The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond
Abstract
:1. Introduction
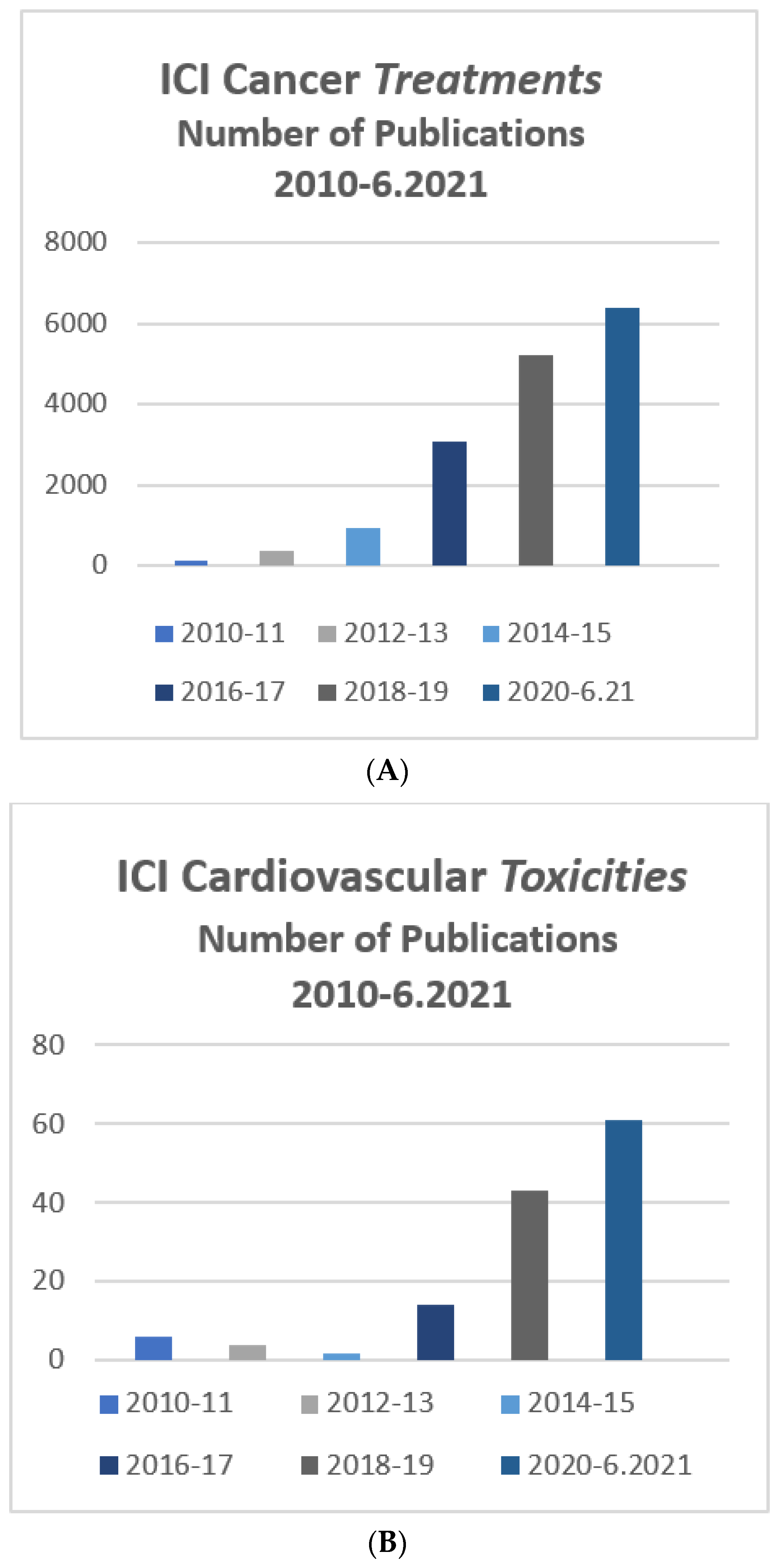
2. Epidemiology of ICI Cardiotoxicity
3. Clinical Manifestations of ICI Cardiotoxicity
3.1. Myocarditis
3.1.1. Clinical Presentation
3.1.2. Electrocardiogram
3.1.3. Biomarkers
3.2. Imaging
3.2.1. Echocardiography
3.2.2. Cardiac Magnetic Resonance (CMR)
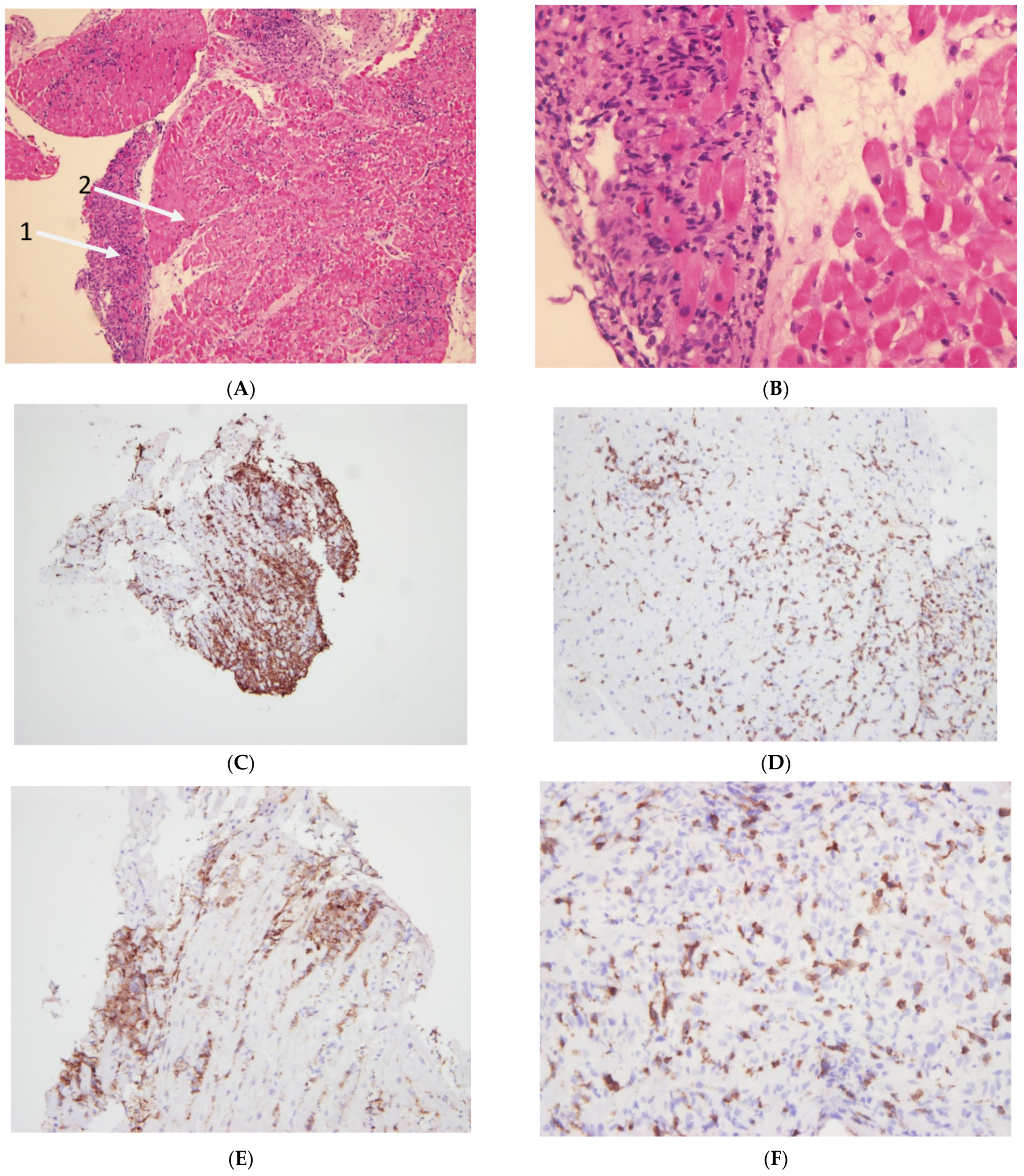
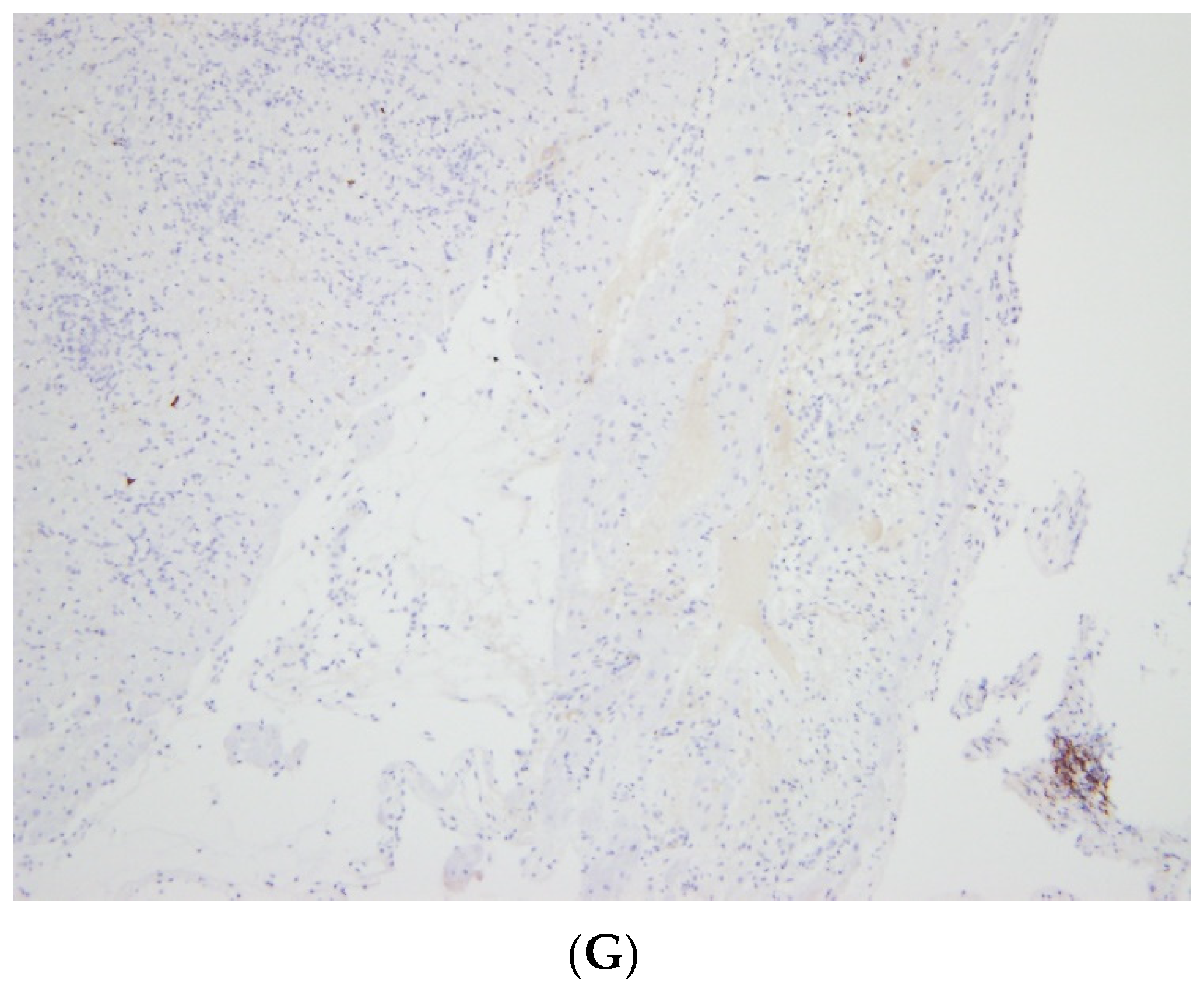
3.2.3. Endomyocardial Biopsy (EMB)
4. Non-Myocarditis ICI-Related Cardiotoxicity
5. Treatment of ICI-Associated Myocarditis
6. Surveillance
7. Rechallenge of ICI Therapy after Myocarditis
8. Open Questions and Future Directions
8.1. Two of the Important Issues That Need Further Consideration
8.1.1. Diagnosis and Monitoring by Imaging
8.1.2. Combination Therapy
9. Take Home Messages: ICI Cardiovascular Toxicities
- Major (issues and concepts):
- -
- High degree of suspicion for myocarditis is mandatory in ICI treated patients
- -
- Combination ICI therapy is an important risk factor, beyond other known risk factors, for myocarditis
- Diagnosis is based on:
- -
- Usually on variable combination of: clinical signs and, physical examination, ECG,
- -
- Echo (global function, segmental wall evaluation, and strain),
- -
- Cardiac Magnetic Resonance (CMR) imaging.
- -
- Endomyocardial Biopsy—considered gold standard
- Treatment Approach:
- -
- ICI discontinuation.
- -
- Immunosuppressive treatment based on steroids
- -
- Relevant pharmacological and/or mechanical cardiac support is needed if steroid treatment fails or is insufficient
- Minor, but relevant and important to remember:
- -
- Global left ventricular function (LVEF) may be normal in up to ~50% of patients with proven myocarditis.
- -
- No consensus on screening, surveillance and prevention for ICI-associated myocarditis.
- -
- When myositis is diagnosed or suspected, the possibility of myocarditis should be also fully evaluated, due to high likelihood of co-existence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor—Associated myocarditis: Manifestations and mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef]
- Zaha, V.G.; Meijers, W.C.; Moslehi, J. Cardio-Immuno-Oncology. Circulation 2020, 141, 87–89. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse effects associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Okazaki, I.-M.; Yoshida, T.; Chikuma, S.; Kato, Y.; Nakaki, F.; Hiai, H.; Honjo, T.; Okazaki, T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 2010, 22, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Gröschel, C.; Sasse, A.; Röhrborn, C.; Monecke, S.; Didié, M.; Elsner, L.; Kruse, V.; Bunt, G.; Lichtman, A.H.; Toischer, K.; et al. T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Sci. Rep. 2017, 7, 15998. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Zhang, H.; Berry, G.; Goronzy, J.J.; Weyand, C.M. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am. J. Physiol. Circ. Physiol. 2017, 312, H1052–H1059. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Zhou, L.; Alexander, G.S.; Park, K.; Yang, L.; Wang, N.; Zaorsky, N.G.; Ma, X.; Wang, Y.; Dicker, A.P.; et al. PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes. J. Thorac. Oncol. 2018, 13, 510–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waheed, N.; Fradley, M.G.; DeRemer, D.L.; Mahmoud, A.; Shah, C.P.; Langaee, T.Y.; Lipori, G.P.; March, K.; Pepine, C.J.; Cooper-DeHoff, R.M.; et al. Newly diagnosed cardiovascular disease in patients treated with immune checkpoint inhibitors: A retrospective analysis of patients at an academic tertiary care center. Cardio-oncology 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.J.; Qdaisat, A.; Chaftari, P.; Lipe, D.; Merlin, J.; Rajha, E.; Wechsler, A.; Sandoval, M.; Viets, J.; Al-Breiki, A.; et al. Diagnosis and management of immune-related adverse effects of immune checkpoint therapy in the emergency department. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1637–1659. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Maisonobe, T.; Knauss, S.; Salem, O.B.H.; Hervier, B.; Auré, K.; Szwebel, T.-A.; Kramkimel, N.; Lethrosne, C.; Bruch, J.-F.; et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018, 91, e985–e994. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.-E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef] [Green Version]
- Zamami, Y.; Niimura, T.; Okada, N.; Koyama, T.; Fukushima, K.; Izawa-Ishizawa, Y.; Ishizawa, K. Factors associated with im-mune checkpoint inhibitor myocarditis. JAMA Oncol. 2019, 5, 1635–1637. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Zlotoff, D.A.; Hassan, M.Z.; Zafar, A.; Alvi, R.M.; Awadalla, M.; Mahmood, S.S.; Zhang, L.; Chen, C.L.; Ederhy, S.; Barac, A.; et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J. Immunother. Cancer 2021, 9, e002007. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.-J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor–Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor—Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef]
- Hauck, A.J.; Kearney, D.L.; Edwards, W.D. Evaluation of Postmortem Endomyocardial Biopsy Specimens From 38 Patients with Lymphocytic Myocarditis: Implications for Role of Sampling Error. Mayo Clin. Proc. 1989, 64, 1235–1245. [Google Scholar] [CrossRef]
- Mirabel, M.; Callon, D.; Bruneval, P.; Lebreil, A.-L.; Mousseaux, E.; Oudard, S.; Hulot, J.-S.; Andreoletti, L. Late-Onset Giant Cell Myocarditis Due to Enterovirus During Treatment with Immune Checkpoint Inhibitors. JACC Cardio Oncol. 2020, 2, 511–514. [Google Scholar] [CrossRef]
- Lehmann, L.H.; Cautela, J.; Palaskas, N.; Baik, A.H.; Meijers, W.C.; Allenbach, Y.; Alexandre, J.; Rassaf, T.; Müller, O.J.; Aras, M.; et al. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor—Associated Myocarditis. JAMA Cardiol. 2021, 6, 1329. [Google Scholar] [CrossRef]
- Anderson, R.D.; Brooks, M. Apical takotsubo syndrome in a patient with metastatic breast carcinoma on novel immunotherapy. Int. J. Cardiol. 2016, 222, 760–761. [Google Scholar] [CrossRef]
- Morel, O.; Sauer, F.; Imperiale, A.; Cimarelli, S.; Blondet, C.; Jesel, L.; Trinh, A.; De Poli, F.; Ohlmann, P.; Constantinesco, A.; et al. Importance of Inflammation and Neurohumoral Activation in Takotsubo Cardiomyopathy. J. Card. Fail. 2009, 15, 206–213. [Google Scholar] [CrossRef]
- Serzan, M.; Rapisuwon, S.; Krishnan, J.; Chang, I.C.; Barac, A. Takotsubo Cardiomyopathy Associated with Checkpoint Inhibitor Therapy. JACC Cardio Oncol. 2021, 3, 330–334. [Google Scholar] [CrossRef]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-Like Syndrome in Cancer Patients Treated with Immune Checkpoint Inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Drobni, Z.D.; Zafar, A.; Quinaglia, T.; Hartmann, S.; Gilman, H.K.; Raghu, V.K.; Gongora, C.; Sise, M.E.; Alvi, R.M.; et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002771. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Bar, J.; Markel, G.; Gottfried, T.; Percik, R.; Leibowitz-Amit, R.; Berger, R.; Golan, T.; Daher, S.; Taliansky, A.; Dudnik, E.; et al. Acute vascular events as a possibly related adverse event of immunotherapy: A single-institute retrospective study. Eur. J. Cancer 2019, 120, 122–131. [Google Scholar] [CrossRef]
- Solinas, C.; Saba, L.; Sganzerla, P.; Petrelli, F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: A systematic review. Thromb. Res. 2020, 196, 444–453. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Poels, K.; van Leent, M.M.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.; Reiche, M.E.; Kusters, P.J.; Malinova, T.; et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell–Driven Plaque Inflammation in Atherosclerosis. JACC Cardio Oncol. 2020, 2, 599–610. [Google Scholar] [CrossRef]
- Poels, K.; Neppelenbroek, S.I.; Kersten, M.J.; Antoni, M.L.; Lutgens, E.; Seijkens, T.T. Immune checkpoint inhibitor treatment and atherosclerotic cardiovascular disease: An emerging clinical problem. J. Immunother. Cancer 2021, 9, e002916. [Google Scholar] [CrossRef]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Giles, A.J.; Hutchinson, M.K.N.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor–Associated Myocarditis. Circulation 2020, 141, 2031–2034. [Google Scholar] [CrossRef]
- Thuny, F.; Alexandre, J.; Salem, J.-E.; Mirabel, M.; Dolladille, C.; Cohen-Solal, A.; Cohen, A.; Ederhy, S.; Cautela, J. Management of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC Cardio Oncol. 2021, 3, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Mohebtash, M.; Rodrigo, M.E.; Ruiz, G.; Atkins, M.B.; Barac, A. Autoimmune Myocarditis Caused by Immune Checkpoint Inhibitors Treated with Antithymocyte Globulin. J. Immunother. 2018, 41, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC Cardio Oncol. 2021, 3, 137–139. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; DA Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Nishino, M.; Hatabu, H.; Hodi, F.S. Imaging of Cancer Immunotherapy: Current Approaches and Future Directions. Radiol. 2019, 290, 9–22. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Maute, R.L.; Gordon, S.R.; Mayer, A.T.; McCracken, M.N.; Natarajan, A.; Ring, N.G.; Kimura, R.; Tsai, J.M.; Manglik, A.; Kruse, A.C.; et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and im-mune-PET imaging. Proc. Natl. Acad. Sci. USA 2015, 112, E6505–E6514. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Lesniak, W.G.; Gabrielson, M.; Lisok, A.; Wharram, B.; Sysa-Shah, P.; Azad, B.B.; Pomper, M.G.; Nimmagadda, S. A humanized antibody for imaging immune check point ligand PD-L1 expression in tumors. Oncotarget 2016, 7, 10215–10227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dorst, D.C.; van Doorn, L.; Colafella, K.M.M.; Manintveld, O.C.; Hassing, H.C.; Danser, A.J.; Mathijssen, R.H.; Versmissen, J. Cardiovascular toxicity of angiogenesis inhibitors and immune checkpoint inhibitors: Synergistic anti-tumour effects at the cost of increased cardiovascular risk? Clin. Sci. 2021, 135, 1649–1668. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Neuzillet, C.; Calderaro, J.; Lafdil, F.; Pawlotsky, J.-M.; Rousseau, B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: Current knowledge and future research directions. J. Immunother. Cancer 2019, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilon, D.; Iakobishvili, Z.; Leibowitz, D. The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond. Vaccines 2022, 10, 304. https://doi.org/10.3390/vaccines10020304
Gilon D, Iakobishvili Z, Leibowitz D. The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond. Vaccines. 2022; 10(2):304. https://doi.org/10.3390/vaccines10020304
Chicago/Turabian StyleGilon, Dan, Zaza Iakobishvili, and David Leibowitz. 2022. "The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond" Vaccines 10, no. 2: 304. https://doi.org/10.3390/vaccines10020304
APA StyleGilon, D., Iakobishvili, Z., & Leibowitz, D. (2022). The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond. Vaccines, 10(2), 304. https://doi.org/10.3390/vaccines10020304




