COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance
Abstract
:1. Introduction
2. Challenges Obstructing Global COVID-19 Vaccine Rollout
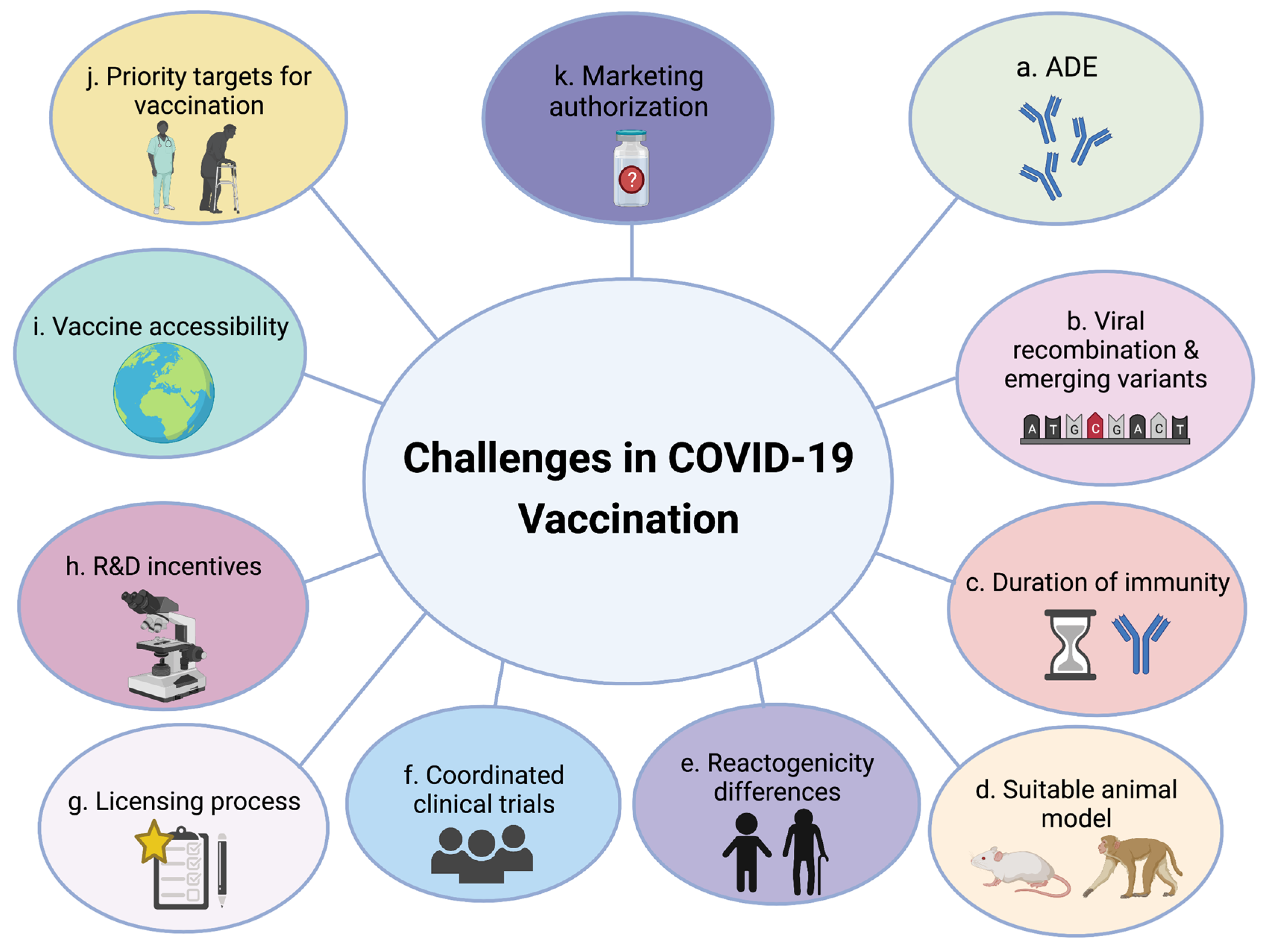
2.1. Challenges in Vaccine Development
2.2. Challenges in Vaccine Dissemination and Deployment
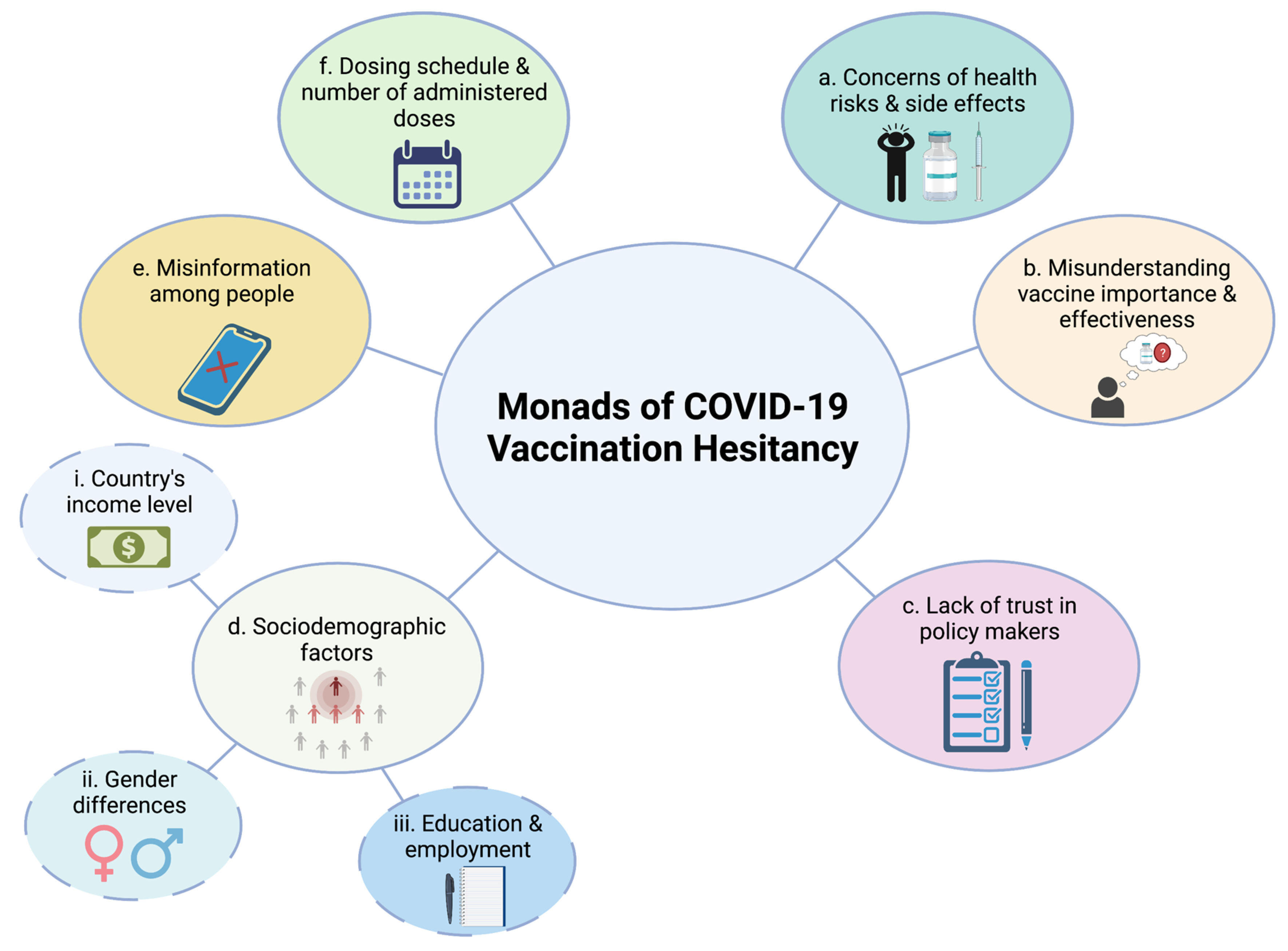
3. Monads of COVID-19 Vaccination Hesitancy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 1 July 2020).
- Güner, R.; Hasanoğlu, I.; Aktaş, F. COVID-19: Prevention and control measures in community. Turk. J. Med. Sci. 2020, 50, 571–577. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A review of the progress and challenges of developing a vaccine for COVID-19. Front. Immunol. 2020, 11, 2413. [Google Scholar] [CrossRef]
- Jung, F.; Krieger, V.; Hufert, F.; Küpper, J.-H. Herd immunity or suppression strategy to combat COVID-19. Clin. Hemorheol. Microcirc. 2020, 75, 1–5. [Google Scholar] [CrossRef]
- Daoust, J.F. Elderly people and responses to COVID-19 in 27 countries. PLoS ONE 2020, 15, e0235590. [Google Scholar] [CrossRef]
- Ko, J.Y.; Danielson, M.L.; Town, M.; Derado, G.; Greenlund, K.J.; Kirley, P.D.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; Ryan, P.A.; et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin. Infect. Dis. 2021, 72, e695–e703. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA73/A73_R1-en.pdf (accessed on 19 May 2020).
- Al-Jighefee, H.T.; Najjar, H.; Ahmed, M.N.; Qush, A.; Awwad, S.; Kamareddine, L. COVID-19 vaccine platforms: Challenges and safety contemplations. Vaccines 2021, 9, 1196. [Google Scholar] [CrossRef]
- Machingaidze, S.; Wiysonge, C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021, 27, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- McGill COVID19 Vaccine Tracker Team. Available online: https://covid19.trackvaccines.org/vaccines/ (accessed on 27 June 2021).
- Bahans, C.; Leymarie, S.; Malauzat, D.; Girard, M.; Demiot, C. Ethical considerations of the dynamics of clinical trials in an epidemic context: Studies on COVID-19. Ethics Med. Public Health 2021, 16, 100621. [Google Scholar] [CrossRef]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Reno, C.; Cocchio, S.; Fantini, M.P. SARS-CoV-2/COVID-19 vaccines: The promises and the challenges ahead. Vaccines 2021, 9, 21. [Google Scholar] [CrossRef]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef] [Green Version]
- Wiysonge, C.S.; Ndwandwe, D.; Ryan, J.; Jaca, A.; Batouré, O.; Anya, B.M.; Cooper, S. Vaccine hesitancy in the era of COVID-19: Could lessons from the past help in divining the future? Hum. Vaccines Immunother. 2021, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Böhm, R.; Chapman, G.B. Using Behavioral Insights to Increase Vaccination Policy Effectiveness. Policy Insights Behav. Brain Sci. 2015, 2, 61–73. [Google Scholar] [CrossRef]
- Brewer, N.T.; Chapman, G.B.; Rothman, A.J.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science Into Action. Psychol. Sci. Public Interest 2017, 18, 149–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Hesitancy, S. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Robinson, K.; Vallée-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef]
- Hotez, P.J.; Corry, D.B.; Bottazzi, M.E. COVID-19 vaccine design: The Janus face of immune enhancement. Nat. Rev. Immunol. 2020, 20, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020, 94, e02015-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Zhang, T.; Gu, Y.; Li, S.; Xia, N. Current progress and challenges in the design and development of a successful COVID-19 vaccine. Fundam. Res. 2021, 1, 139–150. [Google Scholar] [CrossRef]
- Agrawal, A.S.; Tao, X.; Algaissi, A.; Garron, T.; Narayanan, K.; Peng, B.H.; Couch, R.B.; Tseng, C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccines Immunother. 2016, 12, 2351–2356. [Google Scholar] [CrossRef]
- Tseng, C.T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Gribble, J.; Stevens, L.J.; Agostini, M.L.; Anderson-Daniels, J.; Chappell, J.D.; Lu, X.; Pruijssers, A.J.; Routh, A.L.; Denison, M.R. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021, 17, e1009226. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, W.G. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed. Pharmacother. 2021, 136, 111272. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Platt, D.; Parida, L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 2020, 98, 495. [Google Scholar] [CrossRef]
- Wu, K.; Werner, A.P.; Moliva, J.I.; Koch, M.; Choi, A.; Stewart-Jones, G.B.E.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; Graham, B.S.; et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020, 53, 925–933. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of covid-19 vaccination against risk of symptomatic infection, hospitalization, and death up to 9 months: A Swedish total-population cohort study. Lancet 2021, 9. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Z.; Duan, C.; Chen, Z.; Wang, G.; Lu, Y.; Li, M.; Lu, J. Long-term persistence of IgG antibodies in SARS-CoV infected healthcare workers. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.D. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe 2021, 29, 44–57. [Google Scholar] [CrossRef]
- Prado-Vivar, B.; Becerra-Wong, M.; Guadalupe, J.J. COVID-19 re-infection by a phylogenetically distinct SARS-CoV-2 variant, first confirmed event in South America. First Confirmed Event S. Am. 2020. [Google Scholar] [CrossRef]
- Takeda, C.F.V.; de Almeida, M.M.; Gomes, R.G.d.; Souza, T.C.; Mota, M.A.d.; Cavalcanti, L.P.d.; Colares, J.K.B. Case report: Recurrent clinical symptoms of COVID-19 in healthcare professionals: A series of cases from Brazil. Am. J. Trop. Med. Hyg. 2020, 103, 1993. [Google Scholar] [CrossRef] [PubMed]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, D. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- To, K.K.; Hung, I.F.; Ip, J.D.; Chu, A.W.; Chan, W.-M.; Tam, A.R.; Fong, C.H.; Yuan, S.; Tsoi, H.-W.; Ng, A.C. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 73, e2946–e2951. [Google Scholar] [CrossRef]
- Van Elslande, J.; Vermeersch, P.; Vandervoort, K.; Wawina-Bokalanga, T.; Vanmechelen, B.; Wollants, E.; Laenen, L.; André, E.; van Ranst, M.; Lagrou, K. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin. Infect. Dis. 2020, 73, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Muik, A.; Wallisch, A.-K.; Sänger, B.; Swanson, K.A.; Mühl, J.; Chen, W.; Cai, H.; Sarkar, R.; Türeci, Ö.; Dormitzer, P.R.; et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. BioRxiv 2021, 371, 1152–1153. [Google Scholar]
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Tanne, J.H. Covid-19: Moderna plans booster doses to counter variants. BMJ 2021, 372, n232. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- News. Pfizer to Seek FDA Authorization for Third, Booster Dose of Its Covid-19 Vaccine. 8 July 2021. Available online: https://www.statnews.com/2021/07/08/pfizer-to-seek-fda-authorization-for-third-booster-dose-of-covid19-vaccine/ (accessed on 5 November 2021).
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- News. US Panel Backs COVID-19 Boosters Only for Seniors, High-Risk. 17 September 2021. Available online: https://apnews.com/article/fda-panel-rejects-widespread-pfizer-booster-shots-1cd1cf6a5c5c02b63f8a7324807a59f1 (accessed on 5 November 2021).
- Moderna. Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emerging Variants First Identified in the U.K. and the Republic of South Africa. 2021. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against (accessed on 5 November 2021).
- New York Times. F.D.A. Panel Recommends Booster for Many Moderna Vaccine Recipients. Available online: https://www.nytimes.com/2021/10/14/us/politics/fda-moderna-vaccine-boosters.html (accessed on 5 November 2021).
- Moderna. Moderna Announces Submission of Initial Data to U.S. FDA for Its COVID-19 Vaccine Booster. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-submission-initial-data-us-fda-its-covid-19 (accessed on 5 November 2021).
- Kyei-Barffour, I.; Addo, S.A.; Aninagyei, E.; Ghartey-Kwansah, G.; Acheampong, D.O. Sterilizing immunity against COVID-19: Developing helper T cells I and II activating vaccines is imperative. Biomed. Pharmacother. 2021, 144, 112282. [Google Scholar] [CrossRef]
- Dobrowolski, Z. The strategy of vaccination and global pandemic: How framing may thrive on strategy during and after Covid-19. Eur. Res. Stud. J. 2021, 24, 532–541. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Hansen, J. Intranasal COVID-19 vaccine candidate shows sterilizing immunity in preclinical tests. UAB News, 10 May 2021. [Google Scholar]
- Altimmune. Altimmune Announces Update on AdCOVID™ Phase 1 Clinical Trial; Altimmune Inc.: Gaithersburg, MD, USA, 2021. [Google Scholar]
- Kojima, N.; Klausner, J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Doshi, P. Covid-19: Do many people have pre-existing immunity? BMJ 2020, 370, m3563. [Google Scholar] [CrossRef] [PubMed]
- Redd, A.D.; Nardin, A.; Kared, H.; Bloch, E.M.; Pekosz, A.; Laeyendecker, O.; Abel, B.; Fehlings, M.; Quinn, T.C.; Tobian, A.A. CD8+ T cell responses in COVID-19 convalescent individuals target conserved epitopes from multiple prominent SARS-CoV-2 circulating variants. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. COVID Week 19 Vaccine Surveillance Report 36; PHE: London, UK, 2021. [Google Scholar]
- Deb, B.; Shah, H.; Goel, S. Current global vaccine and drug efforts against COVID-19: Pros and cons of bypassing animal trials. J. Biosci. 2020, 45, 82. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Campbell, J.D.; Creech, C.B.; Frenck, R.; Kamidani, S.; Munoz, F.M.; Nachman; Spearman, S.P. Warp speed for coronavirus disease 2019 (COVID-19) vaccines: Why are children stuck in neutral? Clin. Infect. Dis. 2021, 73, 336–340. [Google Scholar] [CrossRef]
- Zimet, G.D.; Silverman, R.D.; Fortenberry, J.D. Coronavirus disease 2019 and vaccination of children and adolescents: Prospects and challenges. J. Pediatrics 2021, 231, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhao, Y.; Wang, F.; Chen, Y.; Kaminga, A.C.; Xu, H. Comorbidities’ potential impacts on severe and non-severe patients with COVID-19: A systematic review and meta-analysis. Medicine 2021, 100, e24971. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Pepperrell, T.; Rodgers, F.; Tandon, P.; Sarsfield, K.; Pugh-Jones, M.; Rashid, T.; Keestra, S. Making a COVID-19 vaccine that works for everyone: Ensuring equity and inclusivity in clinical trials. Glob. Health Action 2021, 14, 1892309. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pan, D.; Nevill, C.R.; Gray, L.J.; Martin, C.A.; Nazareth, J.; Minhas, J.S.; Divall, P.; Khunti, K.; Abrams, K.R.; et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClin. Med. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Chakrabarti, A.; Robinson, D.; Chirgwin, K.; Lumpkin, M. Navigating facilitated regulatory pathways during a disease X pandemic. NPJ Vaccines 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Ritchie, H.; Ortiz, O.E.; Beltekian, D.; Mathieu, E.; Hasell, J.; Macdonald, B.; Giattino, C.; Appel, C.; Rodés-Guirao, L.; Roser, M. Coronavirus Pandemic (COVID-19). Our World Data. 2020. Available online: https://ourworldindata.org/coronavirus/country/south-africa?country=~ZAF (accessed on 5 November 2021).
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.; Anderson, M.; Jit, M.; Mossialos, E. Ensuring access and affordability through COVID-19 vaccine research and development investments: A proposal for the options market for vaccines. Vaccine 2020, 38, 6075. [Google Scholar] [CrossRef]
- Castillo, J.C.; Ahuja, A.; Athey, S.; Baker, A.; Budish, E.; Chipty, T.; Glennerster, R.; Kominers, S.D.; Kremer, M.; Larson, G.; et al. Market design to accelerate COVID-19 vaccine supply. Science 2021, 371, 1107–1109. [Google Scholar] [CrossRef]
- Soy, A. Africa’s long wait for the Covid-19 vaccine. BBC News, 22 January 2021. [Google Scholar]
- Yan, Y.; Pang, Y.; Lyu, Z.; Wang, R.; Wu, X.; You, C.; Zhao, H.; Manickam, S.; Lester, E.; Wu, T.; et al. The COVID-19 Vaccines: Recent Development, Challenges and Prospects. Vaccines 2021, 9, 349. [Google Scholar] [CrossRef]
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746. [Google Scholar] [CrossRef]
- Gavi. Cold Supply for Hot Demand; Gavi: Geneva, Switzerland, 2021. [Google Scholar]
- Fidler, D.P. Negotiating equitable access to influenza vaccines: Global health diplomacy and the controversies surrounding avian influenza H5N1 and pandemic influenza H1N1. PLoS Med. 2012, 7, e1000247. [Google Scholar]
- Whalen, J. Rich nations lock in flu vaccine as poor ones fret. Wall Street Journal, 16 May 2009. [Google Scholar]
- Yamey, G.; Schäferhoff, M.; Hatchett, R.; Pate, M.; Zhao, F.; McDade, K.K. Ensuring global access to COVID-19 vaccines. Lancet 2020, 395, 1405–1406. [Google Scholar] [CrossRef]
- Brown, D. Most of any vaccine for new flu strain could be claimed by rich nations’ preexisting contracts. Wash. Post, 7 May 2009. [Google Scholar]
- Markus, D. Medical apartheid: The dark history of medical experimentation on black Americans from colonial times to the present. Harv. J. Afr. Am. Public Policy 2008, 14, 85–88. [Google Scholar]
- Schoch-Spana, M.; Brunson, E.K.; Long, R.; Ruth, A.; Ravi, S.J.; Trotochaud, M.; Borio, L.; Brewer, J.; Buccina, J.; Connell, N.; et al. The public’s role in COVID-19 vaccination: Human-centered recommendations to enhance pandemic vaccine awareness, access, and acceptance in the United States. Vaccine 2020, 39, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- SteelFisher, G.K.; Blendon, R.J.; Bekheit, M.M.; Lubell, K. The public’s response to the 2009 H1N1 influenza pandemic. N. Engl. J. Med. 2010, 362, e65. [Google Scholar] [CrossRef] [Green Version]
- Persad, G.; Peek, M.E.; Emanuel, E.J. Fairly prioritizing groups for access to COVID-19 vaccines. JAMA 2020, 324, 1601–1602. [Google Scholar] [CrossRef]
- Schmidt, H. Vaccine rationing and the urgency of social justice in the Covid-19 response. Hastings Cent. Rep. 2020, 50, 46–49. [Google Scholar] [CrossRef]
- Mukumbang, F.C. Are asylum seekers, refugees and foreign migrants considered in the COVID-19 vaccine discourse? BMJ Glob. Health 2020, 5, e004085. [Google Scholar] [CrossRef]
- European Commission. Preparedness for COVID-19 Vaccination Strategies and Vaccine Deployment; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Centre for Disease Prevention and Control. Key Aspects Regarding the Introduction and Prioritisation of COVID-19 Vaccination in the EU/EEA and the UK; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Rutten, L.J.F.; Zhu, X.; Leppin, A.; Ridgeway, J.L.; Swift, M.; Griffin, J.M.; St Sauver, J.L.; Virk, A.; Jacobson, R.M. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clinic. Proc. 2020, 96, 699–707. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Mereckiene, J.; Cotter, S.; Weber, J.T.; Nicoll, A.; Ancona, F.; Lopalco, P.L.; Johansen, K.; Wasley, A.M.; Jorgensen, P.; Lévy-Bruhl, D.; et al. Influenza A (H1N1) pdm09 vaccination policies and coverage in Europe. Eurosurveillance 2012, 17, 20064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.A.; Ruiter, R.A.C.; Chapman, G.; Kok, G. The intention to get vaccinated against influenza and actual vaccination uptake of Dutch healthcare personnel. Vaccine 2014, 32, 6986–6991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.-L. Barriers of influenza vaccination intention and behavior—A systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccine Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar]
- Gender Differences in the Intention to Get Vaccinated Against COVID-19—A Systematic Review and Meta-Analysis. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3803323 (accessed on 1 December 2021).
- de Figueiredo, A.; Simas, C.; Larson, H.J. COVID-19 vaccine acceptance and its socio-demographic and emotional determinants: A multi-country cross-sectional study. medRxiv 2021. [Google Scholar] [CrossRef]
- Carrieri, V.; Madio, L.; Principe, F. Vaccine hesitancy and (fake) news: Quasi-experimental evidence from Italy. Health Econ. 2019, 28, 1377–1382. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Mello, M.M.; Silverman, R.D.; Omer, S.B. Ensuring uptake of vaccines against SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1296–1299. [Google Scholar] [CrossRef]
- Megget, K. Even covid-19 can’t kill the anti-vaccination movement. BMJ 2020, 369, m2184. [Google Scholar] [CrossRef]
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; van Exel, J.; Schreyögg, J.; Stargardt, T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020, 21, 977–982. [Google Scholar] [CrossRef]
- Germani, F.; Biller-Andorno, N. The anti-vaccination infodemic on social media: A behavioral analysis. PLoS ONE 2021, 16, e0247642. [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, G. Anti-Vaccine Activists Use a Federal Database to Spread Fear About COVID Vaccines. 14 June 2021. Available online: https://www.npr.org/sections/health-shots/2021/06/14/1004757554/anti-vaccine-activists-use-a-federal-database-to-spread-fear-about-covid-vaccine (accessed on 1 December 2021).
- Burki, T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit. Health 2020, 2, e504–e505. [Google Scholar] [CrossRef]
- The New York Times. Tracking Coronavirus Vaccinations around the World. 13 November 2021. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 1 December 2021).
- El-Mohandes, A.; White, T.M.; Wyka, K.; Rauh, L.; Rabin, K.; Kimball, S.H.; Ratzan, S.C.; Lazarus, J.V. COVID-19 vaccine acceptance among adults in four major US metropolitan areas and nationwide. Sci. Rep. 2021, 11, 21844. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. Mix-and-match COVID vaccines: The case is growing, but questions remain. Nature 2021, 595, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Shekhar, R.; Kottewar, S.; Upadhyay, S.; Singh, M.; Pathak, D.; Kapuria, D.; Barrett, E.; Sheikh, A.B. COVID-19 vaccine hesitancy and attitude toward booster doses among US healthcare workers. Vaccines 2021, 9, 1358. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, H.; Al-Jighefee, H.T.; Qush, A.; Ahmed, M.N.; Awwad, S.; Kamareddine, L. COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance. Vaccines 2021, 9, 1474. https://doi.org/10.3390/vaccines9121474
Najjar H, Al-Jighefee HT, Qush A, Ahmed MN, Awwad S, Kamareddine L. COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance. Vaccines. 2021; 9(12):1474. https://doi.org/10.3390/vaccines9121474
Chicago/Turabian StyleNajjar, Hoda, Hadeel T. Al-Jighefee, Abeer Qush, Muna Nizar Ahmed, Sara Awwad, and Layla Kamareddine. 2021. "COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance" Vaccines 9, no. 12: 1474. https://doi.org/10.3390/vaccines9121474
APA StyleNajjar, H., Al-Jighefee, H. T., Qush, A., Ahmed, M. N., Awwad, S., & Kamareddine, L. (2021). COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance. Vaccines, 9(12), 1474. https://doi.org/10.3390/vaccines9121474





