARS-CoV-2 Antibodies among Ethiopian Healthcare Workers. BMC Infect. Dis. 2022, 22, 261. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2022, 10, 64. [Google Scholar] [CrossRef]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Antibody Response after First and Second-Dose of ChAdOx1-NCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among Health Care Workers in India: The Final Results of Cross-Sectional Coronavirus Vaccine-Induced Antibody Titre (COVAT) Study. Vaccine 2021, 39, 6492–6509. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunisation (JCVI). Advice on Third Primary Dose Vaccination. GOV.UK. Available online: https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (accessed on 21 December 2021).
- Huang, S.-H.; Huang, C.-H.; Wang, N.-C.; Chen, T.-C.; Lee, Y.-T.; Lin, S.-P.; Lin, T.-Y.; Lin, C.-Y.; Lee, Y.-L.; Lee, C.-H.; et al. Early Seroreversion After 2 Doses of Hepatitis A Vaccination in Human Immunodeficiency Virus–Positive Patients: Incidence and Associated Factors. Hepatology 2019, 70, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological Memory and Neutralizing Activity to a Single Dose of COVID-19 Vaccine in Previously Infected Individuals. Int. J. Infect. Dis. 2021, 108, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020, NEJMc2025179. [Google Scholar] [CrossRef]
- Frieman, M.; Harris, A.D.; Herati, R.S.; Krammer, F.; Mantovani, A.; Rescigno, M.; Sajadi, M.M.; Simon, V. SARS-CoV-2 Vaccines for All but a Single Dose for COVID-19 Survivors. EBioMedicine 2021, 68, 103401. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; Leier, H.C.; Lyski, Z.L.; McBride, S.K.; Coulter, F.J.; Weinstein, J.B.; Goodman, J.R.; Lu, Z.; Siegel, S.A.R.; Sullivan, P.; et al. Neutralization of SARS-CoV-2 Variants by Convalescent and BNT162b2 Vaccinated Serum. Nat. Commun. 2021, 12, 5135. [Google Scholar] [CrossRef]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of Age, Ethnicity, Sex and Prior Infection Status on Immunogenicity Following a Single Dose of the BNT162b2 MRNA COVID-19 Vaccine: Real-World Evidence from Healthcare Workers, Israel, December 2020 to January 2021. Euro Surveill. 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to MRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e00341-21. [Google Scholar] [CrossRef]
- Lee, S.W.; Moon, J.-Y.; Lee, S.-K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.-H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 NCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association with Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Ng, H.; Gordon, M.; Salomonsson, A.-C.; Hellström, C.; Pin, E.; Blom, K.; Mangsbo, S.; et al. Antibody Responses after a Single Dose of ChAdOx1 NCoV-19 Vaccine in Healthcare Workers Previously Infected with SARS-CoV-2. EBioMedicine 2021, 70, 103523. [Google Scholar] [CrossRef]
- Leier, H.C.; Bates, T.A.; Lyski, Z.L.; McBride, S.K.; Lee, D.X.; Coulter, F.J.; Goodman, J.R.; Lu, Z.; Curlin, M.E.; Messer, W.B.; et al. Previously Infected Vaccinees Broadly Neutralize SARS-CoV-2 Variants. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Piccini, G.; Manganaro, N.; Pileri, P.; Hyseni, I.; Leonardi, M.; Pantano, E.; Abbiento, V.; Benincasa, L.; et al. Hybrid Immunity Improves B Cells and Antibodies against SARS-CoV-2 Variants. Nature 2021, 600, 530–535. [Google Scholar] [CrossRef]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef]
- Eggink, D.; Andeweg, S.P.; Vennema, H.; van Maarseveen, N.; Vermaas, K.; Vlaemynck, B.; Schepers, R.; van Gageldonk-Lafeber, A.B.; van den Hof, S.; Reusken, B.E.M.; et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Euro Surveill. 2022, 27, 2101196. [Google Scholar] [CrossRef]
| Character | Subcategory | N a | % b | GMT c |
|---|---|---|---|---|
| Age in years | ≥40 | 26 | 41.3 | |
| <40 | 37 | 58.7 | ||
| Sex | Male (m d) | 36 | 57.1 | |
| Female (f e) | 27 | 42.9 | ||
| Naïvef | Pre Vacc h | 39 (23 m,16 f) | 200 | |
| 1d + 8–12w i | 34 (19 m, 15 f) | 1632.16 | ||
| 2d + 8–12w j | 16 (10 m, 6 f) | 6607.31 | ||
| P.I. g | Pre Vacc | 16 (10 m, 6 f) | 1674.94 | |
| 1d + 8–12w | 23 (13 m, 10 f) | 4913.5 | ||
| 2d + 8–12w | 16 (10 m, 6 f) | 9804.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelanew, T.; Mulu, A.; Abebe, M.; Bates, T.A.; Wassie, L.; Teferi, M.; Fentahun, D.; Alemu, A.; Tamiru, F.; Assefa, G.; et al. A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers. Vaccines 2022, 10, 859. https://doi.org/10.3390/vaccines10060859
Gelanew T, Mulu A, Abebe M, Bates TA, Wassie L, Teferi M, Fentahun D, Alemu A, Tamiru F, Assefa G, et al. A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers. Vaccines. 2022; 10(6):859. https://doi.org/10.3390/vaccines10060859
Chicago/Turabian StyleGelanew, Tesfaye, Andargachew Mulu, Markos Abebe, Timothy A. Bates, Liya Wassie, Mekonnen Teferi, Dessalegn Fentahun, Aynalem Alemu, Frehiwot Tamiru, Gebeyehu Assefa, and et al. 2022. "A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers" Vaccines 10, no. 6: 859. https://doi.org/10.3390/vaccines10060859
APA StyleGelanew, T., Mulu, A., Abebe, M., Bates, T. A., Wassie, L., Teferi, M., Fentahun, D., Alemu, A., Tamiru, F., Assefa, G., Bayih, A. G., Tafesse, F. G., Mihret, A., & Abdissa, A. (2022). A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers. Vaccines, 10(6), 859. https://doi.org/10.3390/vaccines10060859






