Abstract
While the role of active smoking on response to vaccines is yet to be fully understood, some real-world studies have outlined a possible link between smoking and humoral response to COVID-19 vaccines. Thus, the present rapid systematic review aimed at summarizing the current epidemiological evidence on this association. Following PRISMA and WHO guidelines on rapid systematic reviews, we systematically reviewed published literature on this topic and discussed the findings according to the aim of analysing smoking and its impact on humoral response to COVID-19 postvaccination antibody titres. The search strategy yielded a total of 23 articles. The sample size amongst the studies ranged between 74 and 3475 participants (median, 360), with the proportion of smokers being between 4.2% and 40.8% (median, 26.0%). The studies included in this review analysis investigated the dynamics of antibody response to different type of COVID-19 vaccines. In 17 out of 23 studies, current smokers showed much lower antibody titres or more rapid lowering of the vaccine-induced IgG compared with nonsmokers. This rapid systematic review indicates that active smoking negatively impacts humoral response to COVID-19 vaccines, although the pathophysiologic mechanisms for this association have not been entirely suggested. The results advocate targeted policies to promote tailored health promotion initiatives, which can increase risk perception and ensure appropriate protection measures to be taken to avoid the health consequences of COVID-19 in smokers.
1. Introduction
Smoking habit is one of the most common unhealthy behaviours widely prevalent around the globe [1]. Many efforts have been put in place to contrast this habit; however, despite the steady decrement observed in smoking prevalence, the total number of smokers has increased due to the population grown [2,3]. In 2019, more than 1.1 billion tobacco users were censused globally, making smoking one of the most important preventable causes of illness and premature death. In particular, smoking accounts for around 8 million deaths and 200 million disability-adjusted life years each year globally, posing a great challenge to healthcare systems all over the world [3]. The health consequences of smoking include a wide range of illnesses, being a risk factor for lung cancer, chronic obstructive pulmonary disease, cardiovascular diseases, viral and bacterial infections of the respiratory system, and others [4].
Smoking affects the immune system and response. In particular, there is evidence of the association between cigarette smoking and higher risk of several immunological diseases, which range from autoimmune diseases (such as allergies or transplant rejection) to systemic inflammatory diseases (e.g., rheumatoid arthritis), to a lower protection against external antigens, impairing the immunological response to infections [5,6,7,8]. As part of undertakings exploring the impact of cigarette smoking on the immune system, effects on the humoral response after immunization and maintenance of protection induced by several vaccines have been also investigated. Some studies described a link between active smoking and lower levels of vaccine-induced antibodies (such as after immunisation against hepatitis B, and boosters of tetanus and diphtheria) [9,10,11], or increased odds of low-avidity immunoglobulins G (IgG) in smokers (in case of the adjuvanted human papillomavirus type 16 and 18 vaccine) [12]. In contrast, another study on influenza vaccination suggested that smoking does not interfere with the quantity of vaccine-induced antibodies [13]. However, while the effect of cigarette smoking on the humoral response after immunization is generally accepted, the current evidence does not seem to be reliable enough to draw firm conclusions or to generate a consensus, likely due to differences according to vaccine types or in study populations—for instance, in terms of age, comorbidities, and smoking exposure—across the studies. Only limited specific information is available about seroconversion after COVID-19 vaccination in smokers.
With the rapid global diffusion of the coronavirus disease 2019 (COVID-19)—the disease due to the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) [14,15], research efforts have been focused on the swift development of treatments and vaccines. Several COVID-19 vaccines have been developed and authorized since December 2020, and effective immunologic response to after immunization is crucial to limit the negative health outcomes of the pandemic [16,17]. Along with immunization efforts, real-world data have been collected worldwide in order to confirm the safety, immunogenicity, and efficacy of COVID-19 vaccines, and to understand the impact of societal and health factors that might affect the massive vaccination campaigns [17,18]. Some of these analyses have described that vaccine-induced antibody titres are lower or decrease faster among smokers compared with nonsmokers, offering suggestions for further research about the impact of smoking on the humoral response to COVID-19 vaccines [19,20].
In light of the above and the prevalence of smokers, and considering the crucial role of vaccines against COVID-19, we conducted the present systematic review with the objective of summarizing the real-world data from epidemiological studies investigating the impact of smoking and on the humoral response after COVID-19 vaccination.
2. Materials and Methods
We carried out a rapid systematic review of evidence on COVID-19 following the World Health Organization (WHO) guidelines: “Rapid reviews to strengthen health policy and systems: a practical guide” [21], and the findings were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 2020 [22]. Indeed, COVID-19 requires solid and up-to-date evidence to support decision- and policy-making in circumstances of emergency. In this context, the WHO defines a rapid review as a timely and affordable tool that can provide actionable and relevant evidence to strengthen health policy and system research, and promotes its use as practical and suitable approach for collecting and synthetizing growing evidence that can be easily and promptly used to inform stakeholders [21,23].
We surfed PubMed/Medline, Scopus, and Embase databases to retrieve relevant literature. In order to identify eligible articles and documents the lists of references of the included studies were manually screened, and the medRxiv preprints platform and the webpages of international health authorities (including WHO, Centers for Disease Control and Prevention—CDC, and European Centre for Disease Prevention and Control—ECDC) were also consulted. The databases were trawled from inception to December 31, 2021, exploring evidence published during 2021. Only original reports published in English were considered eligible. Our search terms comprised two main aspects, namely COVID-19 vaccine and smoking. The full search string—developed using controlled vocabulary (e.g., MeSH terms) and free-text keywords—is: (“COVID-19 Vaccines”[Mesh] OR “COVID-19 Vaccines”[TIAB] OR ((“COVID-19”[Mesh] OR “COVID-19” OR “SARS-CoV-2”[Mesh] OR “SARS-CoV-2”[TIAB]) AND (“Vaccines”[Mesh] OR “Vaccination”[Mesh] OR vaccin*[TIAB])) AND (“Smokers”[Mesh] OR “Ex-Smokers”[Mesh] OR “Non-Smokers”[Mesh] OR smoker*[TIAB] OR nonsmoker*[TIAB] OR exsmoker*[TIAB] OR smoking[TIAB] OR tobacco[TIAB] OR “heat-not-burn”[TIAB] OR “e-cigarette*”[TIAB] OR “e-cig*”[TIAB] OR “nicotine”[TIAB])). The search strategy was adjusted slightly according to the evaluated databases. Retrieved reports were first evaluated based on title and abstract and only eligible papers were evaluated in full by two authors (PF and VG). In order to be included in the systematic review, papers must fulfil the following criteria: (i) full-text accessible primary epidemiological studies; (ii) reporting immunogenicity data after immunization with any available COVID-19 vaccine; (iii) including smokers. Records that met the following criteria were excluded: (i) studies without data on humoral response to COVID-19 vaccines, (ii) not considering smoking as predictors for vaccine-induced antibody dynamics, (iii) published as review, case report, conference abstract, editorial or letter to editor; (iv) published in language other than English. Data extraction was performed using a prepiloted spreadsheet elaborated in Microsoft Excel® for Windows (Microsoft Corporation, Redmond, WA, USA).
Due to the lack of comparable outcome measures, variability in time points for blood sampling, and quite high methodological heterogeneity across the reports, the results were not pooled in a meta-analysis, but discussed according to the aim to analyse smoking and its impact on humoral response to COVID-19 postvaccination antibody titres.
We also assessed the methodological quality of the body of found evidence through the Grading of Recommendation Assessment, Development and Evaluation (GRADE) guidelines [24]. The assessment of risk of bias across studies was based on the following conditions: (i) representativeness, (ii) selection bias, (iii) reporting bias, (iv) laboratory confirmation of humoral response after vaccination, (v) time between vaccination and sampling, and (vi) proportion of smokers.
3. Results
The flow chart of included studies and selection process is presented in Figure 1.
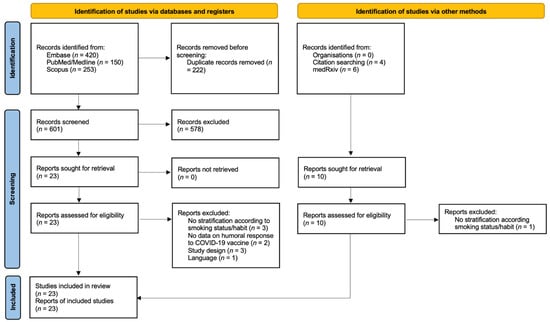
Figure 1.
PRISMA flow chart of included studies and selection process.
The search strategy yielded a total of 833 articles. After the reading of the titles and abstracts, and the detection of those that met the exclusion criteria (Supplementary Materials, Table S1), 23 were selected for inclusion in this rapid systematic review, 17 being scientific articles and six being preprints [19,20,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Characteristics of included studies are presented in Table 1. All included studies were published from April 2021, mostly being research carried out in Europe (13 out 23). The sample size amongst the studies ranged between 74 and 3475 participants (median, 360), with a proportion of smokers between 4.2% and 40.8% (median, 26.0%). All reports assessed the impact of cigarette smoking, while in Yamamoto et al. users of heat-not-burn (HNB) tobacco products were also included [31]. Sixteen studies enrolled healthcare workers (HCW), two general population, and the others recruited patients with multiple sclerosis, cancer, inflammatory bowel disease, and obesity. Another report did not specify the enrolled population. Across the included reports, authors measured the humoral response to different available COVID-19 vaccines: 15 included participants immunized with BNT162b2 mRNA vaccine. in three CoronaVac was used, while the others presented various combination of BNT162b2 with ChAdOx1, mRNA-1273, mRNA-1273 and ChAdOx1, or BBIBP-CorV. Collection of a blood sample by venepuncture was performed at different time points across the reports, ranging from around 21 days to six months after the completion of the vaccination cycle. The studies included in the review analysis reflected a variety of serology tests for the research of IgG that bind the SARS-CoV-2 spike (S) protein receptor-binding domain (RBD), with specific positivity cutoff.

Table 1.
Characteristics and main findings of studies included in the systematic review.
In 17 out of 23 studies, current smokers showed significant lower antibody titre, and in a few reports, highlighted a more rapid lowering of the vaccine-elicited IgG compared with nonsmokers [19,20,26,27,28,29,30,32,34,36,37,38,39,40,41,43,44]. In particular, accelerated antibody decline was reported in the prospective assessments by Ferrara et al. [20], Zhang et al. [27], and Malavazos et al. [30]. Of note, magnitude and timing of smoking-attributable lower antibody levels varied greatly across these studies, according to the type of serological test used and unit of measurement, the time since vaccination, and the analysis and adjustment performed. In all but two reports, the smoking time and quantity were not assessed. Indeed, duration of smoking or number of cigarettes per day did not predict the effect of smoking on the IgG titre in Nomura (b) et al. [37]; while in Yamamoto et al., smokers consuming 11 or more cigarettes per day showed a greater reduction in IgG than those consuming less than 11 cigarettes per day [31].
Regarding the studies that did not find a relationship between exposure to smoking and COVID-19 vaccine response, current smokers tended to have predominant lower antispike IgG levels than the past and never smoker groups, but the difference was not statistically significant in two reports [25,26]. Similarly, smoking status did not correlate with titres of IgG against the spike protein induced by BNT162b2 mRNA COVID-19 vaccine in the study of Modenese et al. [43], or those induced by either BNT162b2 or BBIBP-CorV COVID-19 vaccine in Alqassieh et al. [36]. In both the studies by Kato et al., which enrolled seven and five current smokers, respectively, there was no significant association between the titre of IgG against the spike protein induced by the vaccine and smoking habit [32,34].
In Yamamoto et al., HNB tobacco product users and dual users showed lowered geometric mean titres, but the differences from never smokers were not statistically significant, although the reduction reached statistical significance by combining the two categories of HNB tobacco users [31].
A complete overview of the studies’ findings is presented in Table 1.
The assessment with the GRADE approach found that quality of studies was moderate-to-high, and confirmed that the quality of the body of found evidence is acceptable for assessing the impact of smoking on humoral response to COVID-19 vaccines (Supplementary Materials, Table S2).
4. Discussion
To our knowledge, the present rapid systematic review is the first to show the impact of smoking on postvaccination antibody titres in relation to the use of cigarettes and HNB tobacco products. The vast majority of the current body of evidence suggests that smoking has a negative impact on the humoral response to COVID-19 vaccines, with both potential lower response and more rapid lowering of the vaccine-elicited IgG titres. However, the literature available so far does not allow us to firmly ascertain whether the effect is related to duration of smoking or number of cigarettes smoked per day [20,31,38].
The negative effects played by smoking on the immune system seem to be determined by several mechanisms that influence both innate and adaptive immunity. Regarding the first, certain studies have indicated a direct effect of smoking on alterations in immune cell counts (including monocytes, macrophages, dendritic cells, and lymphocytes), but the effect of the complex mixture of tobacco chemicals varies depending on the individual smoking habits, as well as several subsets of cells explored in different studies. Previous reports have also showed that smoking induced inflammatory cytokines and chemokines. Similarly, consistent animal and human studies have observed that cigarette smoking induces chronic inflammation and downregulates CD4+ T cells and B cells. In cigarettes smokers, the T cells also exhibit differences in proliferation response, indicating defective adaptive immunity responses. Furthermore, analyses of Ig revealed a decreased production of IgA, IgG, and IgM associated with smoking [5,20,46,47,48,49].
Across the found body of evidence, the pathophysiologic bases for the impact of active or ever smoking on the humoral response to COVID-19 vaccines have not been entirely suggested. Linardou et al. speculated that the smoking-attributable immunosuppressive effect is mediated by direct effects on T cells and the dendritic-cell system, which impairs host response to vaccination [38,48,49,50]. A recently published study from the VASCO project, an ongoing broad Italian study on the response to BNT162b2 mRNA COVID-19 vaccine in HCWs [16,17,18,20], underlined that cigarette smoking affects the ability to form memory cells that are critical to the maintenance of the protective immune response induced by vaccines, as well as smoking being associated with increased monocyte–macrophages counts, which may influence the clearance of circulating antibodies, whose half-life is of average 3–4 weeks (depending on IgG isotype and attributes) [18,20]. In Yamamoto, a lower decrease in vaccine-induced IgG levels was also seen in users of HNB tobacco products (compared with never smokers), although to a lesser extent than that associated with cigarette smoking. Authors attributed their findings to effects of nicotine—which is contained within HNB tobacco products at the same amounts as conventional cigarettes [51,52]—that might inhibit antibody-forming cell response, impair antigen-mediated signalling in T cells, and induce T-cell anergy [31,48].
Although a few analyses have separately described whether smoking affects the humoral response to vaccines or the antibody maintenance, the vast majority of the retrieved studies only investigated the IgG titres on defined time points, and we were unable to classify findings as reduced response or more rapid decay. Further research should investigate this important aspect of the overall impact of smoking on immunological response and maintenance [20]. It is worth also mentioning that some studies included in this review did not detect a correlation between smoking status and postvaccination IgG titres. However, most of these reports include a very low sample size and/or proportion of smokers [25,32,34,43], or examined the antibody levels in the early weeks after the completion of vaccination cycle [25,32,43], making it difficult to appreciate possible differences between smokers and nonsmokers [20].
Understanding possible factors that might influence the interindividual variability in vaccine response is important to ensure an effective response to vaccines [16,17,18,20,53]. While the mechanisms by which smoking impairs the humoral responses to COVID-19 vaccines deserve further research, our rapid review evidences the adverse effect of tobacco product use against immunogenicity to COVID-19 vaccination. Furthermore, some population surveys found more negative attitudes toward vaccination and unwillingness to vaccinate against COVID-19 amongst smokers compared with never and former smokers [54]. All together, these results advocate targeted policies to promote tailored health promotion initiatives tending to increase risk perception and ensure appropriate protection measures to be taken to avoid the infection and its consequences. Indeed, smoking cessation should be encouraged not only for prevention of the well-known smoking attributable diseases—namely cancer, respiratory illness, cardiovascular disease, etc.—but also due to the impact of smoking on immune function.
Of note, this study adds interesting insights on the current vivid research on the relationship between smoking and SARS-CoV-2/COVID-19. Indeed, research about the effects of active smoking on both infection and disease is still controversial, with some studies highlighting a lower proportion of positive SARS-CoV-2 serologies among current smokers and opposite evidence identifying smoking as a possible risk factor for disease progression [55]. In this regard, more research is needed to investigate the secondary health consequences of the effect smoking on vaccines response, in terms of vaccine effectiveness and risks of infection and reinfection.
Some limitations must be considered in this review. First, it was as a rapid review, which, despite being systematic in nature, was limited in the number of surfed databases. However, the assessment of the literature was in line with the minimum requirements (at least two databases) set by the PRISMA guidelines, and the review was conducted in accordance with WHO guidelines on rapid reviews [21,22]. Additionally, despite medRxiv being a preprint platform, without a peer-review process, it collects and publishes scientific reports on a very fast track, allowing us to consider the most updated available evidence [56]. In our view, this represents an important strength, especially considering the large number of studies conducted daily on COVID-19 [15,16,17]. Moreover, grey literature (webpages of international health authorities) was also consulted in order to collect and analyse all the available evidence. Nevertheless, at the time of study, evidence about this topic is still relatively sparse and the literature so far available does not allow us to consider the impact of possible aspects of smoking exposure (including duration of smoking, number of cigarettes per day, passive smoking exposure) and their impact on humoral response to COVID-19 vaccines, allowing us to only draw preliminary conclusions. For these reasons, and due to limited quality of data and reporting, these findings should be interpreted with caution, and require further exploration in studies specifically designed to examine the association between smoking and COVID-19 vaccine response [20,56]. Indeed, most of the found evidence relied on self-reported smoking status as part of wider investigation, and thus failed to assess and correct for possible sources of residual confounding [20,55]. Similarly, it is also worth mentioning that humoral response to vaccines could be affected by factors other than smoking exposure, including age, comorbidities and medication history of the vaccinees, number of vaccine doses. However, the use of multivariate analyses in most of the retrieved studies ensured the possibility of adjusting for well-known predictors of vaccine response, allowing us to estimate the independent effect of smoking. Of course, analyses of confounders will be always limited by the limited knowledge of all the “unknown” factors that can influence vaccine-elicited IgG kinetic. Again, the results presented here are time-limited to COVID-19 vaccines. However, the present research is the first to synthetize epidemiological studies on the impact of smoking on postvaccination antibody titres during the ongoing massive COVID-19 vaccination programs worldwide, providing important insights for public health and policymakers. Another limitation due to the design of a rapid systematic review is the absence of a registered protocol, which might have delayed study conduction and dissemination.
5. Conclusions
This rapid systematic review reveals that active smoking has a negative impact on the humoral response to COVID-19 vaccines. The findings suggest the need for tailored interventions directed towards smokers to ensure appropriate protection measures to be taken to avoid SARS-CoV-2 infection and COVID-19 consequences. This review also informs policymakers on how to draw specific actions to tackle health inequalities between smokers and nonsmokers, while also considering that smoking is closely linked to socioeconomically deprived populations who are at higher risk for health problems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10020303/s1, Table S1: List of full-text reports not accepted for inclusion in the rapid systematic review with exclusion reasons; Table S2: Summary of GRADE’s approach for the quality rating of the body of evidence.
Author Contributions
Conceptualization, P.F.; methodology, P.F.; investigation, P.F. and V.G.; writing—original draft preparation, P.F.; writing—review and editing, all authors; visualization, R.P.; supervision, V.T. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The paper is part of the research line on vulnerability and risk management of the project GRIDAVI—Risk Management, Decision Uncertainties and Social Vulnerabilities (Gestione del Rischio, Incertezze DecisionAli e Vulnerabilità sociali) by the University of Catania Research Incentive Plan 2020/2022 PIACERI.
Conflicts of Interest
Outside this research, RP is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. In relation to his recent work in the area of respiratory diseases, clinical immunology, and tobacco control, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy nonprofit organizations. RP has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network (IRIS) initiative. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., Health Diplomats, and Sermo Inc. RP has served on the Medical and Scientific Advisory Board of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc, Pfizer, and PharmaCielo. RP is also founder of the Center for Tobacco prevention and treatment (CPCT) at the University of Catania and of the Center of Excellence for the acceleration of HArm Reduction (CoEHAR) at the same University, which has received support from Foundation for a Smoke Free World to conduct eight independent investigator-initiated research projects on harm reduction. RP is currently involved in a patent application concerning an app tracker for smoking behaviour developed for ECLAT Srl. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). All other authors have no relevant conflict of interest to declare.
References
- World Health Organization Tobacco. Newsroom. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 2 February 2022).
- Institute for Health Metrics and Evaluation Findings from the Global Burden of Disease Study 2017. Institute for Health Metrics and Evaluation. 2018. Available online: http://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf (accessed on 27 January 2022).
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; US Department of Health and Human Services: Atlanta, GA, USA, 2014.
- Edwards, D. Immunological effects of tobacco smoking in “healthy” smokers. COPD 2009, 6, 48–58. [Google Scholar] [CrossRef]
- Qiu, F.; Fan, P.; Nie, G.D.; Liu, H.; Liang, C.-L.; Yu, W.; Dai, Z. Effects of Cigarette Smoking on Transplant Survival: Extending or Shortening It? Front. Immunol. 2017, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Yang, S.M.; Kim, S.H.; Han, K.H.; Park, S.J.; Shin, J.I. Smoking and Rheumatoid Arthritis. Int. J. Mol. Sci. 2014, 15, 22279–22295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulyte, J.; Regueira, C.; Montes-Martínez, A.; Khudyakov, P.; Takkouche, B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001611. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.P.; Follett, E.A.; McIntyre, J.; Stewart, J.; Symington, I.S. Influence of smoking on immunological responses to hepatitis B vaccine. Vaccine 1994, 12, 771–772. [Google Scholar] [CrossRef]
- Petráš, M.; Oleár, V.; Molitorisová, M.; Dáňová, J.; Čelko, A.M.; Nováková, E.; Štefkovičová, M.; Krištúfková, Z.; Malinová, J.; Lesná, I.K. Factors Influencing Persistence of Diphtheria Immunity and Immune Response to a Booster Dose in Healthy Slovak Adults. Vaccines 2019, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Petráš, M.; Oleár, V. Predictors of the immune response to booster immunisation against tetanus in Czech healthy adults. Epidemiol. Infect. 2018, 146, 2079–2085. [Google Scholar] [CrossRef] [Green Version]
- Namujju, P.B.; Pajunen, E.; Simen-Kapeu, A.; Hedman, L.; Merikukka, M.; Surcel, H.M.; Kirnbauer, R.; Apter, D.; Paavonen, J.; Hedman, K.; et al. Impact of smoking on the quantity and quality of antibodies induced by human papillomavirus type 16 and 18 AS04-adjuvanted virus-like-particle vaccine—A pilot study. BMC Res. Notes 2014, 7, 445. [Google Scholar] [CrossRef] [Green Version]
- Nath, K.D.; Burel, J.G.; Shankar, V.; Pritchard, A.L.; Towers, M.; Looke, D.; Davies, J.M.; Upham, J.W. Clinical factors associated with the humoral immune response to influenza vaccination in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Signorelli, C.; Odone, A.; Gianfredi, V.; Bossi, E.; Bucci, D.; Oradini-Alacreu, A.; Frascella, B.; Capraro, M.; Chiappa, F.; Blandi, L.; et al. COVID-19 mortality rate in nine high-income metropolitan regions. Acta Biomed. 2020, 91, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; d’Alessandro, V.; Ferrara, P.; Smaldone, G.; Vitagliano, L. Analysis of the time evolution of COVID-19 lethality during the first epidemic wave in Italy. Acta Biomed. 2021, 92, e2021171. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, D.; Madotto, F.; Conti, S.; Antonazzo, I.C.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; et al. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up. Intern. Emerg. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Antonazzo, I.C.; Polosa, R. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up—Reply. Intern. Emerg. Med. 2022, 17, 313–314. [Google Scholar] [CrossRef]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel Med. 2021, 28, taab173. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Nikolouzakis, T.K.; Poulas, K.; Papazoglou, G.; Hatzidaki, E.; Papanikolaou, N.C.; Drakoulis, N.; et al. Immune response (IgG) following full inoculation with BNT162b2 COVID-19 mRNA among healthcare professionals. Int. J. Mol. Med. 2021, 48, 200. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.; Mantovani, L.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Tricco, A.C.; Langlois, E.V.; Straus, S.E. Rapid Reviews to Strengthen Health Policy and Systems: A Practical Guide; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gianfredi, V.; Mauer, N.S.; Gentile, L.; Riccò, M.; Odone, A.; Signorelli, C. COVID-19 and Recreational Skiing: Results of a Rapid Systematic Review and Possible Preventive Measures. Int. J. Environ. Res. Public Health 2021, 18, 4349. [Google Scholar] [CrossRef]
- Balshema, H.; Helfanda, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Gümüş, H.H.; Ödemiş, I.; Alışka, H.E.; Karslı, A.; Kara, S.; Özkale, M.; Gül, E. Side effects and antibody response of an inactive severe acute respiratory syndrome coronavirus 2 vaccine among health care workers. Rev. Assoc. Med. Bras. 2021, 67, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Nakashima, R.; Miyoshi, K.; Hara, Y.; Hayashi, J.; Hara, H.; Nomura, H.; Shimono, N. Dynamics of anti-Spike IgG antibody level after full BNT162b2 COVID-19 vaccination in health care workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, F.; Zhang, X.; Wang, H.; Liang, T.; Guo, S.B. Down-regulation of SARS-CoV-2 neutralizing antibodies in vaccinated smokers. medRxiv 2021. [Google Scholar] [CrossRef]
- Pitzalis, M.; Idda, M.L.; Lodde, V.; Loizedda, A.; Lobina, M.; Zoledziewska, M.; Virdis, F.; Delogu, G.; Pirinu, F.; Marini, M.G.; et al. Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients. Front. Immunol. 2021, 12, 781843. [Google Scholar] [CrossRef]
- Herzberg, J.; Vollmer, T.; Fischer, B.; Becher, H.; Becker, A.-K.; Honarpisheh, H.; Guraya, S.Y.; Strate, T.; Knabbe, C. SARS-CoV-2-antibody response in health care workers after vaccination or natural infection in a longitudinal observational study. Vaccine 2021, 40, 206–212. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Basilico, S.; Iacobellis, G.; Milani, V.; Cardani, R.; Boniardi, F.; Dubini, C.; Prandoni, I.; Capitanio, G.; Renna, L.V.; et al. Antibody responses to BNT162b2 mRNA vaccine: Infection-naïve individuals with abdominal obesity warrant attention. Obesity 2021. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tanaka, A.; Ohmagari, N.; Yamaguchi, K.; Ishitsuka, K.; Morisaki, N.; Kojima, M.; Nishikimi, A.; Tokuda, H.; Inoue, M.; et al. Use of heat-not-burn tobacco products, moderate alcohol drinking, and anti-SARS-CoV-2 IgG antibody titers after BNT162b2 vaccination among Japanese healthcare workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Go, H.; Yamaoka, Y.; Yajima, S.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. J. Infect. Chemother. 2021, 28, 273–278. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; Sugiyama, K. Attenuation of antibody titres during 3-6 months after the second dose of the BNT162b2 vaccine depends on sex, with age and smoking as risk factors for lower antibody titres at 6 months. Vaccines 2021, 9, 1500. [Google Scholar] [CrossRef]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Yamaoka, Y.; Yajima, S.; Yamazaki, E.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Vaccine-induced humoral and cellular immunity against SARS-CoV-2 at 6 months post BNT162b2 vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2021, 94, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.-E.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Linardou, H.; Spanakis, N.; Koliou, G.-A.; Christopoulou, A.; Karageorgopoulou, S.; Alevra, N.; Vagionas, A.; Tsoukalas, N.; Sgourou, S.; Fountzilas, E.; et al. Responses to SARS-CoV-2 Vaccination in Patients with Cancer (ReCOVer Study): A Prospective Cohort Study of the Hellenic Cooperative Oncology Group. Cancers 2021, 13, 4621. [Google Scholar] [CrossRef]
- Moncunill, G.; Aguilar, R.; Ribes, M.; Ortega, N.; Rubio, R.; Salmeron, G.; Molina, M.J.; Vidal, M.; Barrios, D.; Mitchell, R.A.; et al. Determinants of early antibody responses to COVID-19 mRNA vaccines in exposed and naive healthcare workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. SARS-CoV-2 antibody titer 3 months post-vaccination is affected by age, gender, smoking and vitamin D. medRxiv 2021. [Google Scholar] [CrossRef]
- Michos, A.; Tatsi, E.; Filippatos, F.; Dellis, C.; Koukou, D.; Efthymiou, V.; Kastrinelli, E.; Mantzou, A.; Syriopoulou, V. Association of total and neutralizing SARS-CoV-2 spike -receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1st and 2nd doses of the BNT162b2 vaccine. Vaccine 2021, 39, 5963–5967. [Google Scholar] [CrossRef]
- Lombardi, A.; Consonni, D.; Oggioni, M.; Bono, P.; Renteria, S.U.; Piatti, A.; Pesatori, A.C.; Castaldi, S.; Muscatello, A.; Riboldi, L.; et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J. Infect. Public Health 2021, 14, 1120–1122. [Google Scholar] [CrossRef]
- Modenese, A.; Paduano, S.; Bargellini, A.; Bellucci, R.; Marchetti, S.; Bruno, F.; Grazioli, P.; Vivoli, R.; Gobba, F. Neutralizing Anti-SARS-CoV-2 Antibody Titer and Reported Adverse Effects, in a Sample of Italian Nursing Home Personnel after Two Doses of the BNT162b2 Vaccine Administered Four Weeks Apart. Vaccines 2021, 9, 652. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/Metab. Res. Rev. 2021, 38, e3465. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Simeng Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.M.; Çolak, Y.; Ellervik, C.; Hasselbalch, H.C.; Bojesen, S.E.; Nordestgaard, B.G. Smoking and Increased White and Red Blood Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sopori, M.L. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Hernandez, C.P.; Morrow, K.; Velasco, C.; Wyczechowska, D.D.; Naura, A.S.; Rodriguez, P.C. Effects of ciga-rette smoke extract on primary activated T cells. Cell Immunol. 2013, 282, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piaggeschi, G.; Rolla, S.; Rossi, N.; Brusa, D.; Naccarati, A.; Couvreur, S.; Spector, T.D.; Roederer, M.; Mangino, M.; Cordero, F.; et al. Immune Trait Shifts in Associa-tion with Tobacco Smoking: A Study in Healthy Women. Front. Immunol. 2021, 12, 637974. [Google Scholar] [CrossRef] [PubMed]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tob. Control. 2019, 28, 582–594. [Google Scholar] [CrossRef]
- Younas, M.; Carrat, F.; Desaint, C.; Launay, O.; Corbeau, P. Immune activation, smoking, and vaccine response. Aids 2017, 31, 171–173. [Google Scholar] [CrossRef]
- Jackson, S.E.; Paul, E.; Brown, J.; Steptoe, A.; Fancourt, D. Negative Vaccine Attitudes and Intentions to Vaccinate Against COVID-19 in Relation to Smoking Status: A Population Survey of UK Adults. Nicotine Tob. Res. 2021, 23, 1623–1628. [Google Scholar] [CrossRef]
- Polosa, R.; Tomaselli, V.; Ferrara, P.; Romeo, A.C.; Rust, S.; Saitta, D.; Caraci, F.; Romano, C.; Thangaraju, M.; Zuccarello, P.; et al. Seroepidemiological Survey on the Impact of Smoking on SARS-CoV-2 Infection and COVID-19 Outcomes: Protocol for the Troina Study. JMIR Res. Protoc. 2021, 10, e32285. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Pennisi, F.; Lume, A.; Ricciardi, G.E.; Minerva, M.; Riccò, M.; Odone, A.; Signorelli, C. Challenges and Opportunities of Mass Vaccination Centers in COVID-19 Times: A Rapid Review of Literature. Vaccines 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).