Avian Orthoavulavirus Type-1 as Vaccine Vector against Respiratory Viral Pathogens in Animal and Human
Abstract
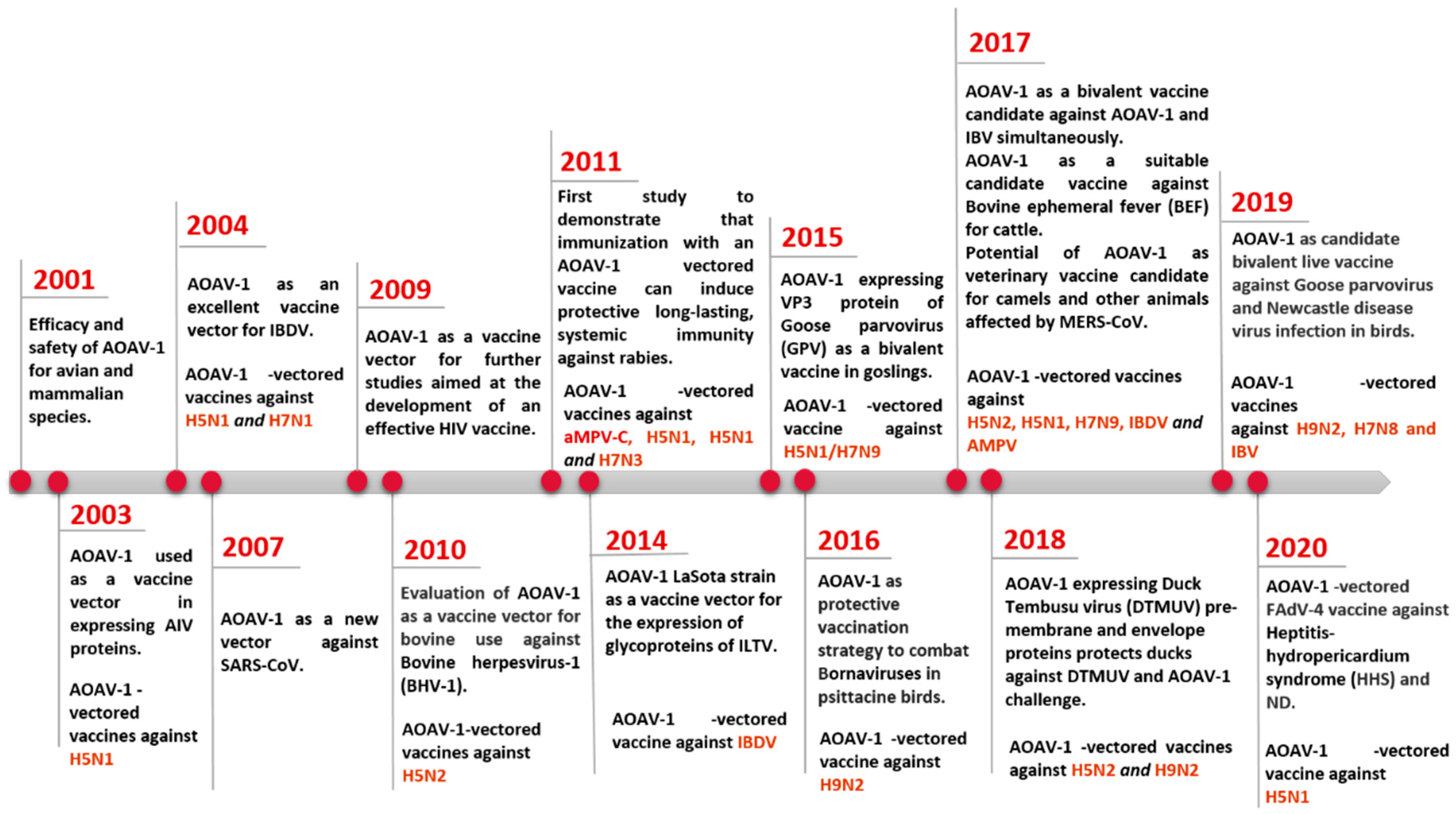
1. Introduction
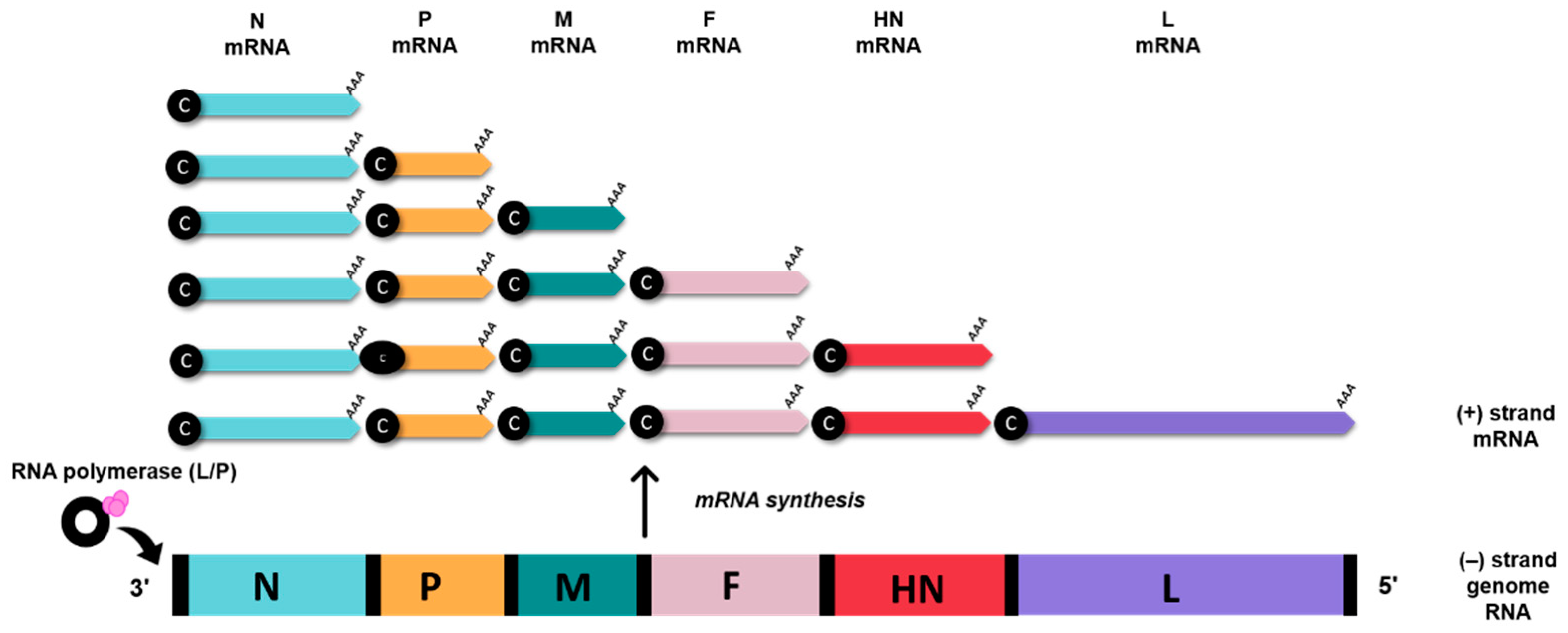
2. Genomic and Biological Features of Avian Orthoavulavirus Type-1
3. AOaV-1 as a Viral Vaccine Vector
4. Current Application of AOaV-1 as Vaccine Vector for Poultry and Animal Viruses
4.1. Recombinant AOaV-1 Based Vaccines for Poultry
4.1.1. Avian Influenza Virus (AIV)
| Pathogen | AOaV-1 Backbone | Antigen | Insert site | Animal Model | Vaccination (Route) | References |
|---|---|---|---|---|---|---|
| H1N1 | Hitchner B1 | HA | P/M | Mouse | i.v. or i.p. | [16] |
| H5N1 | La Sota | HA | P/M | Chicken/Mouse | o.n. (chicken); i.p (mouse] | [35] |
| H5N1 | La Sota | HA HA + AOaV-1 F | P/M | Chicken | o.n. | [29] |
| H5N2 | La Sota | HA + AOaV-1 F | P/M | Chicken | i.m./spray | [34] |
| H5N1/ H7N9 | La Sota | HA + AOaV-1 F | P/M | Chicken | i.m. or o.n. | [31] |
| H5N2 | La Sota/ Chimeric AOaV-1 | HA and NA | HA- P/M NA- M/F | Chicken | i.n | [36] |
| H5N1 | Chimeric AOaV-1 based on AOaV-1 clone 30 | HA | F/HN | Chicken | o.n. | [37] |
| H5N1 | Chimeric AOaV-1 strain BC | HA | N/P or P/M | Chicken | o.n. | [25] |
| H5N1 | La Sota/ Chimeric AOaV-1 | HA HA and NA HA and M1 HA and NS1 | HA- P/M NA, MI, NS1- M/F | Chicken | o.n. | [38] |
| H5N1 | TS09-C | HA/HA1 | P/M | Chicken | i.n/i.o. | [39] |
| H5N1 | La Sota | HA | P/M | Duck | i.o | [40] |
| H5N2 | La Sota | HA | P/M | Chicken | i.o. | [41] |
| H5N1 | Beaudette C strain | HA | P/M | Monkey | i.n/i.t. | [22] |
| H9N2 | La Sota | HA HA + AOaV-1 F | P/M | Chicken | o.n/i.m. | [32] |
| H9N2 | rmNA-1 strain | HA HA + AOaV-1 F | P/M | Chicken | o.n. | [42] |
| H9N2 | Chimeric AOaV-1 | HA | P/M | Chicken | o.n. | [43] |
| H7N2 | Hitchner B1 | HA + AOaV-1 F | P/M | Chicken | i.o. | [28] |
| H7N2 | Hitchner B1 | HA | P/M | Chicken | i.o. | [44] |
| H7N1 | Clone 30 | HA | F/HN | Chicken | i.n. | [45] |
| H7N9 | LX | HA HA + AOaV-1 F | P/M | Chicken | i.n. | [46] |
| H7N3 | La Sota | HA | P/M | Mouse | i.n. | [26] |
| H7N8 | La Sota/ Chimeric AOaV-1 | HA HA and NA | HA- P/M NA- M/F | Chicken | i.n. | [47] |
| H6N2 | Clone 30 | HA | F/HN | Chicken/Turkey | o.n. | [48] |
| IBDV | La Sota | VP2 | Upstream of the NP | Chicken | i.o. | [49] |
| IBDV | F strain | VP2 | P/M | Chicken | i.n. | [50] |
| IBDV | rLaC30L [La Sota; Clone 30] | VP2 | P/M | Chicken embryo | in ovo | [51] |
| ILTV | La Sota | gB or gD | P/M | Chicken | i.n./i.o. | [52] |
| ILTV | La Sota | gB or gC or gD | P/M | Chicken | o.n. | [53] |
| IBV | La Sota | S | P/M | Chicken | o.n. | [54] |
| IBV | La Sota | S1 | P/M | Chicken | o.n. | [55] |
| IBV | La Sota | S1 [multi-epitope] | P/M | Chicken | o.n. | [21] |
| AMPV | La Sota | G | F/HN | Turkey | i.n./i.o. | [56] |
| AMPV | La Sota | G + F | F/HN | Turkey | i.n./i.o. | [57] |
| FAdV | La Sota | fiber 2 | P/M | Chicken | i.m. | [58] |
| GoAstV | SH12 | Cap | P/M | Gosling | o.n. | [59] |
| GPV | NA-1 strain | VP3 | P/M | Gosling | s.c. | [60] |
| DTMUV | GM | prM + E | P/M | Duck | s.c. | [61] |
| Bornavirus | Clone 30 | N or P | F/HN | Cockatiel/Canary | i.m. | [62] |
4.1.2. Infectious Bronchitis Virus (IBV)
4.1.3. Infectious Bursal Disease Virus (IBDV)
4.1.4. Infectious Laryngotracheitis Virus [ILTV]
4.2. Recombinant AOaV-1 Based Vaccines in Animals
4.2.1. Middle East Respiratory Syndrome Corona Virus (MERS-COV)
| Pathogen | AOaV-1 Backbone | Antigen | Insert Site | Animal Model | Vaccination (Route) | References |
|---|---|---|---|---|---|---|
| MERS-CoV | La Sota | S | P/M | Mouse/Camel | i.m. | [32] |
| BEFV | La Sota | G | P/M | Mouse/Cattle | i.m. | [68] |
| BHV-1 | La Sota | gD | P/M | Calf | i.n./i.t. | [67] |
| Rabies | La Sota | G | P/M | Mouse/Cat/Dog | i.m. | [72] |
| PRRSV | La Sota | GP5; GP3 + GP5 | P/M | Piglet | i.m. | [79] |
| NiV | La Sota | G or F | P/M | Pig | i.m. | [73] |
| VSV | La Sota | G | P/M | Mouse | i.m. | [70] |
| CDV | La Sota | F or H | P/M | Mink | i.m. | [71] |
| WNV | La Sota | PrM and E | P/M | Mouse/Horse/Chicken | i.m. | [74] |
| RVFV | La Sota | Gn | P/M | Cattle | i.n./i.m. | [69] |
4.2.2. Bovine Herpesvirus 1 (BoHV-1)
4.2.3. Rift Valley Fever Virus (RVFV)
4.2.4. Canine Distemper Virus (CDV)
4.2.5. Rabies Virus (RV)
4.2.6. Bovine Ephemeral Fever Virus (BEFV]
4.2.7. Vesicular Stomatitis Virus (VSV)
4.2.8. West Nile Virus (WVN)
5. Current Application of AOaV-1 as Vaccine Vector for Emerging and Remerging Human Viruses
5.1. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV 2)
5.2. Severe Acute Respiratory Syndrome-Associated Coronavirus (SARS-CoV)
5.3. Influenza Viruses
5.4. Ebola Virus (EBOV)
5.5. Nipah Virus (NiV)
5.6. Norovirus (NoV)
5.7. Respiratory Syncytial Virus (RSV)
5.8. Human Immunodeficiency Virus (HIV)
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alexander, D.J. Newcastle disease. Br. Poultry Sci. 2001, 42, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, K.; Tan, W.S. Newcastle disease virus: Macromolecules and opportunities. Avian Pathol. 2001, 30, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Huang, Z.; Elankumaran, S.; Rockemann, D.D.; Samal, S.K. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 2004, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.M.; Mahon, P.J. Paramyxoviruses: Different receptors—Different mechanisms of fusion. Trends Microbiol. 2008, 16, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.; Kumar, S.; Khattar, S.K.; Samal, S.K. A single amino acid change, Q114R, in the cleavage-site sequence of Newcastle disease virus fusion protein attenuates viral replication and pathogenicity. J. Gen. Virol. 2011, 92, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Huang, Z.; Samal, S.K. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: Expression of a foreign gene results in growth retardation and attenuation. Virology 2000, 278, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Bashir Bello, M.; Yusoff, K.; Ideris, A.; Hair-Bejo, M.; Hassan Jibril, A.; Peeters, B.P.H.; Omar, A.R. Exploring the prospects of engineered Newcastle disease virus in modern vaccinology. Viruses 2020, 12, 1–23. [Google Scholar]
- Huang, Z.; Elankumaran, S.; Panda, A.; Samal, S.K. Recombinant Newcastle disease virus as a vaccine vector. Poultry Sci. 2003, 82, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.P.H.; De Leeuw, O.S.; Verstegen, I.; Koch, G.; Gielkens, A.L.J. Generation of a recombinant chimeric Newcastle disease virus vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine 2001, 19, 1616–1627. [Google Scholar] [CrossRef]
- Huang, Z.; Krishnamurthy, S.; Panda, A.; Samal, S.K. High-level expression of a foreign gene from the most 3’-proximal locus of a recombinant Newcastle disease virus. J. Gen. Virol. 2001, 82, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Nayak, B.; Samuel, A.; Paldurai, A.; Kanabagattebasavarajappa, M.; Prajitno, T.Y.; Bharoto, E.E.; Collins, P.L.; Samal, S.K. Generation by reverse genetics of an effective, stable, live-attenuated Newcastle disease virus vaccine based on a currently circulating, highly virulent indonesian strain. PLoS ONE 2012, 7, e52751. [Google Scholar]
- Paldurai, A.; Kim, S.-H.; Nayak, B.; Xiao, S.; Shive, H.; Collins, P.L.; Samal, S.K. Evaluation of the contributions of individual viral genes to Newcastle disease virus virulence and pathogenesis. J. Virol. 2014, 88, 8579–8596. [Google Scholar] [CrossRef]
- Rohaim, M.A.; Munir, M. A Scalable Topical Vectored Vaccine Candidate against SARS-CoV-2. Vaccines 2020, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Li, W.; Borderia, A.V.; Moltedo, B.; Moran, T.; García-Sastre, A. Optimization of human immunodeficiency virus gag expression by Newcastle disease virus vectors for the induction of potent immune responses. J. Virol. 2009, 83, 584–597. [Google Scholar] [CrossRef]
- Nakaya, T.; Cros, J.; Park, M.-S.; Nakaya, Y.; Zheng, H.; Sagrera, A.; Villar, E.; García-Sastre, A.; Palese, P. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 2001, 75, 11868–11873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Peeters, B.P.H. Recombinant Newcastle disease virus as a viral vector: Effect of genomic location of foreign gene on gene expression and virus replication. J. Gen. Virol. 2003, 84, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Bukreyev, A.; Huang, Z.; Yang, L.; Elankumaran, S.; St. Claire, M.; Murphy, B.R.; Samal, S.K.; Collins, P.L. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 2005, 79, 13275–13284. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ni, J.; Cao, Y.; Liu, X. Newcastle disease virus as a vaccine vector for 20 years: A focus on maternally derived antibody interference. Vaccines 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S. Newcastle disease virus vectored vaccines as bivalent or antigen delivery vaccines. Clin. Exp. Vaccine Res. 2017, 6, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wen, G.; Qiu, X.; Yuan, Y.; Meng, C.; Sun, Y.; Liao, Y.; Song, C.; Liu, W.; Shi, Y.; et al. A recombinant la sota vaccine strain expressing multiple epitopes of infectious bronchitis virus (Ibv) protects specific pathogen-free (spf) chickens against ibv and ndv challenges. Vaccines 2019, 7, 170. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Yang, L.; Suguitan, A.; Elankumaran, S.; Dorward, D.W.; Murphy, B.R.; Samal, S.K.; Collins, P.L.; Bukreyev, A. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J. Virol. 2007, 81, 11560–11568. [Google Scholar] [CrossRef]
- Kim, S.H.; Samal, S.K. Innovation in newcastle disease virus vectored avian influenza vaccines. Viruses 2019, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Acheson, N.H.; Kolakofsky, D.; Richardson, C. Paramyxoviruses and Rhabdoviruses. In Fundamentals of Molecular Virology; Acheson, N.H., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 175–187. [Google Scholar]
- Kim, S.H.; Paldurai, A.; Samal, S.K. A novel chimeric Newcastle disease virus vectored vaccine against highly pathogenic avian influenza virus. Virology 2017, 503, 31–36. [Google Scholar] [CrossRef]
- Goff, P.H.; Krammer, F.; Hai, R.; Seibert, C.W.; Margine, I.; Garcia-Sastre, A.; Palese, P. Induction of cross-reactive antibodies to novel H7N9 influenza virus by recombinant Newcastle disease virus expressing a north american lineage H7 subtype hemagglutinin. J. Virol. 2013, 87, 8235–8240. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, A.; Steensels, A.C.M.; Lambrecht, A.B.; Desloges, A.N.; Rahaus, M.; Rebeski, D. Potency of a recombinant NDV-H5 vaccine against various HPAI H5N1 virus challenges in SPF chickens. Avian Dis. 2012, 56, 928–936. [Google Scholar] [CrossRef]
- Park, M.S.; Steel, J.; García-Sastre, A.; Swayne, D.; Palese, P. Engineered viral vaccine constructs with dual specificity: Avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. USA 2006, 103, 8203–8208. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Rout, S.N.; Kumar, S.; Khalil, M.S.; Fouda, M.M.; Ahmed, L.E.; Earhart, K.C.; Perez, D.R.; Collins, P.L.; Samal, S.K. Immunization of chickens with Newcastle disease virus expressing H5 hemagglutinin protects against highly pathogenic H5N1 avian influenza viruses. PLoS ONE 2009, 4, e6509. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.K.; Manoharan, V.; Bhattarai, B.; Labranche, C.C.; Montefiori, D.C.; Samal, S.K. Mucosal immunization with newcastle disease virus vector coexpressing HIV-1 env and gag proteins elicits potent serum, mucosal, and cellular immune responses that protect against vaccinia virus env and gag challenges. MBio 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mena, I.; Ma, J.; Bawa, B.; Krammer, F.; Lyoo, Y.S.; Lang, Y.; Morozov, I.; Mahardika, G.N.; Ma, W.; et al. Newcastle disease virus-vectored H7 and H5 live vaccines protect chickens from challenge with H7N9 or H5N1 avian influenza viruses. J. Virol. 2015, 89, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Lee, J.; Mena, I.; Henningson, J.; Li, Y.; Ma, J.; Duff, M.; Li, Y.; Lang, Y.; Yang, J.; et al. Recombinant Newcastle disease virus expressing H9 HA protects chickens against heterologous avian influenza H9N2 virus challenge. Vaccine 2016, 34, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.L.; Rauw, F.; Pirlot, J.F.; Reynard, F.; van den Berg, T.; Bublot, M.; Lambrecht, B. Comparison of single 1-day-old chick vaccination using a Newcastle disease virus vector with a prime/boost vaccination scheme against a highly pathogenic avian influenza H5N1 challenge. Avian Pathol. 2014, 43, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lee, J.; Liu, H.; Mena, I.; Davis, A.S.; Sunwoo, S.Y.; Lang, Y.; Duff, M.; Morozov, I.; Li, Y.; et al. Newcastle disease virus-based H5 influenza vaccine protects chickens from lethal challenge with a highly pathogenic H5N2 avian influenza virus. NPJ Vaccines 2017, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Deng, G.; Wen, Z.; Tian, G.; Wang, Y.; Shi, J.; Wang, X.; Li, Y.; Hu, S.; Jiang, Y.; et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 2007, 81, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lamichhane, B.; Nagy, A.; Chowdhury, I.R.; Samal, S.K.; Kim, S.H. Co-expression of the hemagglutinin and neuraminidase by heterologous Newcastle disease virus vectors protected chickens against H5 clade 2.3.4.4 HPAI viruses. Sci. Rep. 2018, 8, 16854. [Google Scholar] [CrossRef] [PubMed]
- Steglich, C.; Grund, C.; Ramp, K.; Breithaupt, A.; Höper, D.; Keil, G.; Veits, J.; Ziller, M.; Granzow, H.; Mettenleiter, T.C.; et al. Chimeric Newcastle disease virus protects chickens against avian influenza in the presence of maternally derived NDV immunity. PLoS ONE 2013, 8, e72530. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Samal, S.K. Heterologous prime-boost immunization of Newcastle disease virus vectored vaccines protected broiler chickens against highly pathogenic avian influenza and Newcastle disease viruses. Vaccine 2017, 35, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qin, Z.; Qiao, L.; Wen, J.; Shao, H.; Wen, G.; Pan, Z. Characterization of thermostable Newcastle disease virus recombinants expressing the hemagglutinin of H5N1 avian influenza virus as bivalent vaccine candidates. Vaccine 2020, 38, 1690–1699. [Google Scholar] [CrossRef]
- Ferreira, H.L.; Pirlot, J.F.; Reynard, F.; van den Berg, T.; Bublot, M.; Lambrecht, B. Immune responses and protection against H5N1 highly pathogenic avian influenza virus induced by the Newcastle disease virus H5 vaccine in ducks. Avian Dis. 2012, 56, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Sarfati-Mizrahi, D.; Lozano-Dubernard, B.; Soto-Priante, E.; Castro-Peralta, F.; Flores-Castro, R.; Loza-Rubio, E.; Gay-Gutiérrez, M. Protective dose of a recombinant Newcastle disease LaSota–avian influenza virus H5 Vaccine against H5N2 highly pathogenic avian influenza virus and velogenic viscerotropic Newcastle disease virus in broilers with high maternal antibody levels. Avian Dis. 2010, 54, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xue, C.; Liu, X.; Li, J.; Fei, Y.; Liu, Z.; Mu, J.; Bi, Y.; Qian, J.; Yin, R.; et al. A novel recombinant attenuated Newcastle disease virus expressing H9 subtype hemagglutinin protected chickens from challenge by genotype VII virulent Newcastle disease virus and H9N2 avian influenza virus. Vet. Microbiol. 2019, 228, 173–180. [Google Scholar] [CrossRef]
- Liu, J.; Xue, L.; Hu, S.; Cheng, H.; Deng, Y.; Hu, Z.; Wang, X.; Liu, X. Chimeric Newcastle disease virus-vectored vaccine protects chickens against H9N2 avian influenza virus in the presence of pre-existing NDV immunity. Arch. Virol. 2018, 163, 3365–3371. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L.; Schultz-Cherry, S.; Tumpey, T.M.; King, D.J.; Nakaya, T.; Palese, P.; Garcia-Sastre, A. Recombinant paramyxovirus type 1-avian influenza-H7 virus as a vaccine for protection of chickens against influenza and Newcastle disease. Avian Dis. 2003, 47, 1047–1050. [Google Scholar] [CrossRef]
- Schröer, D.; Veits, J.; Grund, C.; Dauber, M.; Keil, G.; Granzow, H.; Mettenleiter, T.C.; Römer-Oberdörfer, A. Vaccination with Newcastle disease virus vectored vaccine protects chickens against highly pathogenic H7 avian influenza virus. Avian Dis. 2009, 4, e5. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Jiao, X.; Liu, X. Newcastle disease virus (NDV) recombinant expressing the hemagglutinin of H7N9 avian influenza virus protects chickens against NDV and highly pathogenic avian influenza A (H7N9) virus challenges. Vaccine 2017, 35, 6585–6590. [Google Scholar] [CrossRef]
- Roy Chowdhury, I.; Yeddula, S.G.R.; Pierce, B.G.; Samal, S.K.; Kim, S.H. Newcastle disease virus vectors expressing consensus sequence of the H7 HA protein protect broiler chickens and turkeys against highly pathogenic H7N8 virus. Vaccine 2019, 37, 4956–4962. [Google Scholar] [CrossRef]
- Schröer, D.; Veits, J.; Keil, G.; Römer-Oberdörfer, A.; Weber, S.; Mettenleiter, T.C. Efficacy of Newcastle disease virus recombinant expressing avian influenza virus H6 Hemagglutinin against Newcastle disease and low pathogenic avian influenza in chickens and turkeys. Avian Dis. 2011, 55, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Elankumaran, S.; Yunus, A.S.; Samal, S.K. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 2004, 78, 10054–10063. [Google Scholar] [CrossRef]
- Dey, S.; Chellappa, M.M.; Pathak, D.C.; Gaikwad, S.; Yadav, K.; Ramakrishnan, S.; Vakharia, V.N. Newcastle disease virus vectored bivalent vaccine against virulent infectious bursal disease and Newcastle disease of chickens. Vaccines 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, X.; Tian, M.; Wen, Z.; Feng, Q.; Qi, X.; Gao, H.; Wang, X.; Bu, Z. Novel in-ovo chimeric recombinant Newcastle disease vaccine protects against both Newcastle disease and infectious bursal disease. Vaccine 2014, 32, 1514–1521. [Google Scholar] [CrossRef]
- Zhao, W.; Spatz, S.; Zhang, Z.; Wen, G.; Garcia, M.; Zsak, L.; Yu, Q. Newcastle disease virus (NDV) recombinants expressing infectious laryngotracheitis virus (ILTV) glycoproteins gB and gD protect chickens against ILTV and NDV challenges. J. Virol. 2014, 88, 8397–8406. [Google Scholar] [CrossRef] [PubMed]
- Kanabagatte Basavarajappa, M.; Kumar, S.; Khattar, S.K.; Gebreluul, G.T.; Paldurai, A.; Samal, S.K. A recombinant Newcastle disease virus (NDV) expressing infectious laryngotracheitis virus (ILTV) surface glycoprotein D protects against highly virulent ILTV and NDV challenges in chickens. Vaccine 2014, 32, 3555–3563. [Google Scholar] [CrossRef]
- Abozeid, H.H.; Paldurai, A.; Varghese, B.P.; Khattar, S.K.; Afifi, M.A.; Zouelfakkar, S.; El-Deeb, A.H.; El-Kady, M.F.; Samal, S.K. Development of a recombinant Newcastle disease virus-vectored vaccine for infectious bronchitis virus variant strains circulating in Egypt. Vet. Res. 2019, 50, 1–13. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, J.; Qi, T.; Zhao, W.; Han, Z.; Yang, X.; Liu, S. Recombinant Newcastle disease virus expressing the infectious bronchitis virus S1 gene protects chickens against Newcastle disease virus and infectious bronchitis virus challenge. Vaccine 2017, 35, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Roth, J.P.; Estevez, C.N.; Zsak, L.; Liu, B.; Yu, Q. Generation and evaluation of a recombinant Newcastle disease virus expressing the glycoprotein (G) of avian metapneumovirus subgroup C as a bivalent vaccine in turkeys. Vaccine 2011, 29, 8624–8633. [Google Scholar] [CrossRef]
- Hu, H.; Roth, J.P.; Zsak, L.; Yu, Q. Engineered Newcastle disease virus expressing the F and G proteins of AMPV-C confers protection against challenges in turkeys. Sci. Rep. 2017, 7, 10–17. [Google Scholar] [CrossRef]
- Tian, K.Y.; Guo, H.F.; Li, N.; Zhang, Y.H.; Wang, Z.; Wang, B.; Yang, X.; Li, Y.T.; Zhao, J. Protection of chickens against hepatitis-hydropericardium syndrome and Newcastle disease with a recombinant Newcastle disease virus vaccine expressing the fowl adenovirus serotype 4 fiber-2 protein. Vaccine 2020, 38, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, C.; Liu, G.; Chen, Z.; Jia, R. Generation and evaluation of a recombinant goose origin Newcastle disease virus expressing Cap protein of goose origin avastrovirus as a bivalent vaccine in goslings. Poultry Sci. 2019, 98, 4426–4432. [Google Scholar] [CrossRef]
- Wang, J.; Cong, Y.; Yin, R.; Feng, N.; Yang, S.; Xia, X.; Xiao, Y.; Wang, W.; Liu, X.; Hu, S.; et al. Generation and evaluation of a recombinant genotype VII Newcastle disease virus expressing VP3 protein of Goose parvovirus as a bivalent vaccine in goslings. Virus Res. 2015, 203, 77–83. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Li, L.; Lin, Q.; Sun, J.; Liu, Z.; Shen, H.; Zhang, J.; Ren, T.; Zhang, C. Recombinant Newcastle disease virus (NDV) expressing Duck Tembusu virus (DTMUV) pre-membrane and envelope proteins protects ducks against DTMUV and NDV challenge. Vet. Microbiol. 2018, 218, 60–69. [Google Scholar] [CrossRef]
- Olbert, M.; Römer-Oberdörfer, A.; Herden, C.; Malberg, S.; Runge, S.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Cavanagh, D.; Gelb, J. Infectious bronchitis. In Diseases of Poultry, 12th ed.; Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2008; pp. 117–135. [Google Scholar]
- Shirvani, E.; Paldurai, A.; Manoharan, V.K.; Varghese, B.P.; Samal, S.K. A recombinant Newcastle disease virus (NDV) expressing S protein of infectious bronchitis virus (IBV) protects chickens against IBV and NDV. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-C.; Giambrone, J.J. Infectious laryngotracheitis virus in chickens. World J. Virol. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Liu, R.Q.; Ge, J.Y.; Wang, J.L.; Shao, Y.; Zhang, H.L.; Wang, J.L.; Wen, Z.Y.; Bu, Z.G. Newcastle disease virus-based MERS-CoV candidate vaccine elicits high-level and lasting neutralizing antibodies in Bactrian camels. J. Integr. Agric. 2017, 16, 2264–2273. [Google Scholar] [CrossRef]
- Khattar, S.K.; Collins, P.L.; Samal, S.K. Immunization of cattle with recombinant Newcastle disease virus expressing bovine herpesvirus-1 (BHV-1) glycoprotein D induces mucosal and serum antibody responses and provides partial protection against BHV-1. Vaccine 2010, 28, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ge, J.; Wen, Z.; Chen, W.; Wang, X.; Liu, R.; Bu, Z. Characterization of a recombinant Newcastle disease virus expressing the glycoprotein of bovine ephemeral fever virus. Arch. Virol. 2017, 162, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, J.; Dekker, A.; de Boer, S.M.; Weerdmeester, K.; Vloet, R.P.M.; de Wit, A.A.; Peeters, B.P.; Moormann, R.J. Intramuscular inoculation of calves with an experimental Newcastle disease virus-based vector vaccine elicits neutralizing antibodies against Rift Valley fever virus. Vaccine 2010, 28, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ge, J.; Li, X.; Chen, W.; Wang, X.; Wen, Z.; Bu, Z. Protective efficacy of a recombinant Newcastle disease virus expressing glycoprotein of vesicular stomatitis virus in mice. Virol. J. 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tian, M.; Gao, Y.; Wen, Z.; Yu, G.; Zhou, W.; Zu, S.; Bu, Z. Recombinant Newcastle disease viral vector expressing hemagglutinin or fusion of canine distemper virus is safe and immunogenic in minks. Vaccine 2015, 33, 2457–2462. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle disease virus-vectored Rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef]
- Kong, D.; Wen, Z.; Su, H.; Ge, J.; Chen, W.; Wang, X.; Wu, C.; Yang, C.; Chen, H.; Bu, Z. Newcastle disease virus-vectored Nipah encephalitis vaccines induce B and T cell responses in mice and long-lasting neutralizing antibodies in pigs. Virology 2012, 432, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Ge, J.; Hua, R.; Liu, R.; Li, X.; Wang, X.; Shao, Y.; Sun, E.; Wu, D.; et al. Newcastle disease virus-vectored West Nile fever vaccine is immunogenic in mammals and poultry. Virol. J. 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Y.; Ong, H.K.; Yeap, S.K.; Ho, K.L.; Tan, W.S. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Q.; Du, L.; Jiang, S. Middle East respiratory syndrome coronavirus (MERS-CoV): Challenges inidentifying its source and controlling its spread. Microbes Infect. 2013, 15, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Han, G.Z. Deciphering MERS-CoV evolution in dromedary camels. Trends Microbiol. 2016, 24, 87–89. [Google Scholar] [CrossRef]
- Chen, X.; Chughtai, A.A.; Dyda, A.; Macintyre, C.R. Comparative epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia and South Korea. Emerg. Microbes Infect. 2017, 6, e51. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, F.; Li, Z.; Zhao, G.; Xie, C.; Ha, Z.; Zhang, J.; Han, J.; Xiao, P.; Zhuang, X.; et al. Construction and immunological evaluation of recombinant Newcastle disease virus vaccines expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP3/GP5 proteins in pigs. Vet. Microbiol. 2019, 239, 108490. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Front. Immunol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Makino, S. Rift Valley fever vaccines. Vaccine 2009, 27, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Kristiansen, P.; Norheim, G.; Yimer, S.A. Rift valley fever: Diagnostic challenges and investment needs for vaccine development. BMJ Glob. Health 2020, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ulbert, S. West Nile virus vaccines—Current situation and future directions. Hum. Vaccines Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Pronker, E.S.; Weenen, T.C.; Commandeur, H.; Claassen, E.H.J.H.M.; Osterhaus, A.D.M.E. Risk in vaccine research and development quantified. PLoS ONE 2013, 8, e57755. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.A.; Goldin, S.; Nannei, C.; Sparrow, E.; Torelli, G. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine 2016, 34, 5410–5413. [Google Scholar] [CrossRef]
- Gilbert, J.A. Seasonal and pandemic influenza: Global fatigue versus global preparedness. Lancet Respir. Med. 2018, 6, 94–95. [Google Scholar] [CrossRef]
- Kim, S.H.; Samal, S.K. Newcastle disease virus as a vaccine vector for development of human and veterinary vaccines. Viruses 2016, 8, 183. [Google Scholar] [CrossRef]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H., 3rd; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Kotelkin, A.; Yang, L.; Elankumaran, S.; Murphy, B.R.; Samal, S.K.; Collins, P.L.; Bukreyev, A. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. USA 2007, 104, 9788–9793. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Yang, L.; Samal, S.K.; Murphy, B.R.; Collins, P.L.; Bukreyev, A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine 2010, 29, 17–25. [Google Scholar] [CrossRef]
- Martinez-Sobrido, L.; Gitiban, N.; Fernandez-Sesma, A.; Cros, J.; Mertz, S.E.; Jewell, N.A.; Hammond, S.; Flano, E.; Durbin, R.K.; García-Sastre, A.; et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus Vector. J. Virol. 2006, 80, 1130–1139. [Google Scholar] [CrossRef]
- Maamary, J.; Array, F.; Gao, Q.; Garcia-Sastre, A.; Steinman, R.M.; Palese, P.; Nchinda, G. Newcastle disease virus expressing a dendritic cell-targeted HIV gag protein induces a potent gag-specific immune response in mice. J. Virol. 2011, 85, 2235–2246. [Google Scholar] [CrossRef][Green Version]
- Manoharan, V.K.; Khattar, S.K.; Labranche, C.C.; Montefiori, D.C.; Samal, S.K. Modified Newcastle Disease virus as an improved vaccine vector against Simian Immunodeficiency virus. Sci. Rep. 2018, 8, 8952. [Google Scholar] [CrossRef]
- Yoshida, A.; Kim, S.H.; Manoharan, V.K.; Varghese, B.P.; Paldurai, A.; Samal, S.K. Novel avian paramyxovirus-based vaccine vectors expressing the Ebola virus glycoprotein elicit mucosal and humoral immune responses in guinea pigs. Sci. Rep. 2019, 9, 5520. [Google Scholar] [CrossRef]
- Viktorova, E.G.; Khattar, S.K.; Kouiavskaia, D.; Laassri, M.; Zagorodnyaya, T.; Dragunsky, E.; Samal, S.; Chumakov, K.; Belov, G.A. Newcastle disease virus-based vectored vaccine against Poliomyelitis. J. Virol. 2018, 92, e00976-18. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Kumar, M.; Yang, X.; Akkoyunlu, M.; Collins, P.L.; Samal, S.K.; Pal, U. A host-restricted viral vector for antigen-specific immunization against Lyme disease pathogen. Vaccine 2011, 29, 5294–5303. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.; Ashraf, S.; Munir, I.; Khalid, H.; Muneer, M.A.; Mukhtar, N.; Amin, S.; Ashraf, S.; Imran, M.A.; Chaudhry, U.; et al. Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health. Emerg. Microbes Infect. 2020, 9, 2222–2235. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, E.; Samal, S.K. Newcastle disease virus as a vaccine vector for sars-cov-2. Pathogens 2020, 9, 619. [Google Scholar] [CrossRef]
- Bukreyev, A.; Rollin, P.E.; Tate, M.K.; Yang, L.; Zaki, S.R.; Shieh, W.-J.; Murphy, B.R.; Collins, P.L.; Sanchez, A. Successful topical respiratory tract immunization of primates against Ebola virus. J. Virol. 2007, 81, 6379–6388. [Google Scholar] [CrossRef]
- Hall, A.J.; Lopman, B.A.; Payne, D.C.; Patel, M.M.; Gastañaduy, P.A.; Vinjé, J.; Parashar, U.D. Norovirus disease in the United States. Emerg. Infect. Dis. 2013, 19, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chen, S.; Jiang, X.; Green, K.Y.; Samal, S.K. Retraction for Kim et al. Newcastle Disease Virus Vector Producing Human Norovirus-Like Particles Induces Serum, Cellular, and Mucosal Immune Responses in Mice. J. Virol. 2014, 88, 9718–9727. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Murphy, B.R. Respiratory syncytial virus: Reverse genetics and vaccine strategies. Virology 2002, 296, 204–211. [Google Scholar] [CrossRef][Green Version]
- Crowe, J.E.J. Respiratory syncytial virus vaccine development. Vaccine 2001, 20 (Suppl. 1), S32–S37. [Google Scholar] [CrossRef]
- Nakaya, Y.; Nakaya, T.; Park, M.-S.; Cros, J.; Imanishi, J.; Palese, P.; García-Sastre, A. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J. Virol. 2004, 78, 9366–9375. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.K.; DeVico, A.L.; LaBranche, C.C.; Panda, A.; Montefiori, D.C.; Samal, S.K. Enhanced immune responses to HIV-1 envelope elicited by a vaccine regimen consisting of priming with Newcastle disease virus expressing HIV gp160 and boosting with gp120 and SOSIP gp140 proteins. J. Virol. 2015, 90, 1682–1686. [Google Scholar] [CrossRef]
- Han, G.Z.; Liu, X.P.; Li, S.S. Caution about Newcastle disease virus-based live attenuated vaccine. J. Virol. 2008, 82, 6782. [Google Scholar] [CrossRef]
- Han, G.Z.; He, C.Q.; Ding, N.Z.; Ma, L.Y. Identification of a natural multi-recombinant of Newcastle disease virus. Virology 2008, 371, 54–60. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, L.; Ma, B.; Cui, Z.; Zhu, Y.; Kitamura, Y.; Liu, W. F gene recombination between genotype II and VII Newcastle disease virus. Virus Res. 2008, 131, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Bukreyev, A.; Murphy, B.R. What are the risks—Hypothetical and observed—Of recombination involving live vaccines and vaccine vectors based on nonsegmented negative-strain RNA viruses? J. Virol. 2008, 82, 9805–9806. [Google Scholar] [CrossRef]
- Song, Q.; Cao, Y.; Li, Q.; Gu, M.; Zhong, L.; Hu, S.; Wan, H.; Liu, X. Artificial recombination may influence the evolutionary analysis of Newcastle disease virus. J. Virol. 2011, 85, 10409–10414. [Google Scholar] [CrossRef] [PubMed]
- Riaz, R.; Muhammad, K.; Rabbani, M.; Iqbal, M.A.; Khan, M.R.; Sarfaraz, S.; Naseer, M.; Majeed, K. Immunomodulatory Effect of Newcastle Disease Virus on Inactivated Mycoplasma gallisepticum Vaccine Response in Broilers. Pakistan J. Zool. 2021, 53, 401–800. [Google Scholar] [CrossRef]
- Qadir, M.F.; Han, X.U.; Qiao, M.L.; Wang, Y.; Zhang, D.; Bi1, Y.H.; Jahejo, A.R.; Cheng, Q.Q.; Tian, W.X. Expression of Prostaglandins-Related Genes in Erythrocytes of Chickens Infected with H9N2 Subtype of Avian Influenza Virus. Pakistan J. Zool. 2021, 53, 1201–1601. [Google Scholar] [CrossRef]
- Ge, Y.; Yao, Q.; Chai, H.; Hua, H.; Deng, G. Differential Cytokine Expression Analyses of Cefs and Defs Infected with H6 Influenza Virus Strains Isolated from Anseriformes in Anhui Province, China. Pakistan J. Zool. 2021, 53, 447–452. [Google Scholar] [CrossRef]
- Yaqub, S.; Yaqub, T.; Shabbir, M.Z.; Nadeem, A.; Rahman, A.U.; Shahid, M.F.; Tahir, Z.; Mukhtar, N. Prevalence, Molecular Epidemiology and Drug Resistance Pattern of Human Immunodeficiency Virus Type 1 among Injecting Drug Users in Lahore, Pakistan. Pakistan J. Zool. 2021, 53, 563–572. [Google Scholar] [CrossRef]
| Pathogen | AOaV-1 Backbone | Antigen | Insert Site | Animal Model | Vaccination (Route) | References |
|---|---|---|---|---|---|---|
| HIV-1 | Hitchner B1 | Gag | P/M | Mouse | i.n. | [15] |
| HIV-1 | La Sota | Gag | P/M | Mouse | i.n. | [94] |
| HIV-1 | La Sota | Gag; Env; Gag + Env | Env- P/M and Gag- HN/L; Gag- O/M and Env- HN/L; Env + Gag- P/M; Env- P/M; Gag- P/M | Guinea pigs/Mouse | i.n. | [30] |
| SIV | La Sota | gp160 | P/M | Guinea pigs/Mouse | i.n. | [95] |
| EBOV | Beaudette C and La Sota | GP | P/M | Rhesus monkeys | i.n/i.t. | [92] |
| EBOV | Chimeric AOaV-1 | GP | N/P, P/M, and M/F | Guinea pigs | i.n. | [96] |
| HPIV-3 | Beaudette C and La Sota | HN | P/M | African green monkeys and rhesus monkeys | i.n./i.t. | [18] |
| RSV | Hitchner B1 | F | P/M | Mouse | i.n. | [93] |
| Poliovirus | La Sota | P1 and 3CD | P1- P/M and 3CD- HN/L | Guinea pigs | i.n. | [97] |
| Lyme | LaSota/VF | BmpA + OspC | P/M | Hamsters | i.n./i.m./i.p. | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, J.; Rohaim, M.A.; Munir, M. Avian Orthoavulavirus Type-1 as Vaccine Vector against Respiratory Viral Pathogens in Animal and Human. Vaccines 2022, 10, 259. https://doi.org/10.3390/vaccines10020259
Vilela J, Rohaim MA, Munir M. Avian Orthoavulavirus Type-1 as Vaccine Vector against Respiratory Viral Pathogens in Animal and Human. Vaccines. 2022; 10(2):259. https://doi.org/10.3390/vaccines10020259
Chicago/Turabian StyleVilela, Julianne, Mohammed A. Rohaim, and Muhammad Munir. 2022. "Avian Orthoavulavirus Type-1 as Vaccine Vector against Respiratory Viral Pathogens in Animal and Human" Vaccines 10, no. 2: 259. https://doi.org/10.3390/vaccines10020259
APA StyleVilela, J., Rohaim, M. A., & Munir, M. (2022). Avian Orthoavulavirus Type-1 as Vaccine Vector against Respiratory Viral Pathogens in Animal and Human. Vaccines, 10(2), 259. https://doi.org/10.3390/vaccines10020259








